Introduction
Recent epidemiological data indicate that consuming
plant-based dietary products offers protection from cancer and
reduces cancer risk. Among the dietary products studied, garlic,
Allium sativum, and related Allium vegetables are
known for their anticancer potential. Garlic is a member of the
lily family and has been widely cultivated and consumed as a food
in numerous countries for the past 10,000 years and has been widely
used as a popular remedy for various disorders for thousands of
years. Compounds in garlic have been recently demonstrated to
suppress carcinogen-induced tumor growth in vitro and in
vivo (1–3). Epidemiological findings also suggest
an inverse relationship between garlic consumption and the
incidence of various types of cancers (4–6).
Cell cycle dysregulation and resistance to apoptosis
are hallmarks of cancer cells; thus, approaches to induce cell
cycle arrest and stimulate apoptotic action could be effective
targets for antitumor intervention. Therefore, recent studies have
offered novel insights into the molecular mechanisms of garlic
component-induced cell cycle arrest and apoptosis (7,8). Modem
et al (9) reported that
fresh garlic clove extract arrests breast cancer cell growth at the
G1 phase. Lund et al (10)
and Frantz et al (11)
reported that a water-soluble extract of garlic arrests breast and
colon cancer cells at the G2/M boundary causing apoptosis. In
addition, a crude garlic extract was found to cause
caspase-dependent apoptosis in colon cancer cells by modulating
Bcl-2 family proteins and mitochondrial dysfunction (12). We previously found that the
generation of cellular reactive oxygen species (ROS) plays a
pivotal role in initiating apoptotic death by garlic clove hexane
extracts in human hepatocarcinoma cells (13). These data suggest that garlic
components may affect different signaling pathways according to
cell type or culture conditions. These effects are selective for
cancer cells, as normal cell lines are resistant to cell cycle
arrest and apoptosis following treatment with garlic components
(14,15). Moreover, a garlic extract was found
to reduce the side effects caused by anticancer agents (1).
We isolated the novel phenylamine derivative
N-benzyl-N-methyldecan-1-amine (NBNMA) from garlic cloves during
the course of our bioactive natural product screening program of
medicinal foods. To date, no studies have reported the anticancer
activity of NBNMA; therefore, we conducted the present study to
investigate the in vitro anti-leukemic properties of this
compound to substantiate its anticancer activity. We used the human
leukemia U937 cell line to identify the molecular effects of NBNMA
and found that NBNMA induced G2/M arrest and apoptosis.
Materials and methods
Plant materials and isolation of the pure
compound
Garlic cloves were purchased directly from the
Danyang Food Co. (Danyang, Korea) in January, 2009. The
freeze-dried garlic cloves (1 kg) were ground to a fine powder and
then successively extracted at room temperature with n-hexane,
ethyl acetate (EtOAc) and 70% ethanol (EtOH) by using 3,000 ml of
each solvent three times to obtain a 3.55 g hexane extract, a 1.12
g EtOAc extract, and a 51.05 g EtOH extract. The EtOH extract (13
g) was evaporated en vacuo and chromatographed on a Diaion
HP20 Resin (0.35 mm; Supelco, Bellefonte, PA, USA) column (30 × 3
cm) with a step gradient (0, 25, 50 and 90%) of EtOH in water and
methanol (MeOH) to obtain 21 fractions. Fraction GDPIEIDIP-II
(221.3 mg) was separated on a Sephadex LH20 (70 μm; Pharmacia
Biotech AB, Uppsala, Sweden) column (100 × 30 cm) with
CHCl3:MeOH:dH2O (65:35:10) to yield the pure
compound (61.2 mg).
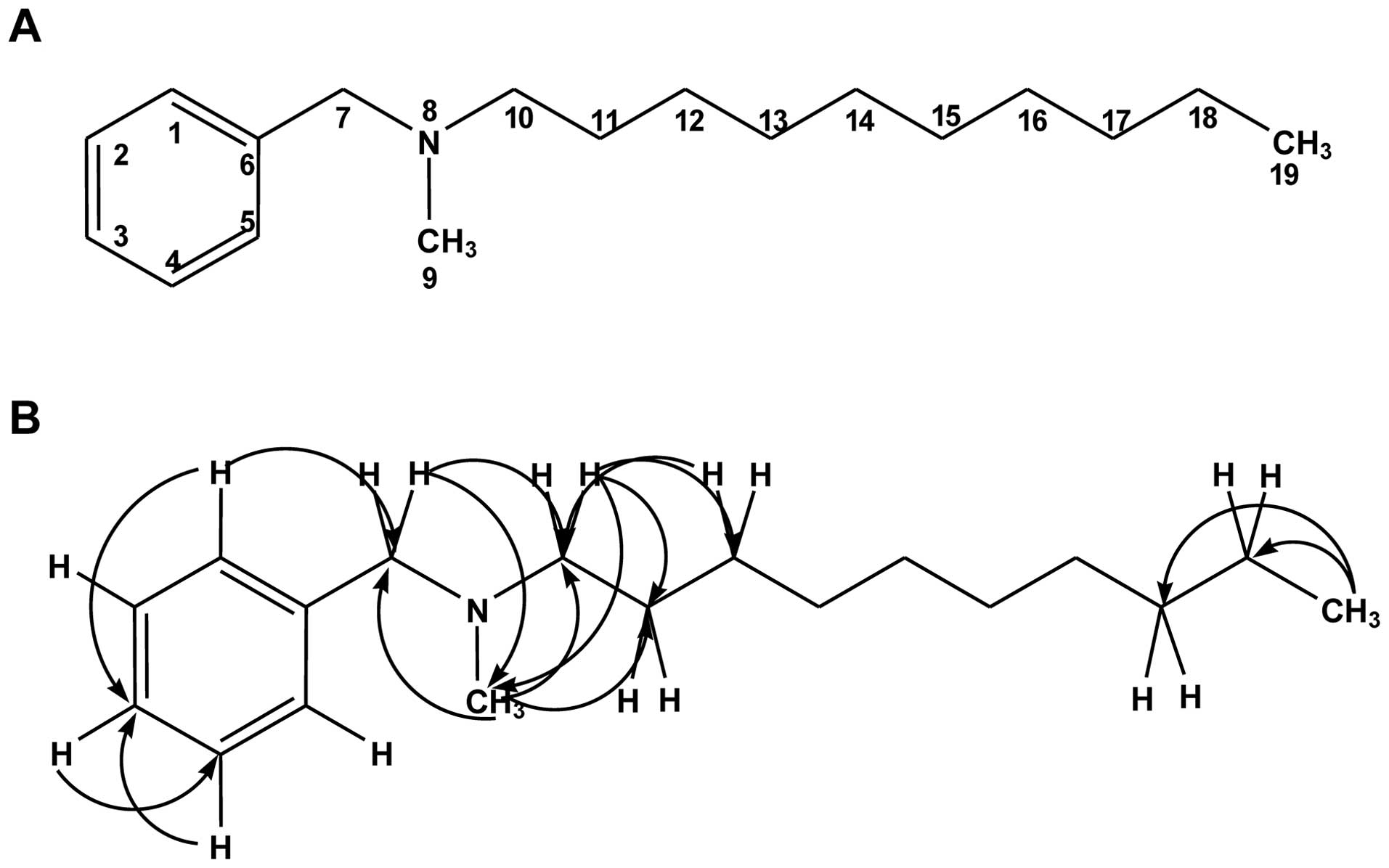
Determination of the NBNMA structure
NBNMA was obtained as a white sticky compound in
MeOH. Liquid chromatography mass-spectrometry analyses indicated a
molecular ion at m/z 283 corresponding to [M + Na]+;
thus, indicating a molecular formula of
C18H30NNa. One- and two-dimensional nuclear
magnetic resonance (NMR) analyses with homonuclear and
heteronuclear direct and long-range correlations permitted
assignments of the 1H and 13C NMR resonances
as listed in Table I. The
13C NMR and distortionless enhanced polarization
transfer spectra showed 18 signals, including six carbons for one
aromatic ring, one phenylic methylene at δC 68.95, one
N-methylene at δC 65.97, one N-methyl at δC
50.34, eight acyclic carbons at δC 23.82–33.17, and one
terminal methyl at δC 14.40. The three aromatic protons
of the phenyl moiety resonated at δH 7.59, 7.53 and
7.55, respectively, and one phenylic methylene group was located at
δH 4.57. One-proton resonance at δH 3.05 was
due to N-methyl protons. The nine-proton multiplet at δH
1.32–1.34 was indicative of an acyclic saturated hydrocarbon group.
Heteronuclear multiple-bond correlation spectroscopy (HMBC)
correlations observed between H-9 and C-7 and C-10 and from H-7 to
C-1, C-5, C-9, and C-10 suggested the presence of an amine group at
N-8 attached to the phenyl ring. Cross-peaks were also observed
between H-10 and C-7 and C-9 and between H-9 and C-7 and C-10.
Correlations between H-19 and C-18 and C-17 established that the
terminal methyl of the acyclic methyl group was at C-19. Moreover,
the HMBC spectrum confirmed the positions of the N-methyl groups,
showing correlations between the N-CH3 protons at
δH 3.05 with N-8, and the N-methylene protons showed
correlations between resonances at δH 3.33
(N-CH2) and C-11 and C-12. The connectivity of NBNMA was
deduced from this information, and the absolute configuration of
the compound was established (Fig.
1).
 | Table I1H (600 MHz) and
13C NMR (150 MHz) data of NBNMA. |
Table I
1H (600 MHz) and
13C NMR (150 MHz) data of NBNMA.
| Position | δC | δH | HMBC |
|---|
| 1, 5 | 134.26 × 2 | CH | 7.59, d J=7.8 | 134.26, 132.02,
68.95 |
| 2, 4 | 130.47 × 2 | CH | 7.53, t | 130.47, 128.10 |
| 3 | 132.02 | CH | 7.55, t | 134.26 |
| 6 | 128.10 | C | | |
| 7 | 68.95 | CH2 | 4.57, s | 134.26, 128.10,
65.97, 50.34 |
| 9 | 50.34 | CH3 | 3.05, s | 68.95, 65.97 |
| 10 | 65.97 | CH2 | 3.33, t | 68.95, 50.34,
27.57, 23.82 |
| 11 | 23.85 | CH2 | 1.90, m, 1.34,
m | 65.97, 27.57 |
| 12 | 27.57 | CH2 | 1.38, m | |
| 13–16 | 30.85 | CH2 | 1.32–1.34 | |
| 30.74 | CH2 | 1.32–1.34 | |
| 30.67 | CH2 | 1.32–1.34 | |
| 30.58 | CH2 | 1.32–1.34 | |
| 30.35 | CH2 | 1.41, t | 27.57 |
| 17 | 33.17 | CH2 | 1.32, m | |
| 18 | 23.82 | CH2 | 1.90, m, 1.34,
m | |
| 19 | 14.60 | CH3 | 0.90, t | 33.17, 23.82 |
Cell culture
U937 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
RPMI-1640 medium containing 10% fetal bovine serum and 1%
antibiotics (penicillin and streptomycin; Gibco-BRL, Grand Island,
NY, USA) under humidified conditions with 5% CO2 at
37°C.
Cell viability assay
Cells (1×105 cells/ml) were seeded in a
6-well plate. After a 24-h incubation, the cells were treated with
various concentrations of NBNMA for 24 h. Then, 0.5 mg/ml MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Sigma-Aldrich Chemical Co., St. Louis, MO, USA)] solution was
added, and the plates were incubated for an additional 2 h at 37°C.
The medium was subsequently removed, and dimethyl sulfoxide
(Sigma-Aldrich) was added. Optical density was measured at 540 nm
using a microplate spectrophotometer (Dynatech Laboratories,
Chantilly, VA, USA).
DAPI staining
A morphological analysis of the treated cells was
conducted by fluorescence microscopy using
4,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining to
determine whether the growth inhibitory activity of NBNMA is
related to the induction of apoptosis. Briefly, the cells were
collected and fixed with 3.7% paraformaldehyde (Sigma-Aldrich) in
PBS for 10 min at room temperature. The fixed cells were washed
with PBS and stained with 2.5 μg/ml DAPI solution for 10 min just
prior to observation using a fluorescence microscope (Carl Zeiss,
Oberkochen, Germany)
Cell cycle analysis
The cells were exposed to NBNMA for 24 h to monitor
their distribution at various phases of the cell cycle. Then, the
cells were incubated with 50 μg/ml propidium iodide (Sigma-Aldrich)
and 0.1% Triton X-100 (Sigma-Aldrich) in the dark. After a 30-min
incubation, the cells were analyzed by FACScan flow cytometry
(Becton-Dickinson, San Jose, CA, USA) equipped with a 488 nm argon
laser (16).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total-RNA was extracted using an RNeasy kit (Qiagen,
La Jolla, CA, USA), and cDNA was synthesized using an RNA PCR kit
(Takara Biomedicals, Osaka, Japan) with the oligo dT primers
supplied (Table II), according to
the manufacturer’s instructions. The resulting amplification
products were separated electrophoretically on a 1% agarose gel and
visualized by ethidium bromide (Sigma-Aldrich) staining. In a
parallel experiment, amplification of glyceraldehyde-3-phosphate
dehydrogenase was used as an internal control to test the integrity
of all cDNA and to provide a measure of relative expression.
 | Table IIGene-specific primers for RT-PCR. |
Table II
Gene-specific primers for RT-PCR.
| Gene | Primer
sequences |
|---|
| Cdk2 | S: 5′-GCT TTC TGC
CAT TCT CAT CG-3′
A: 5′-GTC CCC AGA GTC CGA AAG AT-3′ |
| Cdk4 | S: 5′-ACG GGT GTA
AGT GCC ATC TG-3′
A: 5′-TGG TGT CGG TGC CTA TGG GA-3′ |
| p21 | S: 5′-CTC AGA GGA
GGC GCC ATG-3′
A: 5′-GGG CGG ATT AGG GCT TCC-3′ |
| Cyclin | A S: 5′-TCC AAG AGG
ACC AGG AGA ATA TCA-3′
A: 5′-TCC TCA TGG TAG TCT GGT ACT TCA-3′ |
| Cyclin | B1 S: 5′-AAG AGC
TTT AAA CTT TGG TCT GGG-3′
A: 5′-CTT TGT AAG TCC TTG ATT TAC CAT G-3′ |
| Bcl-2 | S: 5′-CAG CTG CAC
CTG ACG-3′
A: 5′-GCT GGG TAG GTG CAT-3′ |
| Bcl-xL | S: 5′-CGG GCA TTC
AGT GAC CTG AC-3′
A: 5′-TCA GGA ACC AGC GGT TGA AG-3′ |
| Bax | S: 5′-ATG GAC GGG
TCC GGG GAG-3′
A: 5′-TCA GCC CAT CTT CTT CCA-3′ |
| Bad | S: 5′-CAG TGA TCT
GCT CCA CAT TC-3′
A: 5′-TCC AGC TAG GAT GAT AGG AC-3′ |
| XIAP | S: 5′-GAA GAC CCT
TGG GAA CAA CA-3′
A: 5′-CGC CTT AGC TGC TCT CTT CAG T-3′ |
| cIAP-1 | S: 5′-TGA GCA TGC
AGA CAC ATG C-3′
A: 5′-TGA CGG ATG AAC TCC TGT CC-3′ |
| cIAP-2 | S: 5′-CAG AAT TGG
CAA GAG CTG G-3′
A: 5′-CAC TTG CAA GCT GCT CAG G-3′ |
| GAPDH | S: 5′-CGG AGT CAA
CGG ATT TGG TCG TAT-3′
A: 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ |
Western blot analysis
Total cellular protein was isolated from cells
washed once in cold PBS and then suspended in 100 μl lysis buffer
(10 mM Tris-HCl, pH 8.0, 0.32 M sucrose, 1% Triton X-100, 5 mM
EDTA, 2 mM DTT, 1 mM phenylmethanesulfonyl fluoride). Cytosolic and
mitochondrial fractions were prepared using a mitochondrial/cytosol
fractionation kit (Alexis Biochemicals, San Diego, CA, USA),
according to the manufacturer’s instructions. The protein content
was determined with the Bio-Rad protein assay reagent (Hercules,
CA, USA), using bovine serum albumin as the standard. Protein
extracts were reconstituted in sample buffer [0.062 M Tris-HCl, 2%
sodium dodecyl sulfate (SDS), 10% glycerol, 5% β-mercaptoethanol],
and the mixture was boiled for 10 min. Equal amounts of the
denatured protein sample were loaded into each lane, separated by
SDS-polyacrylamide gel electrophoresis, and transferred to
polyvinylidene difluoride membranes (Millipore, Milford, MA, USA).
The membranes were incubated with primary antibodies for 2 h,
washed twice, and stained with enzyme-linked secondary antibodies
(Amersham, Arlington Heights, IL, USA), which were then detected
with an enhanced chemiluminescence kit (Millipore) and
autoradiography using X-ray film. The primary antibodies were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA),
Cell Signaling Technology (Beverly, MA, USA) and Calbiochem (San
Diego, CA, USA).
In vitro caspase activity assay
Caspase activity was determined with a colorimetric
assay kit that used synthetic tetrapeptides [Asp-Glu-Val-Asp (DEAD)
for caspase-3, Ile-Glu-Thr-Asp (IETD) for caspase-8,
Leu-Glu-His-Asp (LEHD) for caspase-9] labeled with p-nitroaniline
(pNA), following the manufacturer’s instructions. Briefly, cells
were lysed in the lysis buffer supplied by the manufacturer and
according to the protocol. The supernatants were collected and
incubated with the supplied reaction buffer containing DTT and
DEAD-pNA, IETD-pNA, or LEHD-pNA as the substrate at 37°C. The
reactions were measured by changes in absorbance at 405 nm using a
microplate reader.
Measurement of mitochondrial membrane
potential (MMP)
MMP values were determined with the dual-emission
potential-sensitive probe
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1; Sigma-Aldrich), which selectively enters mitochondria, and
the color changes reversibly from red to green as the MMP
decreases. Briefly, after treatment with NBNMA for 24 h, the cells
were stained with 10 μM JC-1 for 20 min at 37°C in the dark. Then,
the stained cells were washed with ice-cold PBS and analyzed by
flow cytometry.
Statistical analysis
All data are presented as mean ± standard deviation
values. Statistical analyses were conducted with Prism ver. 5.0
using one-way ANOVA, followed by Dunnett’s or Tukey’s test. A
P<0.05 was considered to indicate a statistically significant
result.
Results
Anti-proliferative effects of NBNMA
against U937 cells
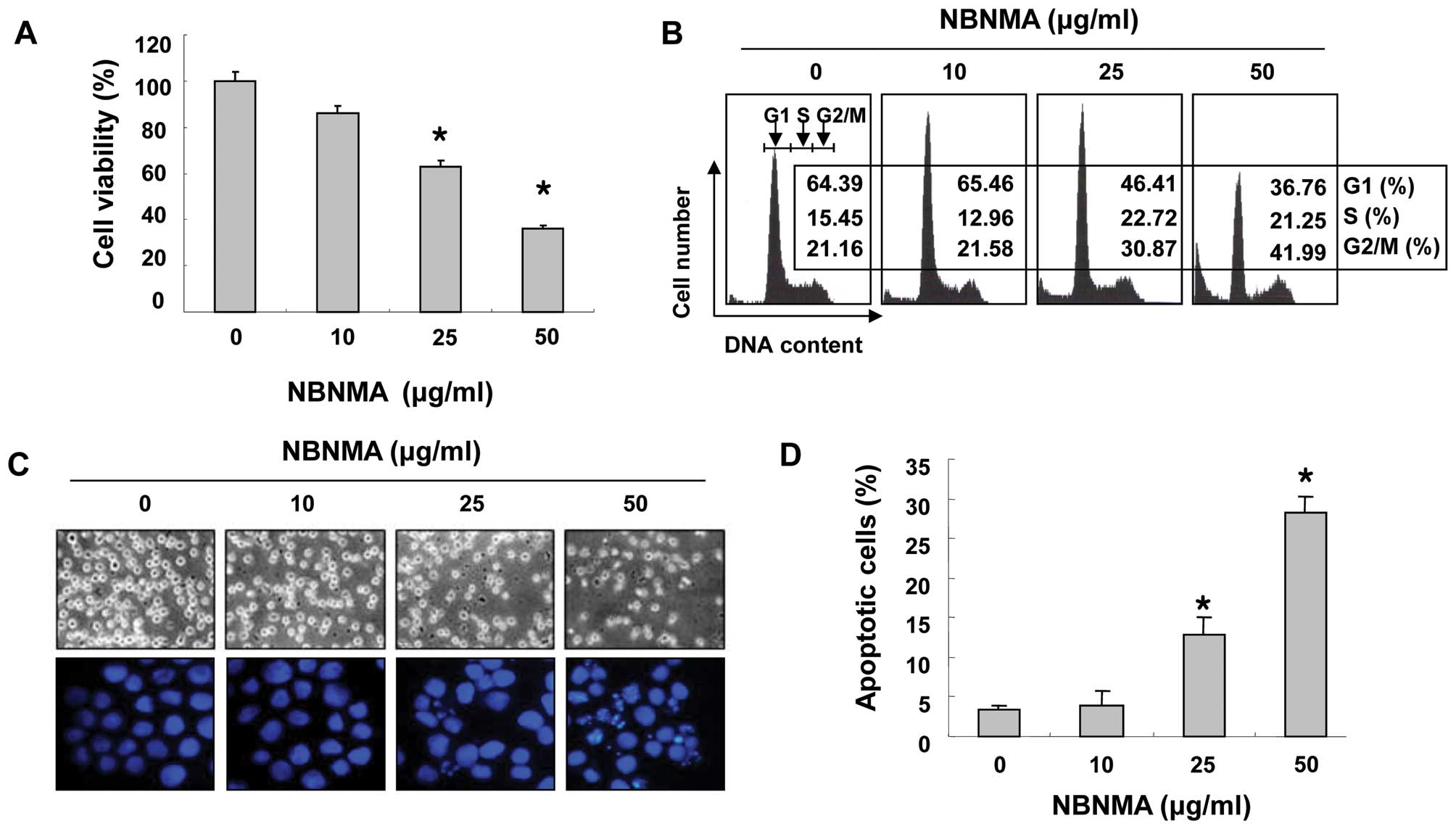
The inhibitory growth effects of NBNMA on U937 cells
were determined by the MTT assay. As shown in Fig. 2A, >38% of cell proliferation was
inhibited by 25 μg/ml NBNMA for 24 h, and 50 μg/ml NBNMA resulted
in >63% inhibition of proliferation after 24 h. Direct
observations using an inverted microscope showed that numerous
morphological changes occurred in the U937 cells following
treatment with NBNMA. In particular, cell shrinkage and cytoplasmic
condensation were noted in a dose-dependent manner after NBNMA
treatment (Fig. 2C).
Induction of G2/M arrest and apoptosis by
NBNMA in U937 cells
U937 cells were treated with different
concentrations of NBNMA and were then subjected to flow cytometric
analysis following DNA staining to test whether NBNMA affects cell
cycle progression. Following a 24-h NBNMA treatment, the percentage
of cells in the G2/M phase increased from 21.16% in the untreated
cells to 21.58, 30.87 and 41.66% in the cells treated with
increasing concentrations of NBNMA (Fig. 2B) with a concomitant decrease in the
percentage of cells in the G1 and S phases. Morphological changes
were examined under a fluorescence microscope after a 24-h exposure
to elucidate whether NBNMA inhibits U937 cell growth by inducing
apoptosis. Following treatment of U937 cells with various NBNMA
concentrations for 24 h, chromatin stained with DAPI had a
characteristic condensed and fragmented appearance and this effect
was concentration-dependent (Fig.
2C). Moreover, treatment of the U937 cells for 24 h with NBNMA
resulted in a concentration-dependent accumulation of cells in the
sub-G1 phase (hypodiploid peak) (Fig.
2D). These data confirmed that NBNMA inhibited U937 cell growth
via cell cycle arrest and induction of apoptosis.
Effects of NBNMA on the expression of
cell cycle-related genes
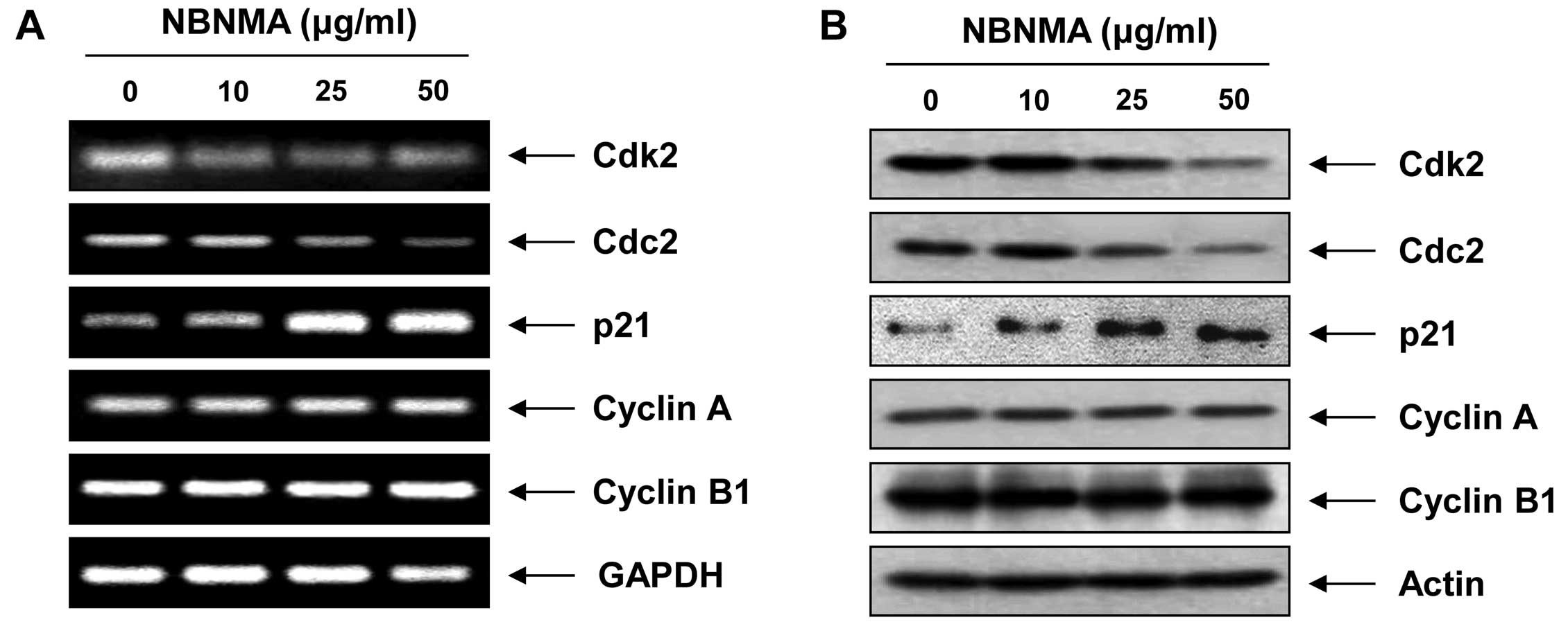
mRNA and protein expression levels of the key cell
cycle regulators between the G2 and M phases were examined by
RT-PCR and immunoblotting to elucidate the molecular mechanism of
NBNMA-induced G2/M arrest in U937 cells. Treatment with NBNMA
resulted in reduced transcriptional and translational levels of
cyclin-dependent kinase (Cdk)2 and Cdc2 in a
concentration-dependent manner, with no effect on cyclin A or
cyclin B1 (Fig. 3). However, the
mRNA and protein levels of the Cdk inhibitor
p21WAF1/CIP1 increased markedly following
treatment with 25 and 50 μg/ml NBNMA for 24 h. Taken together, our
data demonstrated that NBNMA inhibits cell cycle progression and
contributes to reduced growth by modulating Cdk and p21 levels.
Effects of NBNMA on the expression of
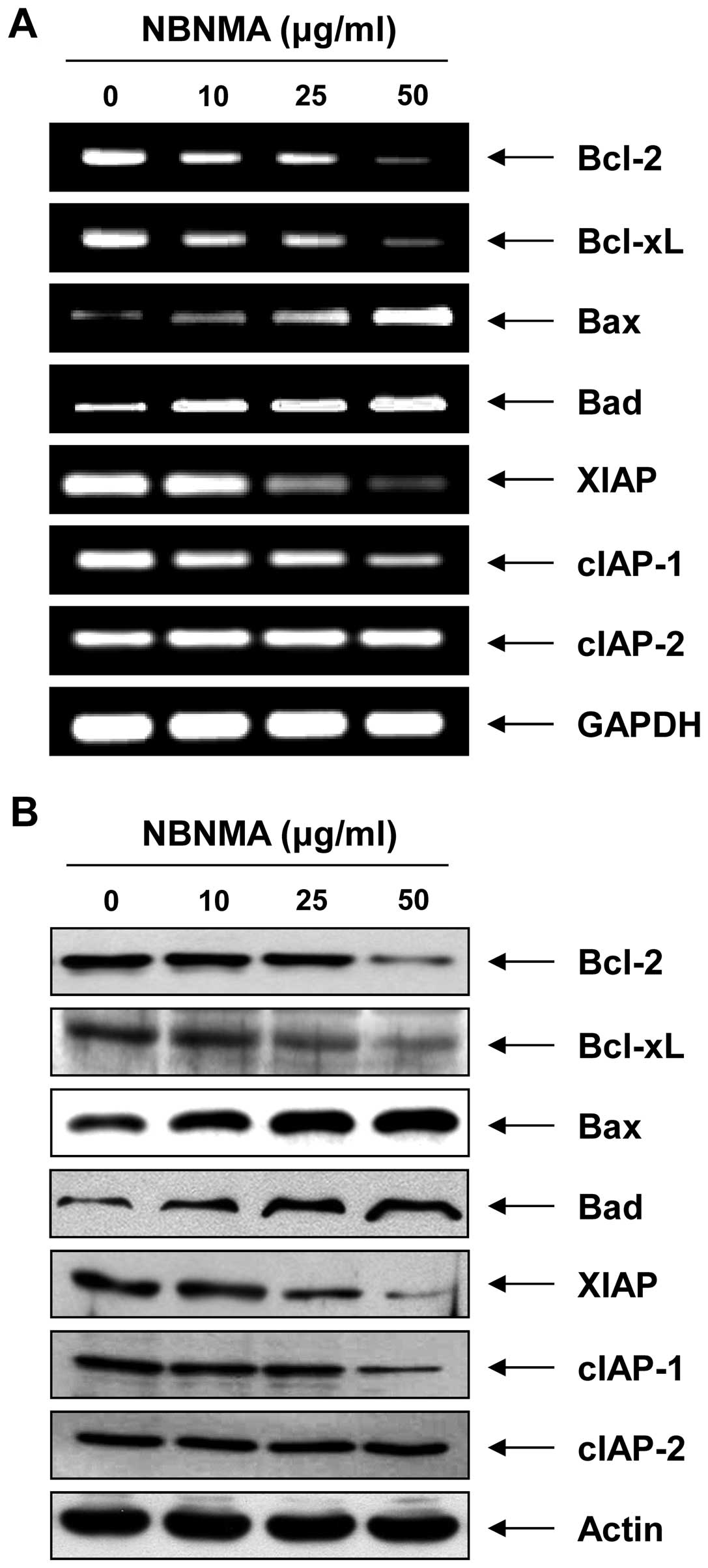
Bcl-2 and IAP family members
As NBNMA treatment induced apoptosis in the U937
cells, we examined the effect of NBNMA on the expression of
apoptosis regulatory genes including Bcl-2 and inhibitor of
apoptosis protein (IAP) family members. The results of RT-PCR and
immunoblotting revealed a marked downregulation of anti-apoptotic
Bcl-2 and Bcl-xL in the U937 cells (Fig. 4). However, treatment with NBNMA
caused an increase in pro-apoptotic Bax and Bad expression. In
addition, relative mRNA and protein expression of anti-apoptotic
XIAP and cIAP-1 decreased in a concentration-dependent manner
compared to that in the control cells, whereas expression of cIAP-1
was relatively constant in the NBNMA-treated U937 cells.
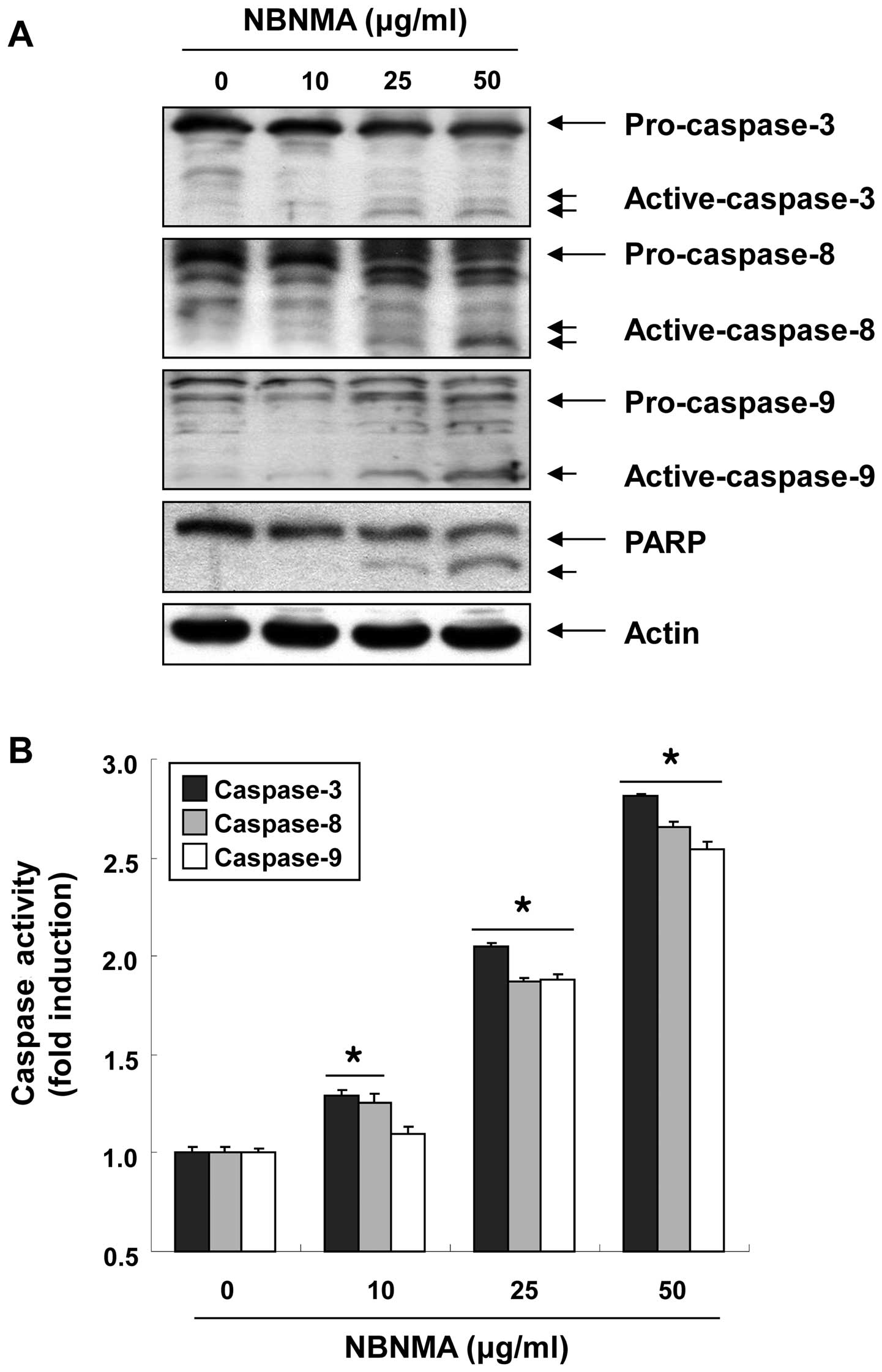
Activation of caspases and degradation of
poly(ADP-ribose) polymerase (PARP)
We next examined whether caspases are activated
during NBNMA-induced U937 cell death to determine the effectors
active in the NBNMA-induced apoptotic pathways. Fig. 5 shows that treatment of U937 cells
with NBNMA increased the levels of active caspase-8 and caspase-9,
the initiator caspases of the extrinsic and intrinsic apoptotic
pathways, respectively, and their in vitro activities in a
concentration-dependent manner. In conjunction with the increase in
caspase-8 and caspase-9 activity, western blot analysis revealed
that NBNMA treatment of U937 cells resulted in proteolytic cleavage
of pro-caspase-3 to active caspase-3, a main executioner caspase,
with subsequent cleavage of PARP into an 85-kDa fragment.
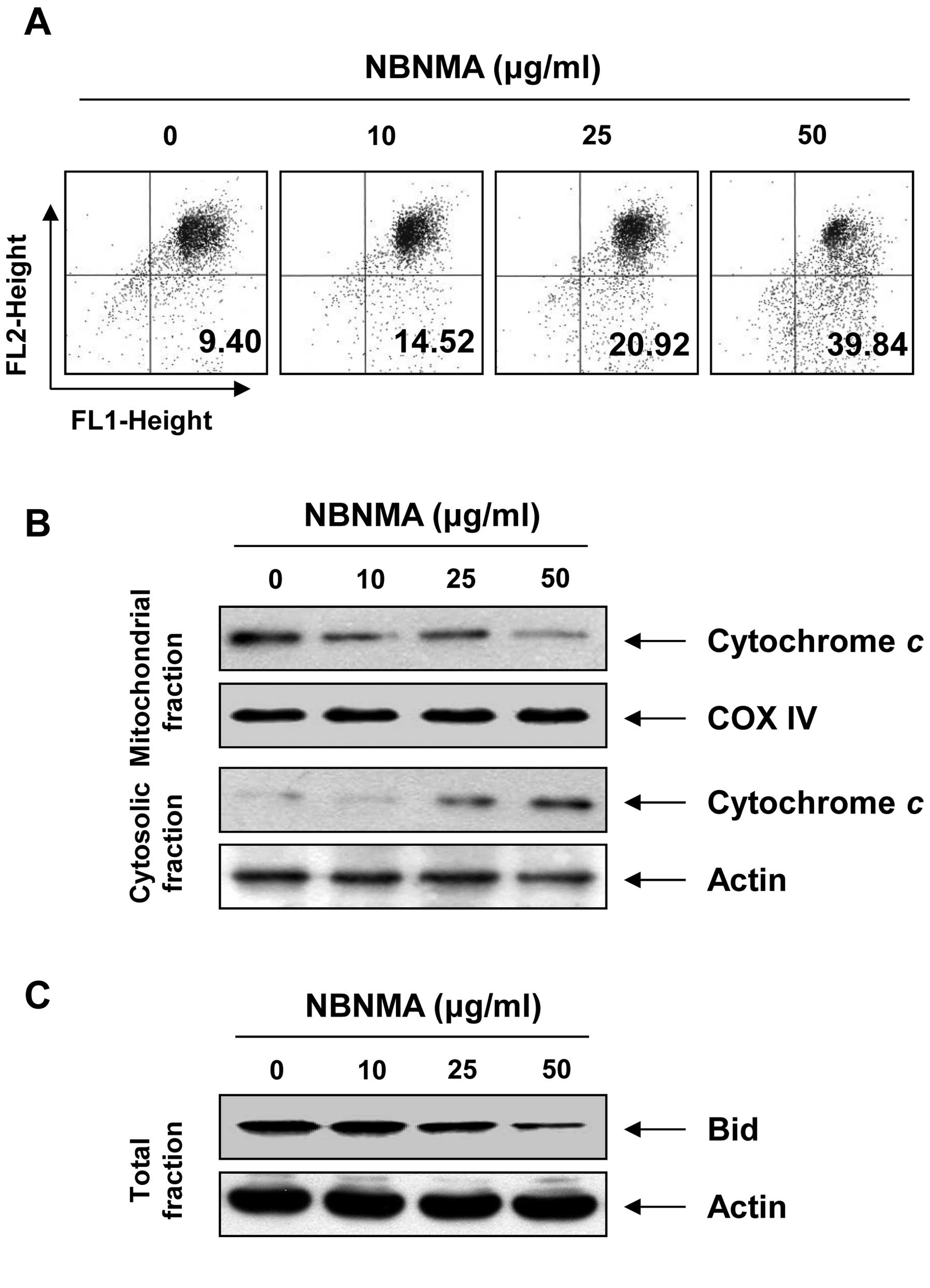
Effects of NBNMA on apoptosis induction
for mitochondrial signaling in U937 cells
Since NBNMA activated caspase-9, we investigated
whether it would affect NBNMA-induced apoptosis associated with
mitochondrial signaling. We examined the effects of NBNMA on
mitochondrial membrane integrity, one of the early events leading
to apoptosis, using the JC-1 fluorescent probe. The results in
Fig. 6A indicate that treatment
with NBNMA clearly elicited dissipation of MMP when compared to
that in the control cells. The loss of MMP is usually accompanied
by release of cytochrome c into the cytosol, which is
involved in activating caspase-3. Therefore, we sought to determine
the effects of NBNMA on cytochrome c levels. As shown in
Fig. 6B, NBNMA triggered the
release of cytochrome c from mitochondria to the cytoplasm,
as determined by immunoblotting using mitochondrial and cytosolic
extracts. The extrinsic apoptotic signaling cascade starts with
activation of caspase-8 and truncation of Bid (tBid), a BH3
pro-apoptotic protein, which translocates to the mitochondrial
membrane, allowing activation of pro-apoptotic proteins such as
caspase-9 and release of cytochrome c. As indicated in
Fig. 6C, NBNMA treatment caused a
decrease in the amount of the Bid pro-form, which is indirect
evidence of protein truncation and activation, suggesting that
NBNMA-induced apoptosis in U937 cells may occur via activation of
caspase-8 and Bid.
Discussion
Despite the early detection and precautions to
minimize the incidence of leukemia, there is a constant effort to
discover alternative strategies to prevent and treat this deadly
disease. To this end, identifying a potent natural molecule that
can specifically target leukemic cells with minimal or no toxicity
to normal cells would be of great benefit. In the present study, we
investigated whether NBNMA, a newly isolated phenylamine derivative
from garlic cloves, could inhibit proliferation of leukemia cells
using the U937 cell line as an experimental model. We found that
NBNMA exerted significant growth inhibitory effects on U937 cells
by inducing G2/M phase arrest and apoptotic cell death.
Molecular analyses of human cancers have revealed
that cell cycle and apoptosis regulators frequently display
encoding gene abnormalities in most common malignancies. Therefore,
agents that alter the regulation of cell cycle machinery, resulting
in arrest at different phases and thereby reducing the growth and
proliferation and even inducing apoptosis of cancer cells may be
useful for the development of new anticancer drugs. Cell cycle
arrest reflects a requirement to repair cell damage; if not
repaired, apoptotic mechanisms are often activated (17,18).
Cell cycle progression in mammalian cells is critically regulated
by sequential activation of Cdks. The activities and specificities
of Cdks are determined by phosphorylation of their corresponding
catalytic subunits and by their associations with cyclins, which
are differentially expressed during the cell cycle. Cell cycle
progression is also regulated by the relative balance between the
cellular concentrations of Cdk inhibitors such as p21, which may
contribute to maintain cell cycle arrest by inactivating the
cyclin/Cdk complex (19,20). Of the Cdks, Cdk2 and Cdc2 kinases
are activated primarily in association with cyclin A and cyclin B1
during progression of the G2/M phase (21–23).
The results of our cell cycle analysis indicated that treatment of
U937 cells with NBNMA resulted in significant accumulation of cells
in the G2/M phase (Fig. 2B). This
cell cycle blockade was associated with a reduction in Cdk2 and
Cdc2 at both the mRNA and protein levels (Fig. 3). G2/M arrest caused by NBNMA was
also supported by a significant increase in the expression of p21,
the first mammalian Cdk inhibitor identified, which is an important
mediator of cell cycle arrest and apoptosis imposed by the tumor
suppressor p53 in response to DNA damage (24,25).
Since the p53 gene is deleted in U937 cells (26), it is most likely that the induction
of p21 was mediated in a p53-independent manner. This result
indicates that NBNMA-induced G2/M arrest in U937 cells might be
mediated through p53-independent upregulation of p21, which
enhances the formation of heterotrimeric complexes with G2/M
cyclins and Cdks, thereby inhibiting their activity.
Apoptosis or programmed cell death is an important
homeostatic mechanism for precisely regulating the number of cells
and as a defense mechanism to remove unwanted cells. Many studies
have shown that an acquired resistance to apoptosis is a hallmark
of most types of cancer. Therefore, inducing apoptosis is a
protective mechanism against cancer progression, and
apoptosis-inducing agents are being investigated as tools to manage
cancer. Apoptosis in mammals is controlled by a
mitochondrial-mediated intrinsic and a membrane death receptor
(DR)-mediated extrinsic pathway (27,28).
Caspases are involved in the intrinsic and extrinsic pathways, each
possessing specific initiator enzymes, caspase-9 and caspase-8,
respectively. The permeability changes and MMP collapse during the
intrinsic pathway induce the formation of apoptosomes between the
apoptotic protease-activating factor-1 and caspase-9 with
cytochrome c following its release from mitochondria into
the cytosol (29,30). Otherwise, activation of DRs by
cross-linking with their respective ligands results in activation
of pro-caspase-8 in the extrinsic pathway. Activated caspase-8
subsequently promotes proteolytic processing of Bid (tBid), a
pro-apoptotic protein in the Bcl-2 family, that converges into the
apoptosis intrinsic pathway downstream from the extrinsic route.
Both activated caspase-8 and caspase-9 activate downstream
executioner caspases such as caspase-3. Activated caspase-3 is
responsible for proteolytic degradation of PARP, which occurs at
the onset of the apoptosis process (30,31).
In the present study, we observed that treatment of U937 cells with
NBNMA induced apoptosis associated with activation of caspase-8 and
caspase-9, along with an increase in the active caspase-3 level and
PARP cleavage (Fig. 5). Our
experiments also clearly showed that mitochondrial depolarization,
release of cytochrome c into the cytosol, and downregulation
of Bid were increased after treatment with NBNMA (Fig. 6), suggesting that both the extrinsic
and intrinsic pathways are involved in NBNMA-induced U937 cell
apoptosis.
Bcl-2 and IAP family members have important
regulatory roles in apoptosis. The Bcl-2 family, which has both
anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic (Bax and Bad)
members, act on mitochondria to either prevent or facilitate the
release of apoptogenic factors. Therefore, the Bax:Bad/Bcl-2:Bcl-xL
ratio is a key factor regulating the apoptotic process (32,33).
In contrast, the IAP family of proteins are all endogenous
inhibitors of apoptosis that bind and inhibit caspases. Thus, they
impede the apoptotic process once it has begun. Caspases targeted
by IAPs include caspase-9 and caspase-3 but not caspase-8 (34,35).
We demonstrated that the increase in NBNMA-induced apoptosis was
associated with dysregulation of Bcl-2 family members (Fig. 4). Our data also indicate that NBNMA
treatment downregulated IAPs and thereby disturbed caspase
activation in U937 cells.
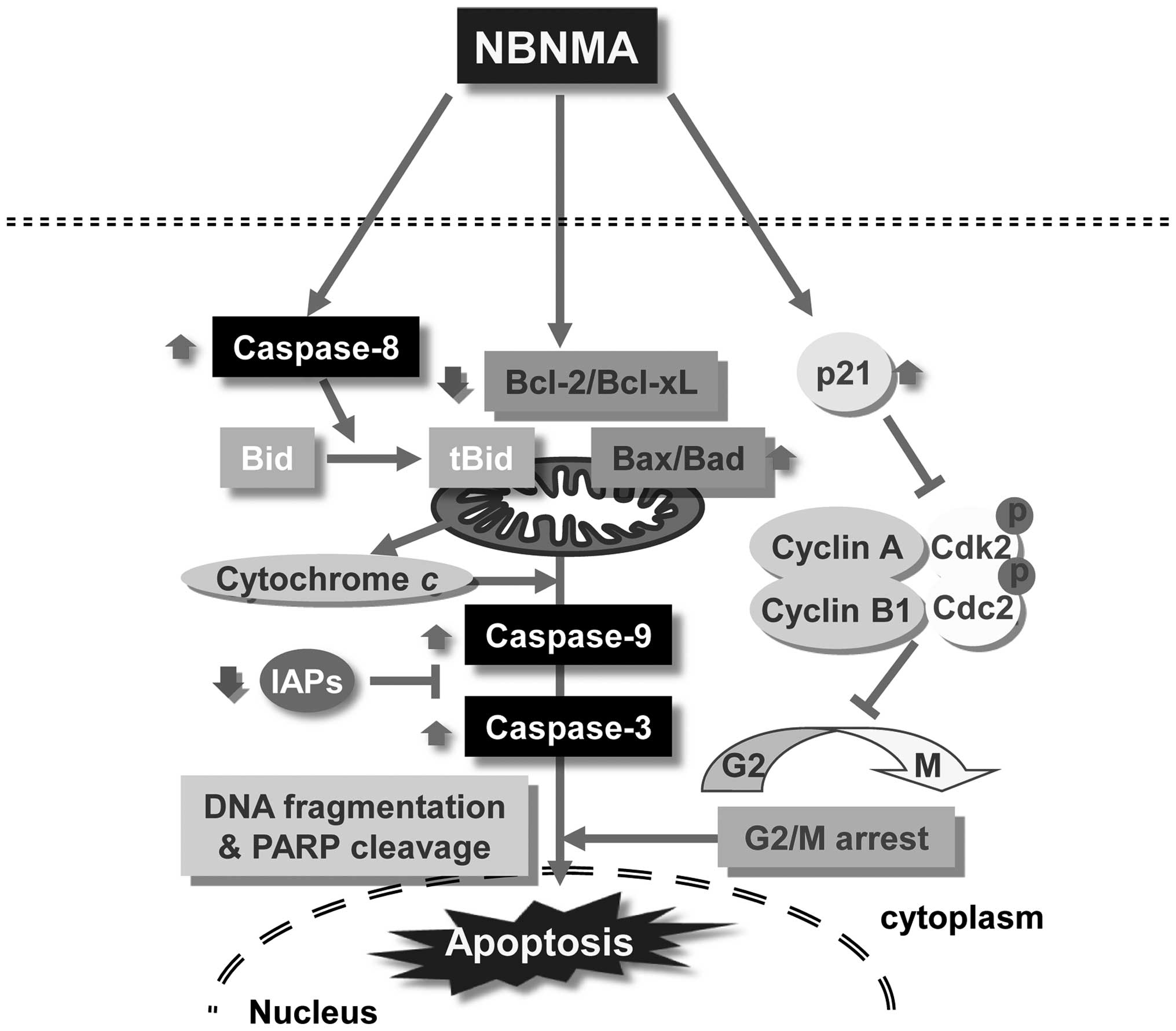
In conclusion, the possible effects of NBNMA on cell
cycle and apoptosis-related genes and the possible mechanism of
action are summarized in Fig. 7.
Taken together, we conclude that NBNMA treatment significantly
inhibited U937 cell proliferation by causing G2/M phase arrest and
inducing apoptosis. Our data provide an important step that might
help model the effects of NBNMA for potential future studies with
animal models and thereby facilitate the development of
nutraceutical products and anticancer drugs using NBNMA.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MSIP) (nos. 2008-0062611 and 2013-041811).
References
|
1
|
Tsubura A, Lai YC, Kuwata M, Uehara N and
Yoshizawa K: Anticancer effects of garlic and garlic-derived
compounds for breast cancer control. Anticancer Agents Med Chem.
11:249–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianchini F and Vainio H: Allium
vegetables and organosulfur compounds: do they help prevent cancer?
Environ Health Perspect. 109:893–902. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milner JA: Mechanisms by which garlic and
allyl sulfur compounds suppress carcinogen bioactivation. Garlic
and carcinogenesis. Adv Exp Med Biol. 492:69–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim JY and Kwon O: Garlic intake and
cancer risk: an analysis using the Food and Drug Administration’s
evidence-based review system for the scientific evaluation of
health claims. Am J Clin Nutr. 89:257–264. 2009.
|
|
5
|
Fleischauer AT and Arab L: Garlic and
cancer: a critical review of the epidemiologic literature. J Nutr.
131:S1032–S1040. 2001.PubMed/NCBI
|
|
6
|
Ngo SN, Williams DB, Cobiac L and Head RJ:
Does garlic reduce risk of colorectal cancer? A systematic review.
J Nutr. 137:2264–2269. 2007.PubMed/NCBI
|
|
7
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Modem S, Dicarlo SE and Reddy TR: Fresh
garlic extract induces growth arrest and morphological
differentiation of MCF7 breast cancer cells. Genes Cancer.
3:177–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lund T, Stokke T, Olsen ØE and Fodstad Ø:
Garlic arrests MDA-MB-435 cancer cells in mitosis, phosphorylates
the proapoptotic BH3-only protein BimEL and induces apoptosis. Br J
Cancer. 92:1773–1781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frantz DJ, Hughes BG, Nelson DR, Murray BK
and Christensen MJ: Cell cycle arrest and differential gene
expression in HT-29 cells exposed to an aqueous garlic extract.
Nutr Cancer. 38:255–264. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su CC, Chen GW, Tan TW, Lin JG and Chung
JG: Crude extract of garlic induced caspase-3 gene expression
leading to apoptosis in human colon cancer cells. In Vivo.
20:85–90. 2006.PubMed/NCBI
|
|
13
|
Kim HJ, Han MH, Kim GY, Choi YW and Choi
YH: Hexane extracts of garlic cloves induce apoptosis through the
generation of reactive oxygen species in Hep3B human
hepatocarcinoma cells. Oncol Rep. 28:1757–1763. 2012.
|
|
14
|
Na HK, Kim EH, Choi MA, Park JM, Kim DH
and Surh YJ: Diallyl trisulfide induces apoptosis in human breast
cancer cells through ROS-mediated activation of JNK and AP-1.
Biochem Pharmacol. 84:1241–1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao D, Herman-Antosiewicz A, Antosiewicz
J, Xiao H, Brisson M, Lazo JS and Singh SV: Diallyl
trisulfide-induced G(2)-M phase cell cycle arrest in human prostate
cancer cells is caused by reactive oxygen species-dependent
destruction and hyperphosphorylation of Cdc 25C. Oncogene.
24:6256–6268. 2005. View Article : Google Scholar
|
|
16
|
Di X, Andrews DM, Tucker CJ, Yu L, Moore
AB, Zheng X, Castro L, Hermon T, Xiao H and Dixon D: A high
concentration of genistein down-regulates activin A, Smad3 and
other TGF-β pathway genes in human uterine leiomyoma cells. Exp Mol
Med. 44:281–292. 2012.PubMed/NCBI
|
|
17
|
Medema RH and Macůrek L: Checkpoint
control and cancer. Oncogene. 31:2601–2613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rozenblat S, Grossman S, Bergman M,
Gottlieb H, Cohen Y and Dovrat S: Induction of G2/M arrest and
apoptosis by sesquiterpene lactones in human melanoma cell lines.
Biochem Pharmacol. 75:369–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sánchez I and Dynlacht BD: New insights
into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005.PubMed/NCBI
|
|
21
|
Jeong AL and Yang Y: PP2A function toward
mitotic kinases and substrates during the cell cycle. BMB Rep.
46:289–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindqvist A, Rodríguez-Bravo V and Medema
RH: The decision to enter mitosis: feedback and redundancy in the
mitotic entry network. J Cell Biol. 185:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porter LA and Donoghue DJ: Cyclin B1 and
CDK1: nuclear localization and upstream regulators. Prog Cell Cycle
Res. 5:335–347. 2003.PubMed/NCBI
|
|
24
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brown L, Boswell S, Raj L and Lee SW:
Transcriptional targets of p53 that regulate cellular
proliferation. Crit Rev Eukaryot Gene Expr. 17:73–85. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danova M, Giordano M, Mazzini G and
Riccardi A: Expression of p53 protein during the cell cycle
measured by flow cytometry in human leukemia. Leuk Res. 14:417–422.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Bertram CC, Shi Q and Zinkel SS:
Proapoptotic Bid mediates the Atr-directed DNA damage response to
replicative stress. Cell Death Differ. 18:841–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shamas-Din A, Brahmbhatt H, Leber B and
Andrews DW: BH3-only proteins: Orchestrators of apoptosis. Biochim
Biophys Acta. 1813:508–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tewari M, Quan LT, O’Rourke K, Desnoyers
S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Low IC, Kang J and Pervaiz S: Bcl-2: a
prime regulator of mitochondrial redox metabolism in cancer cells.
Antioxid Redox Signal. 15:2975–2987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galluzzi L, Larochette N, Zamzami N and
Kroemer G: Mitochondria as therapeutic targets for cancer
chemotherapy. Oncogene. 25:4812–4830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted therapies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|