Introduction
Gastric cancer is one of the most common types of
cancer worldwide, and almost 50% of gastric cancer-related deaths
occur in China (1–3). Surgery offers the only realistic
chance of cure; however, many of the patients present with
unresectable tumors at the time of diagnosis. Even with resection,
more than 50% of patients relapse and eventually die of their
disease (4,5). Therefore, non-surgical methods have
attracted increasing attention. In recent years, 125I
implantation has been widely used to treat prostate cancer
(6,7) and other tumor types (8,9)
because of its ability to offer high precision, little trauma,
strong lethality and few complications (10–12).
Wang and colleagues (13) applied
125I implantation to treat advanced gastric cancer and
found significant improvement in clinical symptoms and the quality
of life of the patients. Although 125I seed
implantations have been successfully applied in the clinic, its
biological effects and underlying molecular mechanisms are far from
fully understood. Takabayashi and colleagues (14) demonstrated that a continuous
low-dose rate of irradiation influenced the proliferation of cells
and the apoptosis rate which possibly was the main mechanism of the
cell-killing effects in CL187 cells. Ma and colleagues (15) demonstrated that 125I
irradiation at 4 Gy significantly induced cell apoptosis and cell
cycle arrest in gastric cancer cells.
The best known and the most efficient growth factors
involved in tumor angiogenesis are vascular endothelial growth
factor (VEGF) and nuclear factor-κB (NF-κB). Their activation has
been connected with multiple aspects of oncogenesis, such as
apoptotic resistance, transformation, growth, metastasis and
angiogenesis. Li et al (16)
found that inhibition of the NF-κB p65 signaling pathway may be
considered as a potential strategy for treating gastric cancer.
Various studies examined the relationship between VEGF expression
and the clinical outcome of patients with gastric cancer and found
that VEGF expression in gastric cancer tissue is associated with
poor survival (17). Thus,
irradiation-induced apoptosis, inhibition of cell proliferation and
VEGF and NF-κB signal transduction may be key mechanisms underlying
the therapeutic effect of low energy 125I seed
implantation. In the present study, we investigated the role of
VEGF and NF-κB in the process of 125I brachytherapy and
125I-induced cell apoptosis and cell cycle changes in a
xenograft model.
Materials and methods
Animal model
Human SGC-7901 cells (3×106/mouse) were
subcutaneously injected into the right dorsal flank of BALB/c-nu/nu
nude mice. After 1–2 weeks of implantation with tumor cells, when
tumors reached ~20–30 mm3, the animals were randomized
into control and treatment groups (30 animals per group). The
125I seeds (0.6 mCi) were injected into the mice in the
treatment group through an 18-gauge needle, while ghost seed were
injected into the mice in the control group. The tumor size was
measured using calipers, and the tumor volume (V) was estimated by
the following formula: (V) (mm3) = (L ×W2) ×
1/2, where L is the length and W is the width of the tumor.
Tumor volumes and body weights were monitored every
3 days over the course of treatment. The tumor weight was measured
when the mice were sacrificed. Mice were sacrificed after 28 days
of treatments, and tumors were removed and fixed in 10% neutral
buffered formalin for histologic and immunohistochemical analyses.
All animal procedures were carried out with the approval of the
Animal Ethics Committee of Kunming Medical College.
Histological analysis of tumors and
immunofluorescence examination of VEGF and NF-κB
Tumors were embedded in paraffin, sectioned (5 μm)
and stained with hematoxylin and eosin (H&E) (Sigma-Aldrich,
St. Louis, MO, USA). For the immunofluorescence staining of NF-κB
and VEGF, the frozen sections were maintained at room temperature
for 30 min, incubated in distilled water for 5 min and in PBS for 5
min and permeabilized in 1 g/l Triton X-100 for 10 min. The
sections were subsequently washed with PBS (5 min × 3), blocked
with 100 ml sheep serum (Sigma) at 37°C for 20 min and incubated in
the primary antibodies: rat anti-mouse NF-κB (BioLegend, San Diego,
CA, USA) and rabbit anti-human VEGF polyclonal antibody (LabVision
Corp., Fremont, CA, USA) at 4°C overnight and washed with PBS (5
min × 3). Incubation in the secondary antibody (goat anti-rat
IgG-conjugated TRITC or sheep anti-rabbit IgG-conjugated FITC;
Sigma) was carried out for 1 h at 37°C. Sections were washed with
PBS (10 min × 3) and then examined under a TCS SP2 laser confocal
microscope. For each group, several field images of VEGF and NF-κB
in each tumor tissue section were captured under a confocal
microscope. The fluorescence intensity of each section in the
confocal fluorescence images was measured using the Leica confocal
analysis system. The mean fluorescence intensity in each section
was then calculated.
Cell cycle distribution analysis
Cells in the mono-dispersed suspension were fixed
with ethanol, followed by propidium iodide staining (PI; Sigma) and
analyzed using the FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA). Percentages of cells resting in the G1, S and G2/M
phases were determined with CellQuest software (BD Biosciences) and
ModFit LT software (Verity Software House, Topsham, ME, USA).
Annexin V/PI assay of
125I-induced apoptosis
The cells were stained with Annexin V-FITC and PI,
and evaluated for apoptosis by flow cytometry according to the
manufacturer’s protocol (BD Pharmingen, San Diego, CA, USA). Both
early (Annexin V-positive, PI-negative) and late (Annexin
V-positive, PI-positive) apoptotic cells were counted as apoptotic
cells.
Total RNA preparation
The samples were ground by using liquid nitrogen.
Total RNA was extracted from lung tissues by using an animal tissue
RNA purification kit (Norgen Biotek Corp., Thorold, ON, Canada) as
recommended by the manufacturer. RNA samples were measured by using
a bioanalyzer to determine RNA integrity number.
Quantitative real-time PCR
The approximate length of miRNA at 21–23 nt resulted
in difficulties in conventional PCR test. We used
TaqMan® MicroRNA Assays (Applied Biosystems, Foster
City, CA, USA) to examine miRNA differential expression profiling
in PTC as recommended by the manufacturer. Sample RNA (10 ng) was
reversely transcribed into cDNA by using specific stem-loop primers
and TaqMan® MicroRNA reverse transcription kit. With
cDNA as the template, TaqMan MicroRNA Assay and the
TaqMan® Universal PCR Master Mix were used for the
serial real-time PCR. RNU48 was used as an internal control to
minimize the variation among reverse transcription, PCR and
samples. The data were collected, analyzed, and normalized by using
the Applied Biosystems analysis software to determine the
differential expression profiles of miRNAs. All of the experiments
were performed in triplicate. Expression levels were calculated by
using the relative quantification method (ΔΔCT) in the ABI PRISM
7500 Sequence Detection system (Applied Biosystems), according to
the manufacturer’s protocol.
Western blot analysis
Proteins were resolved on 12% polyacrylamide gels,
transferred to a nitrocellulose membrane (Bio-Rad Laboratories,
Hercules, CA, USA) and blocked with 5% non-fat dairy milk in
Tris-buffered saline (20 mM Tris, 150 mM NaCl, pH 7.4) with 0.1%
Tween-20.
Statistical analysis
The results of the animal experiments and real-time
PCR were analyzed using SPSS 13.0 software. (SPSS Inc., Chicago,
IL, USA). All data were plotted as mean ± standard deviation.
Student’s t-test was used to compare values between two independent
groups. Differences were considered to be significant at
P<0.05.
Results
Inhibitory effect of 125I seed
irradiation on the growth of gastric cancer
The effectiveness of 125I seed
irradiation to inhibit the growth of implanted SGC-7901 tumors was
examined in a nude mouse model. There were no significant changes
in the tumor volumes for the first 10 days of the 125I
seed treatment. However, after 13 days, the
125I-irradiated tumors were much smaller, and a
significant difference in tumor volume was observed over time
between the control and 125I treatment group (Fig. 1A). On day 28, the mice were
sacrificed and tumor weights were measured. Statistical difference
in the tumor weight was observed between the control and treatment
group (Fig. 1B). All these data
clearly indicated that 125I seed implantation
effectively inhibited tumor growth. In addition, the body weights
of the mice were not affected by the 125I irradiation
and no obvious radiation-induced damage was observed in the vital
organs of the mice (data not shown), indicating the safety of the
125I seed treatment.
Effect of 125I seed
irradiation on morphology of gastric cancer tumors
To investigate the effect of the 125I
irradiation on the histology of the SGC-7901 xenografts, tumor
sections were obtained from mice in the control and the
125I treatment group and were stained using H&E. As
shown in Fig. 2, the histological
appearance of the tumors in the control group was quite different
from that in the 125I treatment group. In the control
group, the cancer cells were densely arranged with large darkly
stained nuclei and obvious karyokinesis. In the treatment group,
large necrotic regions were observed around the 125I
seed. The cancer cells adjacent to the necrotic region were loosely
arranged with condensed nuclei and reduced eosinophilic cytoplasm.
These results indicated that 125I seed implantation
caused growth inhibition of cancer cells in the SGC-7901
xenografts.
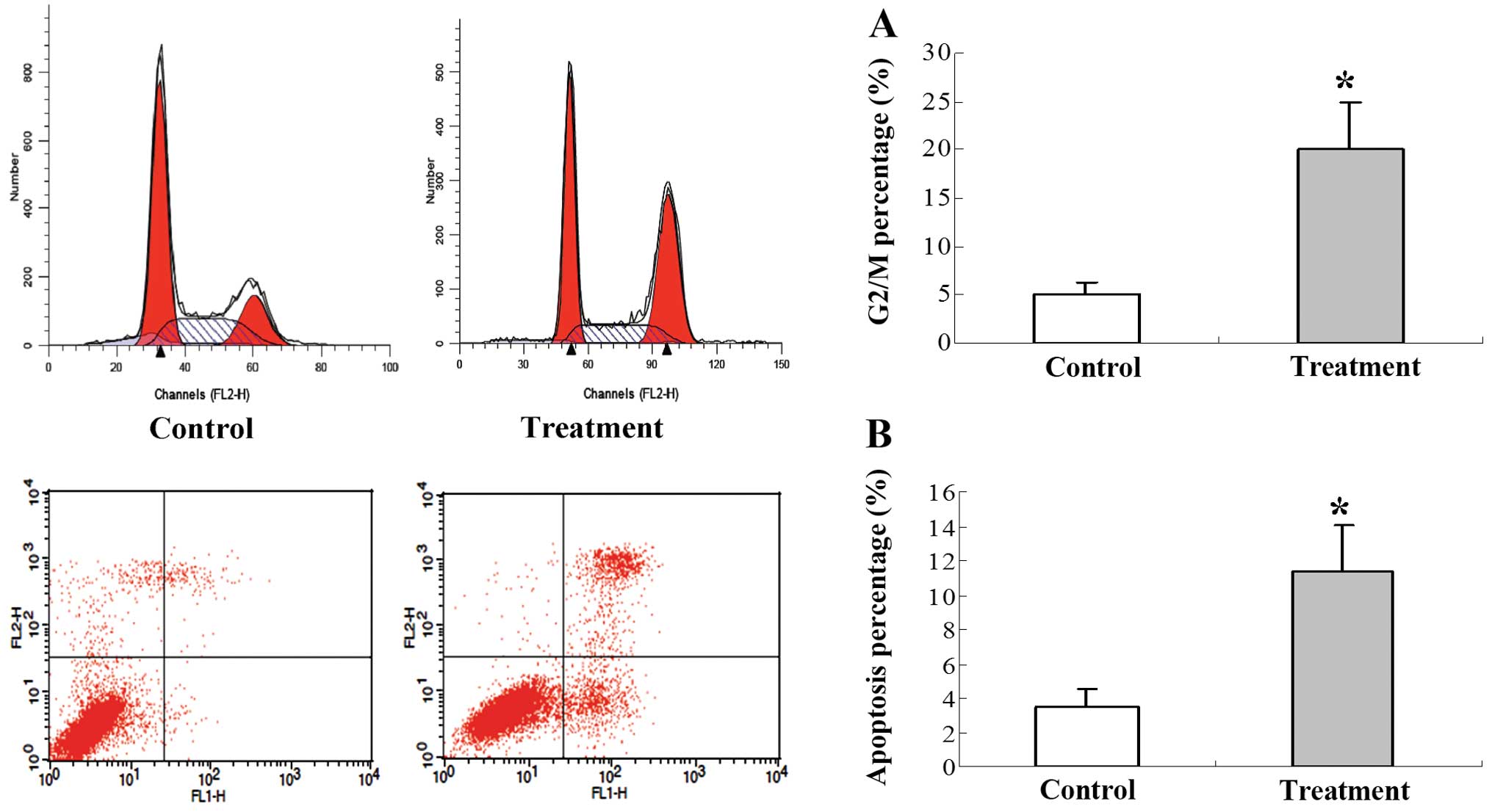
Effect of 125I seed
irradiation on cell apoptosis and cell cycle distribution in the
gastric cancer tumors
To quantitatively compare the cell cycle and
apoptotic index of tumors treated with 125I seed
irradiation, FACS was performed. The cell cycle was blocked in the
G2/M phase in the tumors in the 125I treatment group
when compared to the tumors in the control group (Fig. 3A). The percentage of apoptotic cells
was significantly increased in the 125I treatment group
when compared to the percentage of apoptotic cells in the control
group (Fig. 3B).
Effect of 125I on VEGF and
NF-κB immunofluorescence staining
Expression of VEGF and NF-κB was confirmed by the
presence of fluorescence-stained cytoplasm in the cells. Strong
immunoreactivity to VEGF and NF-κB was found in the SGC-7901 tumor
xenografts of the control group (Fig.
4A-a and -b). Weaker fluorescence intensity expression was
observed in the SGC-7901 tumors of the group treated with
125I (Fig. 4A-c and -d).
The fluorescence intensity levels of VEGF and NF-κB in the tumor
cells were significantly lower in the radiation-treated group than
levels in the control group (Fig.
4B; P<0.01).
Effect of 125I on the
expression of VEGF and NF-κB mRNA and protein in the tumor
tissues
To further analyze the effect of 125I
radiation on VEGF and NF-κB expression in tumors, we assessed VEGF
and NF-κB expression in SGC-7901 cells in vitro using
western blotting and fluorescent quantitative RT-PCR. Western blot
analysis revealed that the expression of VEGF and NF-κB protein was
decreased in the treatment group (Fig.
5B). Furthermore, VEGF and NF-κB mRNA expression was in
agreement with the protein expression results as mRNA data also
revealed a significant difference between the control and the
treatment group (Fig. 5A).
Discussion
Several recent studies suggest that apoptosis and
cell cycle arrest may have important roles in the therapeutic
effects of continuous low-energy 125I irradiation
(14,15,18).
In addition, the VEGF and NF-κB signaling pathway may be involved
in the gastric cancer oncogenic signaling pathway (19). However, comprehensive knowledge on
this topic, particularly at the molecular level, is still lacking.
In the present study, analysis of cell apoptosis and the cell
cycle, as well as VEGF and NF-κB expression analysis of human
gastric cancer xenografts exposed to 125I seed
irradiation were performed to gain insight into the mechanisms
underlying the biological effects of 125I
irradiation.
SGC-7901 gastric cancer cells were implanted into
nude mice to create a xenograft animal model. The growth curves of
tumors indicated that irradiation induced significant tumor growth
inhibition. By observing H&E-stained slides, a large number of
apoptotic cells was observed in the gastric cancer tumors receiving
125I seed implantation. These results showed that
125I suppressed the growth of the gastric cancer
xenografts in the nude mice, while inhibiting cell proliferation
and inducing apoptosis. Our results further demonstrated that
continuous low-dose-rate irradiation by 125I seeds
reduced cell viability and induced cell apoptosis, and led to the
accumulation of cells in the G2/M phase. These data suggest that
the cell cycle was blocked in the G2 phase after radiation. Cells
in the G2 phase are more sensitive to radiation (20), and therefore, more tumor cells were
eliminated.
Our VEGF and NF-κB analyses in the tumor cells
indicated decreased fluorescence intensity implying reduced mRNA
and protein levels in the treatment cells as compared with the
control cells. A previous study demonstrated a correlative
expression relationship between VEGF and NF-κB in 80 ACC clinical
samples (21). Evidence also
indicates that overexpression of NF-κB is the key component of the
angiogenic cascade, which contributes to VEGF-induced angiogenesis
through upregulation of VEGF mRNA expression in many tumor types
(22,23).
NF-κB is a family of homodimeric or heterodimeric
transcription factors formed by proteins of the Rel family.
Recently, it has been suggested that NF-κB plays an important role
in carcinogenesis (24). There is
evidence that NF-κB is constitutively activated in gastric cancer
tissues, with higher levels in gastric carcinoma cells in
comparison to normal adjacent epithelial cells (25). In gastric cancer, abnormal NF-κB
activation has been shown to lead to enhanced proliferation,
evasion of apoptosis, genomic instability, increased rate of
glycolysis and drug resistance (26–28).
VEGF, a dimeric 42-kDa protein, is a multifunctional
cytokine that plays a key role in both physiological and
pathological angiogenesis. It was identified in tumor cells of
gastric cancer more than 10 years ago (29). Several groups of investigators have
reported a correlation between VEGF expression and microvessel
density in human gastric cancer (30). Our data together with previous
research evidence suggest that NF-κB and VEGF play an important
role in the therapeutic effects of continuous low-energy
125I irradiation and are involved in the mechanism of
the 125I seed implantation therapy process.
In conclusion, the present study demonstrated that
human gastric tumor cells following 125I brachytherapy
showed induced cell apoptosis and cell cycle arrest in the G2/M
phase in a xenograft model. Furthermore, suppression of NF-κB
activity through significantly decreased VEGF expression
facilitates the 125I clinical effect on gastric tumors.
The results indicate that brachytherapy is a useful strategy in
gastric tumor therapy. Further study by us will investigate the
function of NF-κB and VEGF signaling in angiogenesis and metastasis
of gastric tumor cells.
Acknowledgements
The present study was supported by a grant from
Yunnan Province (no. 2013FZ189).
References
|
1
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer-slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, et al: Management of gastric cancer in Asia:
resource-stratified guidelines. Lancet Oncol. 14:e535–e547.
2013.PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, et al: Risk of recurrence of
gastrointestinal stromal tumour after surgery: an analysis of
pooled population-based cohorts. Lancet Oncol. 13:265–274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marrelli D, De Stefano A, de Manzoni G,
Morgagni P, Di Leo A and Roviello F: Prediction of recurrence after
radical surgery for gastric cancer: a scoring system obtained from
a prospective multicenter study. Ann Surg. 241:247–255. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrick GS, Wallner KE and Butler WM:
Permanent interstitial brachytherapy for the management of
carcinoma of the prostate gland. J Urol. 169:1643–1652. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roeloffzen EM, Monninkhof EM, Battermann
JJ, et al: Acute urinary retention after I-125 prostate
brachytherapy in relation to dose in different regions of the
prostate. Int J Radiat Oncol Biol Phys. 80:76–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee W, Daly BD, DiPetrillo TA, et al:
Limited resection for non-small cell lung cancer: observed local
control with implantation of I-125 brachytherapy seeds. Ann Thorac
Surg. 75:237–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang HQ, Wang JJ, Liao AY, Wang JD and
Zhao Y: The biological effect of 125I seed continuous
low dose rate irradiation in CL187 cells. J Exp Clin Cancer Res.
28:122009.
|
|
10
|
Ma JX, Jin ZD, Si PR, Liu Y, Lu Z, Wu HY,
Pan X, et al: Continuous and low-energy 125I seed
irradiation changes DNA methyltransferases expression patterns and
inhibits pancreatic cancer tumor growth. J Exp Clin Cancer Res.
30:352011.PubMed/NCBI
|
|
11
|
Wang JJ, Yuan HS, Li JN, Jiang WJ, Jiang
YL and Tian SQ: Interstitial permanent implantation of
125I seeds as salvage therapy for re-recurrent rectal
carcinoma. Int J Colorectal Dis. 24:391–399. 2009.PubMed/NCBI
|
|
12
|
Shi L, Wu C, Wu J, Zhou W, Ji M, Zhang H,
et al: Computed tomography-guided permanent brachytherapy for
localregional recurrent gastric cancer. Radiat Oncol. 7:1142012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Sui A, Jia Y, Xu B, Wei L, Chen J
and Shen W: Treatment of unresectable advanced gastric cancer using
iodine-125 brachytherapy. Chin J Clin Oncol. 3:212–215. 2006.
View Article : Google Scholar
|
|
14
|
Takabayashi K, Kashiwagi K, Kawata T, Sato
T, Matsuoka K, Hisamatsu T, Takaishi H, et al: Continuous low-dose
irradiation by I-125 seeds induces apoptosis of gastric cancer
cells regardless of histological origin. Cancer Biol Ther. 5:81–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma ZH, Yang Y, Zou L and Luo KY: 125I seed
irradiation induces up-regulation of the genes associated with
apoptosis and cell cycle arrest and inhibits growth of gastric
cancer xenografts. J Exp Clin Cancer Res. 31:612012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li ZM, Pu YW and Zhu BS: Blockade of NF-κB
nuclear translocation results in the inhibition of the invasiveness
of human gastric cancer cells. Oncol Lett. 6:432–436. 2013.
|
|
17
|
Cao W, Fan R, Yang W and Wu Y: VEGF-C
expression is associated with the poor survival in gastric cancer
tissue. Tumour Biol. Dec 5–2013.(Epub ahead of print).
|
|
18
|
Yang Z, Jin C, Chen T, Sun H, Yang D,
Huang Y, Zhang J, et al: Changes in cell cycle, apoptosis and
necrosis following the establishment of a 125I
brachytherapy model in the spinal cord in Banna mini-pigs. Oncol
Lett. 3:315–320. 2012.PubMed/NCBI
|
|
19
|
Santos-Silva F: Oncogenic signaling in
gastric carcinoma. Gastric Carcinoma - Molecular Aspects and
Current Advances. Lotfy M: InTech; Rijeka, Croatia: 2011,
View Article : Google Scholar : Available from:
http://www.intechopen.com/books/gastric-carcinomamolecularaspectsandcurrentdvances/oncogenicsignalingingastriccarcinoma.
|
|
20
|
Yan Y, Greer PM, Cao PT, Kolb RH and Cowan
KH: RAC1 GTPase plays an important role in γ-irradiation induced
G2/M checkpoint activation. Breast Cancer Res.
14:R602012.
|
|
21
|
Zhang J, Peng B and Chen X: Expressions of
nuclear factor κB, inducible nitric oxide synthase and vascular
endothelial growth factor in adenoid cystic carcinoma of salivary
glands: correlations with the angiogenesis and clinical outcome.
Clin Cancer Res. 11:7334–7343. 2005.
|
|
22
|
Zhang J and Peng B: In vitro angiogenesis
and expression of nuclear factor κB and VEGF in high and low
metastasis cell lines of salivary gland adenoid cystic carcinoma.
BMC Cancer. 7:952007.
|
|
23
|
Fujioka S, Sclabas GM, Schmidt C,
Frederick WA, Dong QG, Abbruzzese JL, et al: Function of nuclear
factor κB in pancreatic cancer metastasis. Clin Cancer Res.
9:346–354. 2003.
|
|
24
|
Du ZX, Zhang HY, Gao DX, Wang HQ, Li YJ
and Liu GL: Significance of VEGF and NF-κB expression in thyroid
carcinoma. Chin J Clin Oncol. 3:166–171. 2006.
|
|
25
|
Sasaki N, Morisaki T, Hashizume K, Yao T,
Tsuneyoshi M, Noshiro H, et al: Nuclear factor κB p65 (RelA)
transcription factor is constitutively activated in human gastric
carcinoma tissue. Clin Cancer Res. 7:4136–4142. 2001.
|
|
26
|
Tsuboi K, Matsuo Y, Shamoto T, Shibata T,
Koide S, Morimoto M, et al: Zerumbone inhibits tumor angiogenesis
via NF-κB in gastric cancer. Oncol Rep. 31:57–64. 2014.PubMed/NCBI
|
|
27
|
Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha
TK, Byun DS, et al: NF-κB activates transcription of the
RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric
tumorigenesis. Gastroenterology. 135:e2031–e2033. 2008.
|
|
28
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, et al: Warburg effect revisited: an epigenetic link
between glycolysis and gastric carcinogenesis. Oncogene.
29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raica M, Mogoantă L, Cîmpean AM, Alexa A,
Ioanovici S, Mărgăritescu C, et al: Immunohistochemical expression
of vascular endothelial growth factor (VEGF) in intestinal type
gastric carcinoma. Rom J Morphol Embryol. 49:37–42. 2008.PubMed/NCBI
|
|
30
|
Kitadai Y: Angiogenesis and
lymphangiogenesis of gastric cancer. J Oncol. 2010:4687252010.
View Article : Google Scholar
|