Introduction
According to clinical and epidemiological studies,
vitamin D and calcium may reduce the risk of colorectal cancer via
various mechanisms (1–7). Moreover, the impact of vitamin D or
its analogs on colon cancer cell proliferation, apoptosis,
differentiation and cell cycle regulation is being investigated.
The regulation of gene expression by vitamin D and its analogs
progresses via binding to specific vitamin D receptors (VDR) upon
ligand activation and dimerization with retinoid X receptors (RXR).
Target genes possess specific nucleotide sequences: vitamin D
response elements (VDREs) which bind VDR-RXR heterodimers to
activate or suppress their expression (8–10).
Post-transcriptional regulatory mechanisms of gene expression have
also been proposed (11). The
expression of VDR is low in normal colonic epithelial cells, but
increases with malignant transformation and then decays with
progressive tumor growth. This phenomenon is correlated with a
decreasing level of VDR in the nucleus as compared with the
cytoplasm. At the same time, high VDR expression correlates with an
advantageous prognosis in colorectal patients, suggesting an
important role for VDR in the pathogenesis of colon cancer
(1,6,7,12).
Vitamin D deficiency in mice has been shown to
result in the aggressive growth of mouse MC-26 colon cancer
(13). Other experimental data have
shown that dietary vitamin D significantly reduced the incidence of
colonic tumors in rats or mice treated with the carcinogen
(14,15). Moreover, deletion of the VDR gene in
mice alters the balance between proliferation and apoptosis,
increases oxidative DNA damage and enhances susceptibility to
carcinogenesis (16).
Studies on combined treatment with
1,25(OH)2D3 or its analogs and different
chemotherapeutic agents have been reported in vitro
(17–22) and in vivo (23,24).
Previously, we examined the biological activity against various
cancer and normal cell lines of a series of side-chain modified and
diastereomeric and geometric analogs of vitamin D (21,25,26).
We also evaluated the influence of vitamin D analogs on the
activity of a range of anticancer drugs in vitro and in
vivo against the human and murine cancer cells (19–21,25–30).
On the basis of these results, we selected two analogs for further
studies: PRI-2191 (tacalcitol, 1,24-dihydroxyvitamin D3)
and PRI-2205 (5,6-trans calcipotriol) (chemical structures are
shown in Fig. 1). The selected
analogs reveal higher antitumor and lower calcemic activity as well
as lower toxicity than 1,25(OH)2D3 (26,28).
In the present study, we analyzed the effect of the
vitamin D analogs PRI-2191 and PRI-2205 on the antitumor activity
in vivo of 5-fluorouracil (5-FU) in mice bearing human
(HT-29) colon cancer. The mechanism of action of the
anti-metabolite agent 5-FU includes its ability to induce the level
and activity of the tumor suppressor gene p53 and to
stabilize p53 protein (31,32). Moreover, it has been shown that the
target gene of p53 -
p21waf1/cip1 is a primary
1,25(OH)2D3-responding gene with VDR binding
promoter regions, in which p53 also co-localizes (10). In this connection, the
1,25(OH)2D3 can induce the expression of p21
independently on the activity of p53 protein, what consequently
leads to the cell cycle arrest and inhibition of cell
proliferation. This fact is important especially when the colon
cancer cells exhibit mutated p53 protein, such as for example the
HT-29 cell line used in our studies. Previous studies have shown
that the human parathyroid calcium-sensing receptor (CaSR) is
expressed in human colon epithelium and regulates epithelial
proliferation and differentiation. Moreover,
1,25(OH)2D3 is involved in regulation of CaSR
expression (33–35). Notably,
1,25(OH)2D3, as well as calcipotriol,
promoted the sensitivity of human colon carcinoma cells to
anticancer drugs, including 5-FU, which may be mediated through the
CaSR (36,37). These data suggest that combined
therapy with the use of vitamin D analogs and 5-FU may be
promising.
Materials and methods
Compounds
1,25(OH)2D3 (calcitriol),
PRI-2191, calcipotriol (PRI-2201) and PRI-2205 were obtained as
certified synthetic materials from the Pharmaceutical Research
Institute, Warsaw, Poland. Samples of the compounds were stored in
amber ampoules, under argon at −20°C. Prior to usage, in the case
of in vitro studies, compounds were dissolved in 99.8%
ethanol to the concentration of 10−4 M and subsequently
diluted in culture medium in order to reach the concentration of
100 nM. For animal experiments, compounds were dissolved in 99.8%
ethanol, then diluted in 80% propylene glycol in order to reach the
required concentrations and administered subcutaneously (s.c.) to
mice in a volume of 5 μl/1 g of body weight.
5-FU (ICN Polfa, Rzeszów, Poland) solution at a
concentration of 50 mg/ml was diluted in culture medium prior to
usage in in vitro studies in order to reach the required
concentrations and for in vivo experiments in saline in
order to reach the required concentrations and then administered
either intravenously (i.v.) or intraperitoneally (i.p.) to mice at
a volume of 10 μl/1 g of body weight.
Capecitabine (CPC) (Pharmaceutical Research
Institute, Warsaw, Poland) was dissolved in 40% ethanol, then
diluted in water for injection in order to reach the required
concentration and administered orally (p.o.) to mice at a volume of
10 μl/1 g of body weight.
Cells
The human colon cancer cell line HT-29 was obtained
from the German Cancer Research Center (Deutsches
Krebsforschungszentrum, DKFZ, Heidelberg, Germany) the origin of
the cell line, Leibniz Institute DSMZ-German Collection of
Microorganisms and Cell Cultures, Braunschweig, Germany. The cell
line was cultured in vitro at the Cell Culture Collection of
the Institute of Immunology and Experimental Therapy, Wroclaw,
Poland.
Mice
The mice, female 6–8-week old NOD/SCID, Nu/J and
NCr-nu/nu mice, weighing 20–25 g, were supplied by the University
Children’s Hospital in Krakow (Poland) and the Medical University
of Bialystok (Bialystok, Poland), Charles River Labs., National
Cancer Institute, Frederic, USA respectively. Mice were maintained
in specific pathogen-free (SPF) conditions. All animal experiments
were performed according to EU Directive 2010/63/EU for animal
experiments and were approved by the 1st Local Committee for
Experiments with the Use of Laboratory Animals, Wroclaw,
Poland.
Design of the in vivo experiments
Human colon cancer HT-29 cells were harvested with
the use of 0.05% trypsin/0.02% EDTA, washed twice with serum-free
minimum essential medium (α-MEM) and resuspended in Hank’s medium.
A single-cell suspension (3.5×106/200 μl per mouse) with
cell viability >90% was inoculated subcutaneously (s.c.). For
orthotopic transplantation (i.i.), the anesthetized mouse was
placed on a wooden board in the right lateral position and the
incision was made through the left upper abdominal pararectal line
and peritoneum. The cecal wall was carefully exposed, placed and
fixed between layers of sterile gauze. A 1–2 mm piece of specimen,
derived from a primary tumor grown s.c. in another mouse, was fixed
to the serosal part of the cecal wall with 5-0 surgical sutures.
After implantation, the peritoneum and abdominal wall were sutured
with 4-0 surgical sutures (Dexon-‘S’; Polfa, Poznań, Poland). Tumor
cell transplantations were performed under general anesthesia with
a mixture of ketamine hydrochloride (100 mg/kg; Ketamina 10%;
Biowet, Puławy, Poland) and xylazine hydrochloride (20 mg/kg;
XylaRiem; Riemser Arzneimittel AG, Germany).
Details of the treatment schedules
used
Determination of the dose and scheme
of 1,25(OH)2D3 analog treatment
The treatment of NOD/SCID mice bearing subcutaneous
HT-29 tumors was started either on day 12 (Fig. 4A left graph) or on day 5 (Fig. 4A right graph).
In the first experiment, 5-FU was administered
intravenously (i.v.) at a dose of 75 mg/kg/day once a week for 4
weeks (on days 12, 19, 26, 33; total dose, 300 mg/kg). PRI-2191 or
PRI-2205 were injected s.c. at doses of 0.2 μg/kg/day or 5.0
μg/kg/day, respectively, 5 times a week on days 12, 13, 14, 15, 16,
19, 20, 21, 22, 23, 26, 27, 28, 29, 30, 33, 34, 35 (total dose of
PRI-2191, 3.6 μg/kg; PRI-2205, 90 μg/kg). This experiment was ended
on day 36 and, after cell inoculation, the tumors were harvested
for further analyses.
In the second experiment, 5-FU was administered
intravenously (i.v.) at a dose of 75 mg/kg/day once a week, for 5
weeks (on days 5, 12, 19, 26, 33; total dose, 375 mg/kg). PRI-2191
or PRI-2205 were injected s.c. at doses of 1.0 μg/kg/day or 10.0
μg/kg/day, respectively, 3 times a week on days 7, 10, 12, 14, 17,
19, 21, 24, 26, 28, 31, 33, 35, 38, 40, 42, 45, 47 (total dose of
PRI-2191, 18 μg/kg; PRI-2205, 180 μg/kg). This experiment was ended
on day 49 and, after cell inoculation, the tumors were harvested
for further analyses.
Treatment after orthotopic
transplantation
The treatment of NCr-nu/nu mice bearing orthotopic
HT-29 tumors was started either on day 17 (Fig. 5A left graph) or on day 11 (Fig. 5A right graph).
In the first experiment, 5-FU was administered i.v.
at a dose of 75 mg/kg/day once a week, for 4 weeks (on days 17, 24,
31, 38; total dose, 300 mg/kg). PRI-2191 or PRI-2205 were injected
s.c. at doses of 1 μg/kg/day or 10 μg/kg/day, respectively, 3 times
a week on days 17, 19, 21, 24, 26, 28, 31, 33, 35, 38, 40, 42, 45,
47 (total dose of PRI-2191, 14 μg/kg; PRI-2205, 140 μg/kg). This
experiment was ended on day 53 after cell inoculation. The blood
was also collected and the calcium serum level was analyzed.
In the second experiment, mice were i.p. injected
with 75 mg/kg/day 5-FU (on days 11, 18, 25, 32; total dose, 300
mg/kg) and/or vitamin D analog PRI-2205 at a dose of 10 μg/kg/day
administered s.c. (3 times a week, on days 11, 13, 15, 18, 20, 22,
25, 27, 29, 32, 34, 36; total dose, 120 μg/kg). The experiment was
ended on day 39 and, after cell inoculation, the tumors were
harvested for further analyses. The blood was also collected and
morphological analyses were performed.
PRI-2191 or PRI-2205 used in combined
colon cancer treatment with oral 5-FU prodrug CPC
Nu/J mice were s.c. inoculated with human colon
cancer HT-29 (data not shown). The treatment was started on day 5
after tumor cell inoculation. CPC was administered orally with the
use of gastric tubes at a dose of 450 mg/kg/day 5 times a week (on
days 5, 6, 7, 10, 11, 12, 13, 14, 17, 18, 19; total dose, 4.95
g/kg). Analogs PRI-2191 or PRI-2205 were administered s.c., 3 times
a week, at doses of 1 mg/kg/day or 10 mg/kg/day, respectively (on
days 5, 7, 10, 12, 14, 17, 19, 21, 24, 26, 28, 31, 33, 35, 38, 40,
42; total dose PRI-2191, 17 μg/kg; PRI-2205, 170 μg/kg). The
experiment was terminated on day 46.
Evaluation of the therapeutic effect
Tumor diameters were measured three times a week by
caliper. Tumor volume was calculated using the formula:
(a2 × b)/2, where a, a shorter tumor diameter in mm and
b, a longer tumor diameter in mm. Inhibition of tumor growth was
calculated from the following formula: tumor growth inhibition
(TGI) (%) (TGI) = 100 − [(WT/WC) × 100],
where WT is the median tumor weight of treated mice and
WC that of untreated control animals. The mice were also
weighed three times a week.
Evaluation of combination effects
The minimal expected inhibition used to estimate the
effect of the combination of two compounds was evaluated using the
formula: TGI hypothetical values (HTGI) (%)= 100 − [(100 − E for
cytostatic) × (100 − E for 1,25(OH)2D3
analog)/100] (38). Where E was
TGI.
As a result of the comparison of TGI to % HTGI, the
type of interactions between two compounds in combined treatment
was designated and these could be: (i) synergy, when the
experimental value of TGI is greater than HTGI; (ii) additive
effect, when the two values are comparable; (iii) subadditive
effect, if the experimental TGI value is smaller than the
hypothetical, but larger than TGI for cytostatic given alone; (iv)
antagonism, if the experimental TGI is smaller than the
experimental TGI for cytostatic.
Blood leukocytes and calcium
evaluation
The level of blood leukocytes and serum calcium was
measured in each individual blood sample using the following
devices: Sysmex K4500SL, serial number F2872, Japan and Olympus
AU400, Olympus America, Melville, New York, USA, respectively.
Design of in vitro experiments for cell
cycle distribution, cell death and p53 expression analysis
Cultured HT-29 cells were seeded at a density of
1×105 cells/ml of culture medium on 6-well plates
(Corning, NY, USA) at a volume of 2 ml. On the following day, the
cells were exposed to compounds for 48 h; vitamin D compounds 100
nM and 5-FU 100 or 200 μg/ml. Ethanol, used as a solvent for all
compounds and diluted corresponding to its highest concentration,
produced no toxicity.
Cell cycle analysis
Preparation of cells from in vitro
culture
After 48 h of incubation, the cells were collected
with the use of 0.05% trypsin/0.02% EDTA. The cell suspension was
washed once in phosphate-buffered saline (PBS) supplemented with 2%
of fetal bovine serum (2% PBS). Then, the cells were suspended in
2% PBS and counted in a hemacytometer.
Tissue preparation
HT-29 colon cancer cells from primary tumors were
obtained by mincing fresh tumor tissue with a scalpel, passing them
through a plasma filter and suspending them in PBS. After
centrifugation, the cells were disaggregated with the use of 0.05%
trypsin/0.02% EDTA. The cell suspension was washed once with PBS
supplemented with 2% of fetal bovine serum (2% PBS). Then, the
cells were suspended in 2% PBS and counted in a hemacytometer.
Cells (1×106) were fixed for 24 h in 70%
ethanol at −20°C. Then, the cells were washed twice in PBS and
incubated with RNAse (8 μg/ml, Fermentas, Germany) at 37°C for 1 h.
The cells were stained for 30 min with propidium iodide (PI) (0.5
mg/ml; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) at 4°C and
the cellular DNA content was determined using a BD FACSCalibur
instrument (Becton-Dickinson, San Jose, CA, USA.) and either ModFit
LT 3.0 or WinMDI software.
Death cell analysis with PI staining
(sub-G1 stage)
After 48 h of incubation, the cells were prepared in
the same manner as cells for the cell cycle distribution assay
described above. Data analysis was performed by flow cytometry
using a BD LSRFortessa instrument (Becton-Dickinson). Next, data
were analyzed in a BD FACSDiva 6.2 program. The experiment was
repeated 4 times.
Apoptosis determination by Annexin V
staining
After 48 h of incubation, the cells were collected
using non-enzymatic cell dissociation solution (Sigma-Aldrich
Chemie GmbH), washed in PBS supplemented with 1% of fetal bovine
serum (1% FBS) and counted in a hemacytometer. The cells
(2×105) were washed twice with PBS. Annexin V-FITC
(Alexis Biochemicals, San Diego, CA, USA) was diluted to a
concentration of 1 mg/ml in binding buffer [Hepes buffer: 10 mM
HEPES/NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2,
1.8 mM CaCl2, (IIET, Wroclaw, Poland)] and the cells
were suspended in 200 μl of this solution (freshly prepared each
time). Then, after 15 min of incubation in the dark at room
temperature, PI solution (0.1 mg/ml) was added prior to analysis to
give a final concentration of 0.01 mg/ml. Data acquisition was
performed by flow cytometry on a BD FACSCalibur instrument
(Becton-Dickinson) using the CellQuest program. The data were
displayed as a two-color dot plot with FITC-Annexin V (FL1-H, Y
axis) vs. PI (FL3-H, X axis). Double-negative cells were live
cells, PI+/Annexin V+ were late apoptotic or necrotic cells and
PI−/Annexin V+ early apoptotic cells. Data were analyzed in the
WinMDI 2.9 program. The experiment was repeated 3 times.
Mitochondrial membrane potential (Ψmt)
determination
Mitochondrial injury was assessed by JC-1
(Sigma-Aldrich) staining. This dye, existing in the cytosol as a
monomer, remained unprocessed due to a breakdown of Ψmt and
fluoresces green. JC-1 can assume a dimeric configuration in the
mitochondria and fluoresces red in a reaction driven by the
mitochondrial transmembrane potential (39).
After 48 h of incubation, the cells were collected
with the use of 0.05% trypsin/0.02% EDTA. The cell suspension was
washed once with 2% PBS. Then, the cells were suspended in 2% PBS
and counted in a hemacytometer. The HT-29 cells (5×105)
were washed in 2% PBS. Pelleted cells were resuspended in 100 μl of
warm cultured medium with the addition of 10 μl JC-1 (the final
concentration of JC-1 was 3 μg/ml) and were then incubated for 15
min at 37°C. Next, the cells were washed with 1 ml of 2% PBS and
were then resuspended in 300 μl of 2% PBS. Data acquisition was
performed by flow cytometry on a BD FACSCalibur instrument
(Becton-Dickinson) using the CellQuest program. Next, data were
analyzed in WinMDI 2.8.
As a positive control of cells with low potential,
we used cells which were incubated for 24 h with valinomycin
(Sigma-Aldrich) at a concentration of 1 μM.
Active caspase 3 analysis
After 48 h of incubation, the cells were collected
using non-enzymatic cell dissociation solution (Sigma-Aldrich
Chemie GmbH), washed in PBS supplemented with 1% of fetal bovine
serum (1% FBS) and counted in a hemacytometer. The cells
(2×105 per sample) were washed once with 1% PBS and then
suspended in 0.1 ml solution to fixing and permeabilization (BD
Pharmingen, CA, USA) and incubated for 30 min at 4°C. After
incubation, the cells were washed using buffer with added saponin
(Perm/Wash Buffer; BD Pharmingen, CA, USA). Next, the cells were
stained with anti-active caspase 3 conjugated with phycoerythrin
(PE) (BD Pharmingen, CA, USA) for 50 min in the dark at room
temperature. After incubation, the cells were washed using buffer
with added saponin and then suspended in 0.3 ml in the same buffer.
Data analysis was performed by flow cytometry using an LSRFortessa
and FACSCalibur instruments (Becton-Dickinson, San Jose, CA, USA).
Next, data were analyzed with the BD FACS Diva 6.2 program. The
experiment was repeated 4 times.
p53 expression analysis
After 48 h of incubation, the cells were collected
using non-enzymatic cell dissociation solution (Sigma-Aldrich
Chemie GmbH), washed in PBS supplemented with 1% FBS and counted in
a hemacytometer. The cells (2×105/sample) were washed
once with 1% FBS and then suspended in 0.1 ml solution to fixing
and permeabilization (BD Pharmingen, CA, USA) and incubated for 30
min at 4°C. After incubation, the cells were washed using buffer
with added saponin (Perm/Wash Buffer; BD Pharmingen, CA, USA).
Next, the cells were stained either with anti-p53-PE or with an
IgG1,κ-PE isotype control (BD Pharmingen) for 50 min in
the dark at room temperature. After incubation, the cells were
washed using buffer with added saponin and then suspended in 0.3 ml
in the same buffer. Data analysis was performed by flow cytometry
using a BD LSRFortessa instrument (Becton-Dickinson). Next, data
were analyzed with BD FACS Diva 6.2. The experiment was repeated 4
times.
Western blot analysis
Tissue preparation
Specimens of tumor tissue from euthanized animals
were collected in liquid nitrogen and stored at −80°C. To determine
protein expression via western blot analysis, frozen tumors were
mechanically homogenized (Rotilabo, Carl Roth, Karlsruhe, Germany)
in RIPA buffer (Sigma-Aldrich Chemie GmbH) supplemented with a
complete mixture of phosphatase and protease inhibitors
(Sigma-Aldrich Chemie GmbH) and then kept on ice for 45 min.
Lysates were cleared via microcentrifugation at 17968 rcf × g for
20 min.
Preparation of cells from in vitro
culture
Cultured HT-29 cells were seeded to a volume of 6 ml
and at a density of 2×105 cells/ml of culture medium on
a glass Petri dish. Next, after 24 h of incubation the cells were
exposed to the test vitamin D compounds at the concentrations of
100 nM and/or 200 μg/ml 5-FU for 48 h.
Protein concentrations were determined using a
protein assay (DC Protein Assay; Bio-Rad Laboratories, Hercules,
CA, USA). Equal amounts of protein (25 or 50 μg for detecting VDR;
100 μg for p21, p27; ERK and p-ERK and 25, 50 or 100 μg for
β-actin) were separated in a 10% (VDR, ERK1/2, p-ERK1/2, β-actin)
or 15% (p21, p27) sodium dodecyl sulfate (SDS) polyacrylamide gel
and transferred to either a polyvinylidene difluoride (PVDF)
membrane (0.45 μm; GE Healthcare, Amersham, Little Chalfont, UK) or
a nitrocellulose membrane (0.22 μm; NitroBind, GE Water and Process
Technologies, Osmonics, Hopkins, MN, USA). Protein loading and
transfer efficiency were monitored via 0.1% Ponceau S-Red staining.
Membranes were blocked overnight (4°C) in 1% blocking reagent
(membrane blocking agent; GE Healthcare, Amersham) in PBS. On the
following day, the membrane was washed three times (×10 min) with
0.05% PBS/Tween-20 (PBST) and then incubated for 1 h at room
temperature with a primary antibody: rabbit anti-VDR, anti-p21,
anti-27, anti-ERK1/2 or anti-p-ERK1/2 polyclonal antibody (all from
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or rabbit
anti-β-actin (Sigma-Aldrich, Poznan, Poland). After incubation, the
blot was washed three times with 0.1% PBST and incubated for 1 h
with the secondary anti-rabbit immunoglobulins (GE Healthcare,
Amersham). The membrane was finally washed three times with 0.1%
PBST and incubated for 30 min with a fluorescent substrate for
alkaline phosphatase-based detection (ECF; GE Healthcare,
Amersham). Fluorescence was detected using a scanner (Typhoon
scanner; GE Healthcare, UK). Densitometric analysis of the western
blots was carried out using ImageJ 1.46r (National Institutes of
Health, Bethesda, MA, USA).
Molecular modeling
Models of proteins were based on available
structural data; vitamin D receptor in complex with vitamin D (PDB
code: 1DB1), vitamin D-binding protein (DBP) in complex with
25-hydroxyvitamin D3 and oleic acid (PDB code: 1J7E) and the
constitutive androstane receptor/retinoid X receptor (CAR/RXR)
complex (PDB code: 1XV9). Structural data were processed with the
Schroedinger LLC software suite Protein Preparation Wizard
(40) and used for docking ligands
prepared with ligand preparation in the GlideXP module (41–43) of
the same software. Solvent molecules were removed from the complex
unless within the 3.5 Å radius and participating in 3 or more
hydrogen bonds. Estimates of binding energies were performed with
Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) method
(VSGB 2.0 energy model) employing implicit solvation (44). The MMGSA model compares favorably
with the more computationally intensive Poisson-Boltzmann method
(45–47).
Statistical analysis
Statistical analysis was performed using STATISTICA
version 7.1 (StatSoft, Inc., USA). The assumptions of ANOVA were
checked using PP-plots, Shapiro-Wilk’s test and Levene’s test. In
the case of violations of the ANOVA assumptions, a nonparametric
stratified permutation Kruskal-Wallis overall test either with
subsequent multiple comparisons or ANOVA followed by Tukey’s HSD
for unequal N or Fisher’s test were used. P-values <0.05
were considered to indicate a statistically significant
difference.
Results
Cell cycle and cell death of HT-29 colon
cancer in vitro after incubation with PRI-2191 and PRI-2205 applied
alone or in combination with 5-FU
The in vitro tests were performed after 48 h
incubation of colon cancer cells with vitamin D analogs.
1,25(OH)2D3 and calcipotriol (PRI-2201) were
used as reference compounds. In such experimental conditions,
vitamin D compounds did not inhibit proliferation of HT-29 colon
cancer cells; however, the tendency to improve the
antiproliferative activity of 5-FU was observed (Table I). Cell cycle analysis in cultured
HT-29 cells showed an increase in G0/G1 and a
simultaneous decrease in S cell cycle stage by reference compounds
or PRI-2191. PRI-2205 did not affect cell cycle distribution in the
concentrations used (100 nM). 5-FU used alone at the dose of 200
μg/ml reduced the number of cells in G0/G1
and increased the number in the G2/M phase. Reference
vitamin D compounds and PRI-2191 further decreased the cells in
G0/G1 and increased those G2/M
phase as compared to 5-FU used alone. Moreover, a significant
decrease in cells in the S stage was observed. PRI-2205 acts
differently from other vitamin D compounds; used in parallel with
5-FU, it increased the number of cells in
G0/G1, S and decreased the number in
G2/M as compared to 5-FU. Moreover, we observed a
tendency for the number of cell deaths to increase when PRI-2205
was used together with 5-FU (Table
I).
 | Table IThe proliferation inhibition and cell
cycle distribution of HT-29 colon cancer cells in vitro. |
Table I
The proliferation inhibition and cell
cycle distribution of HT-29 colon cancer cells in vitro.
| Compounds | Proliferation
inhibition (%) | Cell cycle
distribution (% of cells) |
|---|
|
|---|
|
G0/G1 | S |
G2/M |
Sub-G1 |
|---|
| Control | - | 49.9±4.9 | 38.6±4.2 | 11.5±1.7 | 0.4±0.5 |
| EtOH | 1.5±1.5 | 49.2±4.9 | 37.6±5.8 | 13.2±1.3 | 0.7±0.3 |
|
1,25(OH)2D3 | 7.1±3.9 | 56.1±3.7 | 32.4±4.4 | 11.5±1.6 | 0.1±0.1 |
| PRI-2191 | 1.6±3.0 | 59.0±1.4 | 30.4±1.1 | 10.6±0.7 | 0.1±0.2 |
| Calcipotriol | 3.2±3.1 | 58.5±0.4 | 31.8±1.7 | 9.7±2.3 | 0.1±0.2 |
| PRI-2205 | 4.6±1.8 | 51.2±4.6 | 36.7±4.6 | 12.2±2.2 | 0.9±0.8 |
| 5-FU | 49.9±4.6 | 41.5±7.4 | 33.2±8.7 | 25.3±15.2 | 0.1±0.2 |
|
5-FU+Calcitriol | 54.1±4.4 | 40.1±1.6 | 21.2±0.8 | 38.8±0.8 | 0.2±0.3 |
| 5-FU+PRI-2191 | 52.3±5.1 | 39.1±2.0 | 21.3±2.3a | 39.6±2.4 | 0.1±0.1 |
| 5-FU+PRI-2201 | 50.9±4.4 | 38.6±0.5 | 20.6±1.5a | 40.9±1.1 | 0 |
| 5-FU+PRI-2205 | 53.0±3.9 | 49.6±3.7 | 36.9±4.0 | 13.8±0.7 | 6.9±7.8 |
In further studies using the same experimental
conditions, we analyzed some parameters of the cell death of HT-29
cells (Fig. 2). As shown in
Fig. 2A, incubation with 5-FU
caused significant HT-29 cell death when counted as positive PI
staining. However, simultaneous treatment with vitamin D compounds
led to a decrease in the percentage of cell deaths. This phenomenon
was very profound when PRI-2191 was used with 5-FU. In apoptotic
cells, the externalization of phosphatidylserine is observed from
the cytoplasmic to the extracellular membrane site (39). Using Annexin V staining, we studied
whether the tested compounds are able to induce apoptotic death and
using PI staining, necrotic HT-29 cells were determined. The
results are summarized in Fig. 2B.
5-FU, either used alone or in combination with all vitamin D
compounds (with the exception of PRI-2191), showed the tendency to
increase the number of early apoptotic cells (AV+), as well as to
decrease the percentage of late apoptotic cells (AV+/PI+) (Fig. 2B). During apoptosis, the
electrochemical gradient across the mitochondrial membrane breaks
down (39). In our studies, the
influence of the tested compounds on the Ψmt of HT-29 cells was
analyzed. However, we did not observe any significant changes in
Ψmt independent of the compounds used. Only analog PRI-2191 used
with 5-FU caused a decrease in the number of cells with low Ψmt
(Fig. 2C).
 | Figure 2Effect of combined treatment on cell
death of colon cancer cells in vitro. (A) Propidium iodide
(PI)-stained cells, *P<0.05 as compared to
5-FU-treated cells. (B) Annexin V- and PI-stained cells. (C)
Mitochondrial membrane potential (Ψmt) of HT-29 cells,
*P<0.05 as compared to EtOH-treated cells;
Valinomycin-treated cells, P<0.05 as compared to control.
Dot-plots from representative experiment for selected groups are
presented. (D) Expression of active caspase-3,
*P<0.05 as compared to 5-FU-treated cells. (E) Mean
fluorescence channel of cells stained with antibodies against p53,
*P<0.05 as compared to control. The cells were
treated for 48 h with vitamin D compounds at a concentration of 100
nM and with 5-FU; (A,D,E) 100 or (B,C) 200 μg/ml. Calcitriol,
1,25(OH)2D3; PRI-2201, calcipotriol.
Statistical analysis, Fisher’s test. N=4–5 in each group; mean (±
SD) is presented. 5-FU, 5-fluorouracil. |
5-FU alone induced active caspase-3 expression.
Analogs used alone had no effect on caspase-3 activity; however, in
combination with 5-FU, such analogs reduced the induction of active
caspase-3. 1,25(OH)2D3 diminished active
caspase-3 expression to 50% as compared to 5-FU alone. Also,
PRI-2191 and PRI-2201 diminished the expression of this enzyme. In
contrast, PRI-2205 did not influence the induction of caspase-3 by
5-FU (Fig. 2D).
All vitamin D compounds showed the tendency to
decrease p53 expression. Moreover,
1,25(OH)2D3, PRI-2191 and PRI-2201 decreased
the level of p53 as compared to 5-FU alone (Fig. 2E).
A western blot analysis was also performed on the
expression of cyclin-dependent kinase inhibitors (CDKI) p21 and p27
(Fig. 3A). The expression of p21 in
HT-29 cells was 2.4- and 1.4-fold increased by PRI-2191 and
PRI-2205, respectively. 5-FU used alone increased its expression
4.6-fold and a further increase of p21 expression was observed
after cell incubation with 5-FU combined with vitamin D analogs. In
the case of p27, only a slight induction of this protein expression
was observed in cells incubated with either PRI-2191 or 5-FU alone
or combined (Fig. 3A).
The level of phosphorylated ERK1/2 (p-ERK1/2) was
diminished in cells treated either with 5-FU alone or combined with
both PRI-2191 and PRI-2205. The diminution was more profound in
cells incubated with 5-FU combined with both analogs (Fig. 3B).
Effect of PRI-2191 or PRI-2205
administered alone or in combination with 5-FU on subcutaneous
human colon cancer HT-29
Primary tumor growth
Independent of the treatment schedule, mice treated
with PRI-2191 or PRI-2205 developed similar tumors to those in
control mice. They also did not affect the body weight of the
animals treated (max. 8% decrease). 5-FU decreased tumor volume,
but not in a significant manner; TGI was in the range of 15–50%.
5-FU used alone decreased the body weight by ~5% (data not
shown).
In combined treatment using the first protocol
(analogs administered 5 times a week), analog PRI-2205 did not
influence tumor growth. From day 28 of the experiment, analog
PRI-2191 showed a tendency to reduce tumor volume in combination
with 5-FU and its interaction could be explained as synergism
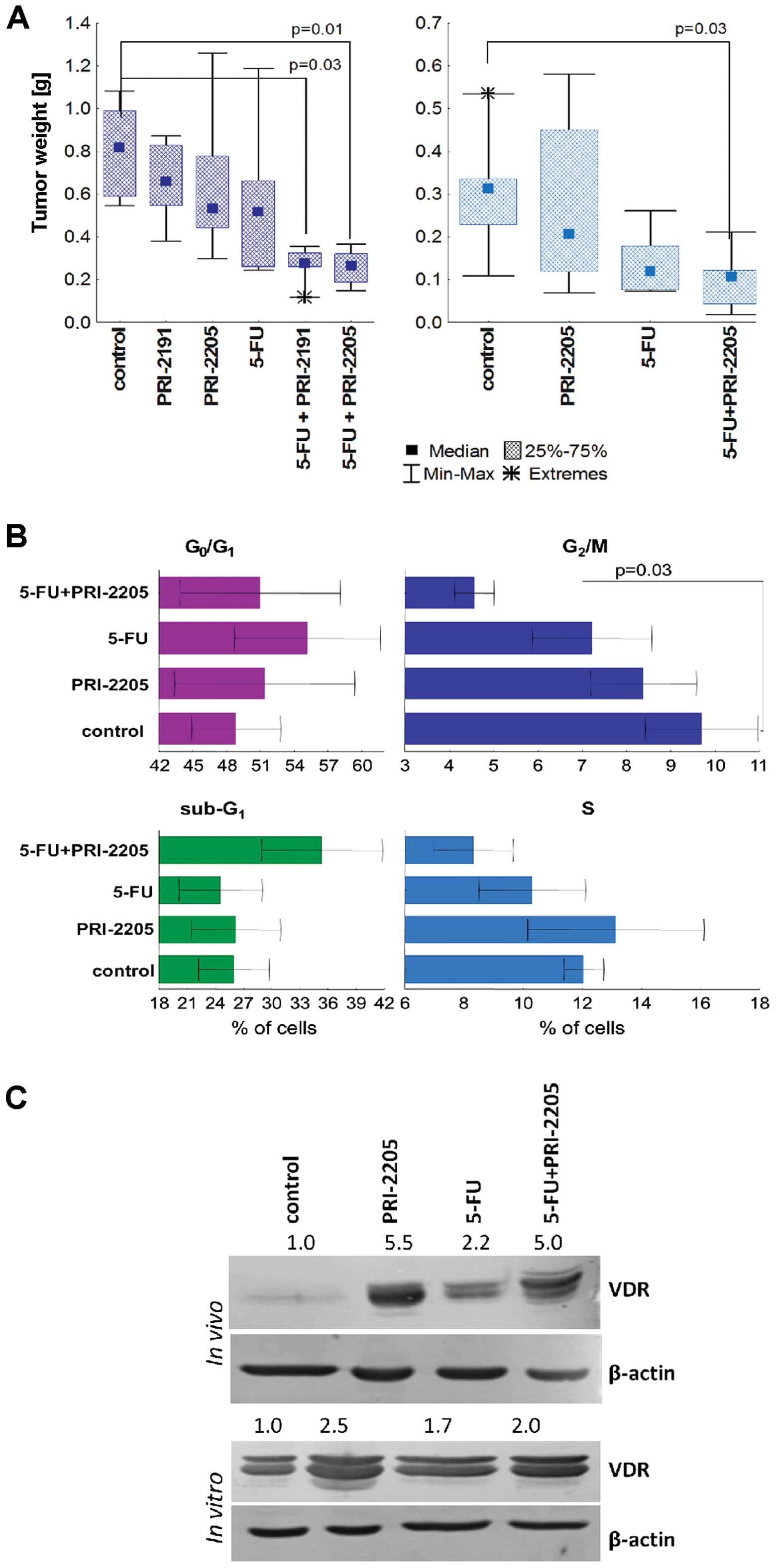
(Fig. 4A, left graph).
The second treatment schedule was considerably more
effective. Both agents potentiate the antitumor activity of 5-FU. A
statistically significant retardation of tumor growth was observed
in mice treated with 5-FU and PRI-2191 from day 28 to the end of
the experiment (with the exception of days 40 and 47). PRI-2205
used with 5-FU significantly retarded tumor growth from day 24 to
the end of measurements. Synergy was observed on all measurement
days (Fig. 4A, right graph).
Cell cycle analysis (analogs
administered 5 times a week)
Cell cycle analysis was performed on tumors
harvested from mice treated both with 5-FU alone and combined with
both analogs. It was observed that, in tumors treated with 5-FU,
the percentage of cells in the G0/G1 stage
significantly decreased. Moreover, these cells were stopped in the
S phase (P<0.05) and the percentage of cell deaths increased.
PRI-2191 used alone did not significantly affect the cell cycle of
HT-29 tumors. However, used in combined treatment with 5-FU, it
increased the number of cells in G0/G1 and in
parallel decreased the cells in both the G2/M and S
stages as well as death cells as compared to 5-FU alone (Fig. 4C). Tumors from mice treated with
PRI-2205 combined with 5-FU showed a similar cell cycle
distribution to that of mice treated with 5-FU alone (Fig. 4C).
Western blot analysis of selected
groups of mice (analogs administered 5 times a week)
For this analysis tumors from mice treated with 5-FU
alone or combined with vitamin D analogs were harvested. The
results of the western blot studies showed that, in tumors from
mice treated with 5-FU combined with PRI-2191 or PRI-2205, the
expression of p27 and VDR was increased as compared to tumors from
animals treated with 5-FU alone. The expression of p21, as well as
p-ERK1/2 was diminished when mice were administered with 5-FU
combined with both analogs. This diminution was well-defined for
PRI-2205 (Fig. 4B).
Effect of PRI-2191 or PRI-2205
administered alone or in combination with 5-FU on mice bearing
orthotopic human colon cancer HT-29
Primary tumor growth
In these experiments, we used the optimal treatment
schedule to assess the antitumor activity of combined treatment
against tumors growing intra-intestinally.
Analyzing the weight of intestinal tumors harvested
from mice, we observed a statistically significant reduction in
tumor growth in mice treated with 5-FU and both analogs (Fig. 5A). In the left-hand graph, the
results of experiments with an i.v. route of injection of 5-FU are
shown. 5-FU alone reduced tumor weight by 37% as compared to the
control, whereas used with PRI-2191 or PRI-2205 the reduction was
66 and 68%, respectively (Table
II) and this effect was synergistic. Similar results were
observed after i.p. administration of 5-FU with PRI-2205 (Fig. 5A, right graph). The schedules of
treatment used did not affect the body weight of animals (data not
shown). Moreover, when the serum calcium level was analyzed, we did
not observe any significant changes (Table II). The blood leukocyte count had
decreased by 5-FU and a further decrease was observed in combined
treatment with PRI-2205 (Table
II). This analysis was not performed for PRI-2191.
 | Table IIThe intestinal tumor growth, calcium
level and blood leukocyte count in mice bearing HT-29 tumors. |
Table II
The intestinal tumor growth, calcium
level and blood leukocyte count in mice bearing HT-29 tumors.
| 5-FU
intraperitoneally | 5-FU
intravenously |
|---|
|
|
|
|---|
| Group | Tumor weight on day
39 (g) | TGI (%) | H (%) | Leukocytes
(thousands/μl) | N | Tumor weight on day
53 (g) | TGI (%) | H (%) | Calcium
(mmol/l) | N |
|---|
| Control | 0.305±0.140 | | | 5.5±1.3 | 6 | 0.808±0.24 | -- | | 2.50±0.07 | 6 |
| PRI-2191 | nt. | nt. | | nt. | | 0.657±0.20 | 20 | | 2.61±0.07 | 5 |
| PRI-2205 | 0.291±0.199 | 5 | | 5.7±0.6 | 7 | 0.663±0.38 | 35 | | 2.42±0.08 | 5 |
| 5-FU | 0.138±0.072 | 55 | | 4.2±1.3 | 6 | 0.527±0.33 | 37 | | 2.48±0.03 | 7 |
| 5-FU +
PRI-2191 | nt. | nt. | | nt. | | 0.268±0.09a | 66 | 49 | 2.55±0.04 | |
| 5-FU +
PRI-2205 | 0.097±0.062a | 68 | 57 | 3.5±1.0 | 7 | 0.259±0.08a | 68 | 59 | 2.49±0.02 | 6 |
Cell cycle analysis and VDR expression
(i.p. administration of 5-FU)
Analyzing cell cycle and cell death in cells derived
from HT-29 intestinal tumors, we showed a significant decrease in
the percentage of cells in the G2M phase and a tendency
to increase the number of cell deaths in tumors from mice treated
with 5-FU and PRI-2205 simultaneously (Fig. 5B). The expression of VDR increased
in treated groups as compared to tumors from control mice. A 5
fold-increase in VDR expression as compared to control tumors was
observed in tumors from mice treated with both agents and with
PRI-2205 alone (Fig. 5C). We
observed a similar tendency in HT-29 cells from in vitro
culture (Fig. 5C).
Effect of PRI-2191 or PRI-2205
administered either alone or in combination with CPC on mice
bearing subcutaneous human colon cancer HT-29
CPC, in the doses and schedules used, did not
influence tumor growth until day 38 of the experiment, when some
retardation was observed. In this model, neither vitamin D analog
improved the anticancer activity of CPC (data not shown).
Molecular modeling
The vitamin D compounds
1,25(OH)2D3, PRI-2191, PRI-2205 and
calcipotriol were docked into the space occupied by the original
ligand [1,25(OH)2D3; 25(OH)D3;
3,20-pregnanedione for VDR, DBP and CAR, respectively] and the
binding energy was estimated using an implicit solvation model. The
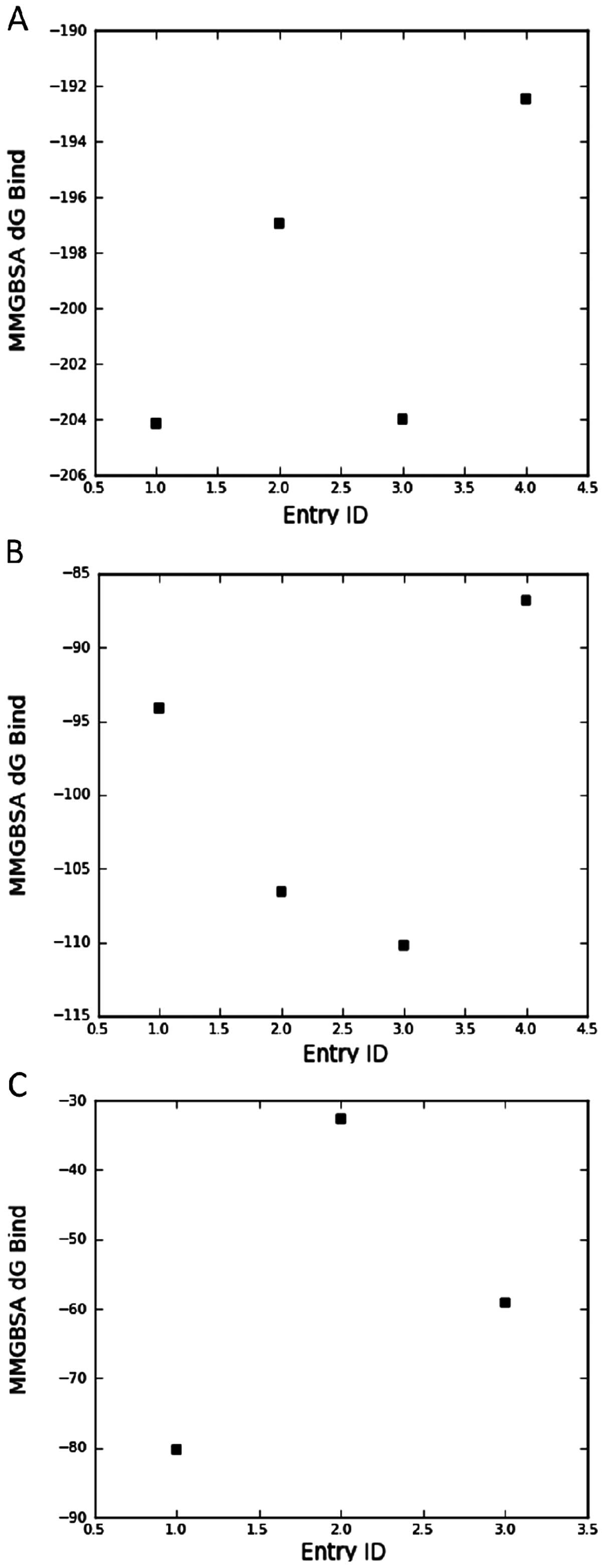
results presented in Fig. 6A show
that the estimated binding energy to VDR calculated for PRI-2205 is
similar to its parent compound, namely calcipotriol, and is higher
than that observed for PRI-2191 and
1,25(OH)2D3. On the other hand, analyzing
binding energy to DBP (Fig. 6B), we
conclude that both analogs tested, PRI-2191 and PRI-2205, have
higher affinity to DBP than the parent compounds,
1,25(OH)2D3 and calcipotriol.
1,25(OH)2D3 did not interact
with the ligand binding domain of CAR/RXR protein complex. However,
PRI-2205 possesses a reasonable affinity to this receptor which was
higher than for tacalcitol (Fig.
6C). The affinity of PRI-2201 was the highest from the three
mentioned compounds.
Discussion
1,25(OH)2D3 (calcitriol) and
its analogs are known to be agents which exert anticancer
activities both alone and by cooperating with anticancer compounds
(17,48). In our previous experiments with
mouse colon cancer MC38 in vivo, we showed that
1,25(OH)2D3 analogs interact synergistically
with 5-FU improving its antitumor and antimetastatic activity, as
well as prolonging the survival time of mice. However, better
results were observed for PRI-2191 than for PRI-2205 (49). In the present study, we analyzed the
antitumor effect of such combined treatment on immunodeficient mice
bearing human HT-29 colon cancer. We showed that the three times a
week schedule of treatment is more effective than the other
schedule. However, analog PRI-2191 in combined treatment with 5-FU
retarded subcutaneous tumor growth in both schedules (three and
five times a week of administration of vitamin D analogs). The
improved antitumor effect of 5-FU by PRI-2191 or PRI-2205 was also
observed in mice bearing human colon cancer HT-29 transplanted
orthotopically.
It has been previously shown that proliferating
HT-29 colon cancer cells exhibit upregulation of VDR and induction
of 24-hydroxylase mRNA, whereas differentiated cells fail to
exhibit either of these biological responses to
1,25(OH)2D3 (50). Moreover, differentiation of colon
cancer cells induced by various treatments occurs via upregulation
of VDR (50,51). Palmer et al reported that
vitamin D analogs promote differentiation only of colon cancer
cells expressing VDR and that this process is related to induction
of E-cadherin and inhibition of β-catenin signaling (52). The analysis of VDR expression in
orthotopic or subcutaneous tumors from mice treated with 5-FU and
PRI-2205 showed either a 2.3- or only a 1.2-fold increase as
compared to 5-FU, respectively. In subcutaneous tumors, PRI-2191
also slightly increased (1.4-fold) expression of VDR as compared to
5-FU alone. In our previous studies, we observed a 6-fold increase
in VDR expression in subcutaneous HT-29 tumors by PRI-2191 used
alone, but not by PRI-2205 (30).
The differences between the significant increase (5.5-fold) in VDR
in orthotopic tumors and that observed subcutaneously (0.8-fold) by
PRI-2205 (30) could be explained
via the importance of the tumor microenvironment in which cancer
cells are implanted (53,54). HT-29 cells incubated with these
agents in in vitro culture showed a similar pattern of VDR
expression to that of orthotopic tumors (Fig. 5C).
The ligand binding domain (LBD) of the VDR protein
has been well characterized using the known amino acid sequence of
the VDR and its crystallized structure. An evaluation of how
1,25(OH)2D3 is oriented within the LBD has
provided important insight into the chemical interactions that are
responsible for ligand-receptor binding. These interactions have
been essential for the elucidation of the structure function
relationship between the VDR, 1,25(OH)2D3 and
the development of new 1,25(OH)2D3 analogs
(55,56). It is known that PRI-2191
[tacalcitol, 1,24-(OH)2D3] has a similar or
higher affinity for the VDR than the parent compound
1,25(OH)2D3 (57). On the other hand, the relative
affinities to the VDR of some analogs with high anticancer
activity, such as KH1060, calcipotriol (MC-903) and EB 1089, were
similar or lower compared to 1,25(OH)2D3
(55). The estimated energy of
binding PRI-2205 to LBD of VDR as calculated in our studies was
higher than that for PRI-2191; moreover, both analogs bind to
ligand binding pocket of vitamin D binding protein (DBP) with
similar affinity. The biological activity of vitamin D metabolites
and analogs depends on their affinity not only for the VDR but also
for vitamin D binding protein. One of the functions of DBP is to
prolong the lifetime of 1,25(OH)2D3 in
circulation. Analogs with a low DBP but good receptor binding
properties display low in vivo biologic activity on calcium
and bone homeostasis, at least partly due to the altered
pharmacokinetics (58). However,
the biological activity in vivo of secosteroids could also
be modulated through its potential interaction with constitutive
androstane receptor (CAR) and a crosstalk CAR-VDR. Constitutive
androstane receptor was originally identified as a xenobiotic
sensor that regulates the expression of cytochrome P450 genes.
However, previous studies suggest that this nuclear receptor is
also involved in the regulation of energy metabolism including
glucose and lipid homeostasis (59,60).
One of the VDR target genes is CYP24 which encodes a mitochondrial
cytochrome P450 that hydroxylates 1,25(OH)2D3
and other vitamin D derivatives at position 24. This
biotransformation converts these molecules to inactive metabolites.
Transactivation of two responsive elements (VDRE-1 and VDRE-2) in
CYP24 by VDR/RXR leads to increased expression of CYP24, reduced
level of the biologically active 1,25(OH)2D3
and downregulation of VDR-target genes (61). Notably, Moreou et al
(62) showed that CAR binds to and
transactivates the VDREs present in the CYP24 promoter and that a
specific human CAR agonist, CITCO, increases CYP24 mRNA expression
in primary human hepatocytes. These findings suggest that the
VDR-CAR crosstalk resulting from the recognition of same response
elements is reciprocal (62). Such
crosstalk provides, at least in part, an objective explanation to
the observation from our studies indicating similar biological
activity of PRI-2191 and PRI-2205, but lower toxicity of the latter
(26). As we showed, PRI-2205 has
stronger affinity for CAR/RXR complex than PRI-2191 and in parallel
possesses higher binding affinity for VDR LBD. This characteristic
of PRI-2205 may be the possible explanation of its low toxicity via
increased generation of CYP24 through VDR and CAR activation, with
a sustained, but lower, anticancer activity through the requirement
for higher doses needed to obtain results similar to PRI-2191. The
last conclusion is also supported by our docking studies showing a
high affinity to VDR as well as the best binding to DBP.
Cell cycle distribution in cells from in
vitro culture as well as from tumors was not influenced by
PRI-2205 used alone. However, we observed a similar general
tendency in all experimental conditions towards a decrease in the
number of cells in G0/G1, G2M and
S as compared to 5-FU alone. Moreover, in orthotopic tumors as well
as in cultured cells, an increase in cell deaths was observed in
combined treatment regimens. PRI-2191 acted differently, increasing
the percentage of cells in G0/G1 and
decreasing it in S and G2/M as compared to 5-FU alone.
This suggests the pro-differentiating activity of this analog,
particularly when we take into consideration our previous results
showing that PRI-2191 increased the expression of E-cadherin on
HT-29 cells in vitro (49).
There, it was shown that the target gene of p53 -
p21waf1/cip1 is a primary
1,25(OH)2D3-responding gene with VDR binding
promoter regions (10). Moreover,
other vitamin D targeted genes which lack VDRE, such as the other
cell cycle inhibitor p27, could be regulated by
1,25(OH)2D3 (63,64).
Several studies suggest that growth inhibition by
1,25(OH)2D3 may be attributed to inhibition
of the G1 to S cell cycle transition, which may be due,
at least in part, to stimulation of the expression of the
cyclin-dependent kinase inhibitors (CDKIs), p21 and p27, as well as
to programmed cell death (10). Our
present observations are in accordance with previous results on the
pro-differentiating activity of PRI-2191. We have shown that HL-60
leukemia cells acquire a mature macrophage phenotype after their
exposure to PRI-2191 in vitro (21). Moreover, we also observed that
PRI-2191 increased the expression of p27 in mouse mammary gland
tumors (29). These data suggest
that the higher antitumor effect of the combined treatment with
PRI-2191 and 5-FU may be the result of the cell cycle arrest in the
G0/G1 cell cycle phase and the increased p21
and p27 expression following treatment with PRI-2191. However, the
lack of increase in the p27 expression following treatment with
PRI-2205 suggests that there may be differences between the
mechanisms of action of these two analogs.
1,25(OH)2D3 or its analogs
could reveal various activities, balanced between pro- and
anti-apoptotic pathways. In particular,
1,25(OH)2D3 increased the level of
pro-apoptotic protein BAK (BCL-2 family member) (65) and decreased the anti-apoptotic
activity of β-catenin (52).
Analysis of the cell death parameters in in vitro culture
suggested antagonism in pro-apoptotic activity, especially of
PRI-2191 and 5-FU. The percentage of PI stained cells, active
caspase-3 as well as p53 positive cells decreased in comparison to
5-FU. PRI-2191 also reduced the number of both cells with lower
mitochondrial potential and Annexin V positive cells. A similar
effect was described after simultaneous incubation of HT-29 cells
with H2O2 and
1,25(OH)2D3. The increased cytotoxic effect
of simultaneous treatment was demonstrated, but the activity of
caspase 3 was decreased by 1,25(OH)2D3 as
compared to H2O2 (66). PRI-2205 slightly decreased only PI
positive cells, but did not affect caspase-3 or p-53 positive cells
as compared to 5-FU alone. HT-29 possessed mutated p53 (67) and, according to recent studies, a
functional and physical interaction between mutated p53 and the
vitamin D transcriptional regulatory pathway exists. This
interaction lies in the cooperation between mutated p53 with
1,25(OH)2D3 to elicit an anti-apoptotic state
(68). Since PRI-2191 possesses a
similar pattern of activity to 1,25(OH)2D3,
the presence of mutated p53 in HT-29 cells may be the reason of the
observed anti-apoptotic effects. However, in spite of these
disadvantageous effects regarding apoptosis, the therapeutic effect
expressed as tumor growth retardation is significant. In contrast,
PRI-2205, several activities of which differ from those observed
for 1,25(OH)2D3 or PRI-2191 (such as no
influence on the expression of p53), did not reveal anti-apoptotic
activity. Moreover, our previous studies, including on HL-60
leukemia cell lines, showed the pro-apoptotic activity of this
analog (26).
5-FU is an important drug used in the chemotherapy
of colorectal cancer, but new strategies for combined treatment
with this agent are still under development. It has been assumed
that the low thymidylate synthase (TS; a direct target of 5-FU)
level increases drug efficacy (69). Previous data as well as our own
results showed that HT-29 cells (with mutated TP53) exposed to 5-FU
are cumulated in S cell cycle phase which is correlated with high
TS level during DNA synthesis (70). High level of TS leads to
insensitivity of tumor cells to 5-FU and is one of the main reasons
of resistance development (71).
Takagi et al (72) showed
that the HCT116 colon cancer cells with silenced expression of p21
protein express higher level of TS than wild-type cells. Both
analogs induced the expression CDKIs p21 and/or p27 and could
probably block the expression of TS and therefore increase the 5-FU
activity. Some agents are known as sensitizing cells to 5-FU via
various mechanisms. An example is trametinib, which induced p15 and
p27 expression and reduced cyclin D1 levels. Another one is
fenofibrate which dephosphorylated ERK1/2 and reduced cyclin D1
levels in HT-29 cells with subsequent G1-phase arrest,
reduced TS expression and sensitized cells to 5-FU (73). PRI-2191 and PRI-2205 possess a
similar profile of activity, also reducing the level of p-ERK1/2 as
compared to 5-FU alone.
In conclusion, our results suggest that the
mechanism of potentiation of 5-FU antitumor action by both analogs
is realized via increased p21 expression and decreased
phosphorylation level of ERK1/2 which may lead to diminution of
thymidylate synthase expression. However, the differences in the
biological activity including mode of action and toxicity between
both analogs was observed. Our molecular modeling studies may in
part clarify some of these differences, namely, in general PRI-2205
possesses higher binding affinity for VDR, DBP, but also for
CAR\RXR ligand binding domain which may, in part, explain its very
low toxicity with sustained anticancer activity. However, the
combined treatment proposed in our studies was ineffective in the
HT-29 colon cancer model, when capecitabine was used in place of
5-FU.
Acknowledgements
The research was supported by the Polish Ministry
of Science and Higher Education Grant No. N N401 014535 and by
Polish National Science Center Grant No. 2012/05/N/NZ5/00883. W.S.
is funded by an internal grant from Wroclaw Research Center EIT+
Sp. z o.o. No. 3.6 ‘Development of inhibitors of pathogenic E.
coli’ and an extension to the same grant ‘Development of efflux
pump inhibitors for chemotherapy’.
References
|
1
|
Lamprecht SA and Lipkin M: Chemoprevention
of colon cancer by calcium, vitamin D and folate: molecular
mechanisms. Nat Rev Cancer. 3:601–614. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCarthy TC, Li X and Sinal CJ: Vitamin D
receptor-dependent regulation of colon multidrug
resistance-associated protein 3 gene expression by bile acids. J
Biol Chem. 280:23232–23242. 2005. View Article : Google Scholar
|
|
3
|
Pritchard RS, Baron JA and Gerhardsson de
Verdier M: Dietary calcium, vitamin D, and the risk of colorectal
cancer in Stockholm, Sweden. Cancer Epidemiol Biomarkers Prev.
5:897–900. 1996.PubMed/NCBI
|
|
4
|
Terry P, Baron JA, Bergkvist L, Holmberg L
and Wolk A: Dietary calcium and vitamin D intake and risk of
colorectal cancer: a prospective cohort study in women. Nutr
Cancer. 43:39–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berkovich L, Sintov AC and Ben-Shabat S:
Inhibition of cancer growth and induction of apoptosis by BGP-13
and BGP-15, new calcipotriene-derived vitamin D3analogs,
in-vitro and in-vivo studies. Invest New Drugs. 31:247–255. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grau MV, Baron JA, Sandler RS, Haile RW,
Beach ML, Church TR and Heber D: Vitamin D, calcium
supplementation, and colorectal adenomas: results of a randomized
trial. J Natl Cancer Inst. 95:1765–1771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hartman TJ, Albert PS, Snyder K, Slattery
ML, Caan B, Paskett E, Iber F, Kikendall JW, Marshall J, Shike M,
Weissfeld J, Brewer B, Schatzkin A and Lanza E: The association of
calcium and vitamin D with risk of colorectal adenomas. J Nutr.
135:252–259. 2005.PubMed/NCBI
|
|
8
|
Baniahmad A and Tsai MJ: Mechanisms of
transcriptional activation by steroid hormone receptors. J Cell
Biochem. 51:151–156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagpal S, Na S and Rathnachalam R:
Noncalcemic actions of vitamin D receptor ligands. Endocr Rev.
26:662–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saramaki A, Banwell CM, Campbell MJ and
Carlberg C: Regulation of the human p21 (waf1/cip1) gene promoter
via multiple binding sites for p53 and the vitamin
D3receptor. Nucleic Acids Res. 34:543–554. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alvarez-Diaz S, Valle N, Ferrer-Mayorga G,
Lombardia L, Herrera M, Dominguez O, Segura MF, Bonilla F, Hernando
E and Munoz A: MicroRNA-22 is induced by vitamin D and contributes
to its antiproliferative, antimigratory and gene regulatory effects
in colon cancer cells. Hum Mol Genet. 21:2157–2165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matusiak D, Murillo G, Carroll RE, Mehta
RG and Benya RV: Expression of vitamin D receptor and
25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant
human colon. Cancer Epidemiol Biomarkers Prev. 14:2370–2376.
2005.
|
|
13
|
Tangpricha V, Spina C, Yao M, Chen TC,
Wolfe MM and Holick MF: Vitamin D deficiency enhances the growth of
MC-26 colon cancer xenografts in Balb/c mice. J Nutr.
135:2350–2354. 2005.PubMed/NCBI
|
|
14
|
Mokady E, Schwartz B, Shany S and
Lamprecht SA: A protective role of dietary vitamin D3in
rat colon carcinogenesis. Nutr Cancer. 38:65–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hummel DM, Thiem U, Hobaus J, Mesteri I,
Gober L, Stremnitzer C, Graca J, Obermayer-Pietsch B and Kallay E:
Prevention of preneoplastic lesions by dietary vitamin D in a mouse
model of colorectal carcinogenesis. J Steroid Biochem Mol Biol.
136:284–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larriba MJ, Ordonez-Moran P, Chicote I,
Martin-Fernandez G, Puig I, Munoz A and Palmer HG: Vitamin D
receptor deficiency enhances Wnt/β-catenin signaling and tumor
burden in colon cancer. PLoS ONE. 6:e235242011.PubMed/NCBI
|
|
17
|
Cho YL, Christensen C, Saunders DE,
Lawrence WD, Deppe G, Malviya VK and Malone JM: Combined effects of
1,25-dihydroxyvitamin D3 and platinum drugs on the
growth of MCF-7 cells. Cancer Res. 51:2848–2853. 1991.PubMed/NCBI
|
|
18
|
Ravid A, Rocker D, Machlenkin A, Rotem C,
Hochman A, Kessler-Icekson G, Liberman UA and Koren R:
1,25-Dihydroxyvitamin D3 enhances the susceptibility of breast
cancer cells to doxorubicin-induced oxidative damage. Cancer Res.
59:862–867. 1999.PubMed/NCBI
|
|
19
|
Siwinska A, Opolski A, Chrobak A, Wietrzyk
J, Wojdat E, Kutner A, Szelejewski W and Radzikowski C:
Potentiation of the antiproliferative effect in vitro of
doxorubicin, cisplatin and genistein by new analogues of vitamin D.
Anticancer Res. 21:1925–1929. 2001.PubMed/NCBI
|
|
20
|
Pelczynska M, Switalska M, Maciejewska M,
Jaroszewicz I, Kutner A and Opolski A: Antiproliferative activity
of vitamin D compounds in combination with cytostatics. Anticancer
Res. 26:2701–2705. 2006.PubMed/NCBI
|
|
21
|
Opolski A, Wietrzyk J, Siwinska A,
Marcinkowska E, Chrobak A, Radzikowski C and Kutner A: Biological
activity in vitro of side-chain modified analogues of calcitriol.
Curr Pharm Des. 6:755–765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kota BP, Allen JD and Roufogalis BD: The
effect of vitamin D3and ketoconazole combination on
VDR-mediated P-gp expression and function in human colon
adenocarcinoma cells: implications in drug disposition and
resistance. Basic Clin Pharmacol Toxicol. 109:97–102. 2011.
|
|
23
|
Abe J, Nakano T, Nishii Y, Matsumoto T,
Ogata E and Ikeda K: A novel vitamin D3analog,
22-oxa-1,25-dihydroxyvitamin D3, inhibits the growth of human
breast cancer in vitro and in vivo without causing hypercalcemia.
Endocrinology. 129:832–837. 1991.
|
|
24
|
Abe-Hashimoto J, Kikuchi T, Matsumoto T,
Nishii Y, Ogata E and Ikeda K: Antitumor effect of
22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic
mice implanted with human breast carcinoma and its synergism with
tamoxifen. Cancer Res. 53:2534–2537. 1993.PubMed/NCBI
|
|
25
|
Chodyński M, Wietrzyk J, Marcinkowska E,
Opolski A, Szelejewski W and Kutner A: Synthesis and
antiproliferative activity of side-chain unsaturated and
homologated analogs of 1,25-dihydroxyvitamin D(2).
(24E)-(1S)-24-Dehydro-24a-homo-1,25-dihydroxyergocalciferol and
congeners. Steroids. 67:789–798. 2002.PubMed/NCBI
|
|
26
|
Wietrzyk J, Chodynski M, Fitak H, Wojdat
E, Kutner A and Opolski A: Antitumor properties of diastereomeric
and geometric analogs of vitamin D3. Anticancer Drugs.
18:447–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wietrzyk J, Nevozhay D, Filip B, Milczarek
M and Kutner A: The antitumor effect of lowered doses of
cytostatics combined with new analogs of vitamin D in mice.
Anticancer Res. 27:3387–3398. 2007.PubMed/NCBI
|
|
28
|
Wietrzyk J, Pelczynska M, Madej J, Dzimira
S, Kusnierczyk H, Kutner A, Szelejewski W and Opolski A: Toxicity
and antineoplastic effect of (24R)-1,24-dihydroxyvitamin D3
(PRI-2191). Steroids. 69:629–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wietrzyk J, Nevozhay D, Milczarek M, Filip
B and Kutner A: Toxicity and antitumor activity of the vitamin D
analogs PRI-1906 and PRI-1907 in combined treatment with
cyclophosphamide in a mouse mammary cancer model. Cancer Chemother
Pharmacol. 62:787–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milczarek M, Rosinska S, Psurski M,
Maciejewska M, Kutner A and Wietrzyk J: Combined colonic cancer
treatment with vitamin D analogs and irinotecan or oxaliplatin.
Anticancer Res. 33:433–444. 2013.PubMed/NCBI
|
|
31
|
Lowe SW, Bodis S, McClatchey A, Remington
L, Ruley HE, Fisher DE, Housman DE and Jacks T: p53 status and the
efficacy of cancer therapy in vivo. Science. 266:807–810. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XX, Dai MS and Lu H: 5-fluorouracil
activation of p53 involves an MDM2-ribosomal protein interaction. J
Biol Chem. 282:8052–8059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chakrabarty S, Radjendirane V, Appelman H
and Varani J: Extracellular calcium and calcium sensing receptor
function in human colon carcinomas: promotion of E-cadherin
expression and suppression of beta-catenin/TCF activation. Cancer
Res. 63:67–71. 2003.
|
|
34
|
Bhagavathula N, Hanosh AW, Nerusu KC,
Appelman H, Chakrabarty S and Varani J: Regulation of E-cadherin
and beta-catenin by Ca2+in colon carcinoma is dependent
on calcium-sensing receptor expression and function. Int J Cancer.
121:1455–1462. 2007.PubMed/NCBI
|
|
35
|
Chakrabarty S, Wang H, Canaff L, Hendy GN,
Appelman H and Varani J: Calcium sensing receptor in human colon
carcinoma: interaction with Ca2+and
1,25-dihydroxyvitamin D3. Cancer Res. 65:493–498.
2005.PubMed/NCBI
|
|
36
|
Wang X, Chen W, Singh N, Promkan M and Liu
G: Effects of potential calcium sensing receptor inducers on
promoting chemosensitivity of human colon carcinoma cells. Int J
Oncol. 36:1573–1580. 2010.PubMed/NCBI
|
|
37
|
Liu G, Hu X and Chakrabarty S: Vitamin D
mediates its action in human colon carcinoma cells in a
calcium-sensing receptor-dependent manner: downregulates malignant
cell behavior and the expression of thymidylate synthase and
survivin and promotes cellular sensitivity to 5-FU. Int J Cancer.
126:631–639. 2010. View Article : Google Scholar
|
|
38
|
Peters GJ, van der Wilt CL, van Moorsel
CJ, Kroep JR, Bergman AM and Ackland SP: Basis for effective
combination cancer chemotherapy with antimetabolites. Pharmacol
Ther. 87:227–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim R, Emi M, Tanabe K, Uchida Y and
Arihiro K: The role of apoptotic or nonapoptotic cell death in
determining cellular response to anticancer treatment. Eur J Surg
Oncol. 32:269–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sastry GM, Adzhigirey M, Day T,
Annabhimoju R and Sherman W: Protein and ligand preparation:
parameters, protocols, and influence on virtual screening
enrichments. J Comput Aided Mol Des. 27:221–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Friesner RA, Murphy RB, Repasky MP, Frye
LL, Greenwood JR, Halgren TA, Sanschagrin PC and Mainz DT: Extra
precision glide: docking and scoring incorporating a model of
hydrophobic enclosure for protein-ligand complexes. J Med Chem.
49:6177–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Halgren TA, Murphy RB, Friesner RA, Beard
HS, Frye LL, Pollard WT and Banks JL: Glide: a new approach for
rapid, accurate docking and scoring. 2. Enrichment factors in
database screening. J Med Chem. 47:1750–1759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Friesner RA, Banks JL, Murphy RB, Halgren
TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK,
Shaw DE, Francis P and Shenkin PS: Glide: a new approach for rapid,
accurate docking and scoring. 1. Method and assessment of docking
accuracy. J Med Chem. 47:1739–1749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Abel R, Zhu K, Cao Y, Zhao S and
Friesner RA: The VSGB 2.0 model: a next generation energy model for
high resolution protein structure modeling. Proteins. 79:2794–2812.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hou T, Wang J, Li Y and Wang W: Assessing
the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy
of binding free energy calculations based on molecular dynamics
simulations. J Chem Inf Model. 51:69–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hou T, Wang J, Li Y and Wang W: Assessing
the performance of the molecular mechanics/Poisson Boltzmann
surface area and molecular mechanics/generalized Born surface area
methods. II. The accuracy of ranking poses generated from docking.
J Comput Chem. 32:866–877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rastelli G, Del Rio A, Degliesposti G and
Sgobba M: Fast and accurate predictions of binding free energies
using MM-PBSA and MM-GBSA. J Comput Chem. 31:797–810.
2010.PubMed/NCBI
|
|
48
|
Danilenko M and Studzinski GP: Enhancement
by other compounds of the anti-cancer activity of vitamin
D3and its analogs. Exp Cell Res. 298:339–358. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Milczarek M, Psurski M, Kutner A and
Wietrzyk J: Vitamin D analogs enhance the anticancer activity of
5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer.
13:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao X and Feldman D: Regulation of
vitamin D receptor abundance and responsiveness during
differentiation of HT-29 human colon cancer cells. Endocrinology.
132:1808–1814. 1993.PubMed/NCBI
|
|
51
|
Gaschott T, Werz O, Steinmeyer A,
Steinhilber D and Stein J: Butyrate-induced differentiation of
Caco-2 cells is mediated by vitamin D receptor. Biochem Biophys Res
Commun. 288:690–696. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Palmer HG, Gonzalez-Sancho JM, Espada J,
Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros
AG, Lafarga M and Munoz A: Vitamin D(3) promotes the
differentiation of colon carcinoma cells by the induction of
E-cadherin and the inhibition of beta-catenin signaling. J Cell
Biol. 154:369–387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wietrzyk J, Opolski A, Madej J and
Radzikowski C: Antitumour and antimetastatic effect of genistein
alone or combined with cyclophosphamide in mice transplanted with
various tumours depends on the route of tumour transplantation. In
Vivo. 14:357–362. 2000.
|
|
54
|
Hidalgo AA, Paredes R, Garcia VM, Flynn G,
Johnson CS, Trump DL and Onate SA: Altered VDR-mediated
transcriptional activity in prostate cancer stroma. J Steroid
Biochem Mol Biol. 103:731–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Spina CS, Tangpricha V, Uskokovic M,
Adorinic L, Maehr H and Holick MF: Vitamin D and cancer. Anticancer
Res. 26:2515–2524. 2006.
|
|
56
|
Tocchini-Valentini G, Rochel N, Wurtz JM
and Moras D: Crystal structures of the vitamin D nuclear receptor
liganded with the vitamin D side chain analogues calcipotriol and
secocalcitol, receptor agonists of clinical importance. Insights
into a structural basis for the switching of calcipotriol to a
receptor antagonist by further side chain modification. J Med Chem.
47:1956–1961. 2004.
|
|
57
|
Matsunaga T, Yamamoto M, Mimura H, Ohta T,
Kiyoki M, Ohba T, Naruchi T, Hosoi J and Kuroki T:
1,24(R)-dihydroxyvitamin D3, a novel active form of vitamin D3 with
high activity for inducing epidermal differentiation but decreased
hypercalcemic activity. J Dermatol. 17:135–142. 1990.
|
|
58
|
Bouillon R, Allewaert K, Xiang DZ, Tan BK
and van Baelen HJ: Vitamin D analogs with low affinity for the
vitamin D binding protein: enhanced in vitro and decreased in vivo
activity. J Bone Miner Res. 6:1051–1057. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu RX, Lambert MH, Wisely BB, et al: A
structural basis for constitutive activity in the human
CAR/RXRalpha heterodimer. Mol Cell. 16:919–928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu B, Li S and Dong D: 3D structures and
ligand specificities of nuclear xenobiotic receptors CAR, PXR and
VDR. Drug Discov Today. 18:574–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sutton AL and MacDonald PN: Vitamin D:
more than a ‘bone-a-fide’ hormone. Mol Endocrinol. 17:777–791.
2003.
|
|
62
|
Moreau A, Maurel P, Vilarem MJ and
Pascussi JM: Constitutive androstane receptor-vitamin D receptor
crosstalk: consequence on CYP24 gene expression. Biochem Biophys
Res Commun. 360:76–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cheng HT, Chen JY, Huang YC, Chang HC and
Hung WC: Functional role of VDR in the activation of p27Kip1 by the
VDR/Sp1 complex. J Cell Biochem. 98:1450–1456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang YC, Chen JY and Hung WC: Vitamin D3
receptor/Sp1 complex is required for the induction of p27Kip1
expression by vitamin D3. Oncogene. 23:4856–4861. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Diaz GD, Paraskeva C, Thomas MG, Binderup
L and Hague A: Apoptosis is induced by the active metabolite of
vitamin D3 and its analogue EB1089 in colorectal adenoma and
carcinoma cells: possible implications for prevention and therapy.
Cancer Res. 60:2304–2312. 2000.PubMed/NCBI
|
|
66
|
Koren R, Wacksberg S, Weitsman GE and
Ravid A: Calcitriol sensitizes colon cancer cells to
H2O2-induced cytotoxicity while inhibiting
caspase activation. J Steroid Biochem Mol Biol. 101:151–160. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Meng J, Zhang HH, Zhou CX, Li C, Zhang F
and Mei QB: The histone deacetylase inhibitor trichostatin A
induces cell cycle arrest and apoptosis in colorectal cancer cells
via p53-dependent and -independent pathways. Oncol Rep. 28:384–388.
2012.PubMed/NCBI
|
|
68
|
Stambolsky P, Tabach Y, Fontemaggi G,
Weisz L, Maor-Aloni R, Siegfried Z, Shiff I, Kogan I, Shay M, Kalo
E, Blandino G, Simon I, Oren M and Rotter V: Modulation of the
vitamin D3 response by cancer-associated mutant p53. Cancer Cell.
17:273–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: an emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
71
|
Mader RM, Muler M and Steger G: Resistance
to 5-fluorouracil. Gen Pharmacol. 31:661–666. 1998. View Article : Google Scholar
|
|
72
|
Takagi K, Sowa Y, Cevik OM, Nakanishi R
and Sakai T: CDK inhibitor enhances the sensitivity to
5-fluorouracil in colorectal cancer cells. Int J Oncol.
32:1105–1110. 2008.PubMed/NCBI
|
|
73
|
Watanabe M, Sowa Y, Yogosawa M and Sakai
T: Novel MEK inhibitor trametinib and other retinoblastoma gene
(RB)-reactivating agents enhance efficacy of 5-fluorouracil on
human colon cancer cells. Cancer Sci. 104:687–693. 2013. View Article : Google Scholar : PubMed/NCBI
|