Introduction
Cancer of the oral cavity and oropharynx is among
the top 10 leading cancers worldwide (1). The most common malignant type is
squamous cell carcinoma (SCC) of the tongue. Even more serious is
that most tongue SCC cases are in advanced stages when diagnosed
and effective treatment is quite rare. Furthermore, little
improvement has been achieved in the past two decades for the
5-year survival rate of tongue SCC patients (2) even though medical and surgical
treatment have made huge advances in cancer therapy. A causal
relationship has been established between tongue SCC and several
risk factors such as drinking and smoking, yet the molecular
pathways underlying tumorigenesis and progression remain elusive.
Exploring the underlying mechanisms will provide valuable
information for the prevention, diagnosis and treatment of tongue
SCC.
Zinc finger protein, X-linked (ZFX), encoded by the
gene ZFX located on the X chromosome, is a member of the ZFY
protein family and has been identified as a transcription factor.
ZFX is composed of three functional domains including a DNA binding
domain containing 13 C2H2-type zinc fingers,
a nuclear localization sequence and an acidic transcriptional
activation domain. Functional analysis has shown that ZFX is
essential for the survival and self-renewal of embryonic and
hematopoietic stem cells (3).
Moreover, further research has unraveled the involvement of ZFX
protein in the propagation of leukemia cells in acute myeloid
leukemia (AML) and acute T-lymphoblastic leukemia (T-ALL) (4). In addition, ZFX also plays important
roles in the initiation and progression of multiple types of
cancer. However, the relationship between ZFX and tongue SCC is
still unclear.
Here, we tried to explore the role of ZFX in tongue
SCC development and progression. We first analyzed the expression
pattern of ZFX protein in tissue samples derived from tongue SCC
patients, and confirmed that ZFX expression is upregulated in
tongue SCC patients. Then an efficient siRNA targeting the human
ZFX gene was designed, and a lentiviral system was used to
manipulate ZFX gene expression in two human tongue SCC cell lines,
Tca-8113 and CAL-27, which were derived from a male and female
patient, respectively. The impact of ZFX knockdown on cell
proliferation, apoptosis and cell cycle distribution was
investigated extensively, confirming the indispensable role of ZFX
for tongue SCC development and progression. Thus, our results
provide evidence that ZFX is a promising target for tongue SCC
treatment.
Materials and methods
Preparation of clinical tumor samples and
immunohistochemical (IHC) staining
In the present study we obtained informed consent
from all patients. This study was approved by the ethics committee
of our institution. Fifty clinical samples from patients with SCC
of the tongue were collected, and patient information is described
in Table I. Ten normal tongue
tissues were used as negative control. ZFX IHC staining was
performed in the tumor samples as described previously (5). Briefly, ZFX expression was detected
with the NBP1-80582 ZFX antibody purchased from Novus Company.
Staining was conducted with an EliVision™ Plus kit (Fuzhou Maixin
Biotechnology Co., Ltd.). Then, ZFX expression by IHC staining was
determined independently by two experienced pathologists blinded to
all clinical data, and ZFX signals were scored as follows: −
(<5% positive staining), + (6–100% positive staining).
 | Table IAssociation between ZFX expression and
pathological parameters of the tongue SCC cases (cases, %) by IHC
staining. |
Table I
Association between ZFX expression and
pathological parameters of the tongue SCC cases (cases, %) by IHC
staining.
| | ZFX | | |
|---|
| |
| | |
|---|
| Parameters | N | + | − | (%) | χ2 | P-value |
|---|
| Gender | | | | | 5.748 | 0.017 |
| Male | 28 | 27 | 1 | 96.42 | | |
| Female | 22 | 16 | 6 | 72.72 | | |
| Age, years | | | | | 0.166 | 0.684 |
| ≥60 | 18 | 15 | 3 | 97.05 | | |
| <60 | 32 | 28 | 4 | 62.5 | | |
| TNM stage | | | | | 4.193 | 0.041 |
| I | 33 | 26 | 7 | 81.81 | | |
| II, III, IV | 17 | 17 | 0 | 100 | | |
| Pathological
grades | | | | | 4.989 | 0.026 |
| I | 31 | 24 | 7 | 77.41 | | |
| II, III | 19 | 19 | 0 | 100 | | |
Lentivirus-mediated siRNA targeting the
ZFX gene
Strategy with a lentiviral system expressing small
interfering RNA (siRNA) targeting the ZFX gene was employed to
manipulate ZFX expression as previously described (6). Briefly, we first designed efficient
siRNA (siRNA sequence, GAGCCTGAGAATGATCATGGA) specifically
targeting the human ZFX gene, while the sequence of the siRNA used
as the negative control was: TTCTCCGAACGTGTCACGT. Then, stem-loop
DNA oligonucleotides were synthesized, annealed and inserted into
the lentiviral vector pGCSIL-GFP. The vector plasmids and two
helper plasmids were then co-transfected into 293T cells to
generate the lentivirus expressing scrambled siRNA or siRNA
targeting the human ZFX gene. Two human tongue SCC cell lines,
Tca-8113 and CAL-27, were infected with the lentivirus expressing
scrambled siRNA or siRNA targeting the human ZFX gene to determine
the knockdown efficiency at the protein and RNA levels using
western blotting and real-time PCR.
Cell cycle analysis by
fluorescence-activated cell sorting technology
The method used to perform cell cycle analysis was
previously described with slight modifications (7). Briefly, two human tongue SCC cell
lines, Tca-8113 and CAL-27, were treated with the lentivirus
expressing siRNA targeting the human ZFX gene or the scrambled
siRNA and incubated for 4 days. Cells were then resuspended and
seeded in 6-cm dishes for further incubation. After ~80% coverage
was achieved, we harvested the cells and fixed them with ice-cold
70% alcohol for at least 1 h. Staining solution was prepared using
40X PI stock (2 mg/ml), 100X RNase stock (10 mg/ml) and 1X
phosphate-buffered saline (PBS) buffer at a dilution of
25:10:1,000. Cells were then stained with the staining buffer after
washing with PBS solutions. Then, FACSCalibur (Becton-Dickinson,
USA) was used to perform cell cycle analysis. At least
1×106 cells/sample were used for cell cycle analysis,
and three independent experiments were carried out.
Cell apoptosis analysis by FACS
Annexin V-APC staining was used to perform cell
apoptosis analysis. Briefly, the lentivirus expressing ZFX siRNA or
scrambled siRNA was added to the Tca-8113 and CAL-27 cells, and the
cells were incubated at 37°C for 4 days. Cells were then collected
and washed with PBS buffer. Staining buffer was used to resuspend
the cells at a final density of 1×106 –
1×107/ml. Then 100 μl of the cell suspensions
were mixed with 5 μl Annexin V-APC and incubated at room
temperature in the dark for 10–15 min. FACSCalibur
(Becton-Dickinson, USA) was used to analyze the cell apoptosis
profiles.
Colony formation assay
A colony formation assay was performed as previously
described (8). Tca-8113 and CAL-27
cells were treated with the lentivirus expressing ZFX siRNA or
scrambled siRNA and then cultured for 48 h. Cells were collected in
the logarithmic phase. Cells were counted with a haemocytometer,
and 800 cells/well were seeded in triplicate into 6-well plates.
Then cells were cultured in a 5% CO2 incubator at 37°C
for another 14 days. After fixation with paraformaldehyde for 30–60
min, the cells were stained with Giemsa for 20 min. Images of the
cell plates were captured using micropublisher 3.3 RTV (Olympus)
after washing with distilled water. Cell colonies under each
condition were counted and analyzed.
Cell proliferation analysis by MTT
assay
Cell proliferation was analyzed using the MTT assay.
The lentivirus expressing ZFX siRNA or scrambled siRNA was added to
the Tca-8113 and CAL-27 cells, and the cells were incubated for 48
h. The cells were then trypsinized in the logarithmic phase and
resus-pended thoroughly with culture medium. After counting with a
haemocytometer, 2,000 cells/well were seeded in 96-well plates in
quintuplicate and cultured in a 5% CO2 incubator at
37°C. Cell proliferation was analyzed each day for 5 days using the
MTT assay. Briefly, 20 μl of MTT solution (5 mg/ml) was
added into each well and incubation was continued for another 4 h.
Then culture medium was discarded and 150 μl of DMSO was
added to dissolve the formazan. The plate was under constant
shaking for 5–10 min and then the absorbance at 490/570 nm was
measured with a microplate reader.
RNA isolation and real-time quantitative
PCR
Tca-8113 and CAL-27 cells were infected with the
lentivirus expressing ZFX siRNA or scrambled siRNA and cultured for
another 2 days. Then total RNA of these cultured tumor cells was
isolated using TRIzol (Invitrogen) according to the manufacturer’s
instructions. Reverse transcription was performed using 2 μg RNA,
M-MLV reverse transcriptase (Promega) and oligo(dT) primers
(Sangon, Shanghai) to produce cDNAs. To quantify ZFX expression in
the Tca-8113 and CAL-27 cells infected with the lentivirus
expressing siRNA targeting ZFX, real-time quantitative PCR was
performed using a real-time PCR machine TP800 (Takara), and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal reference. Primers used in real-time PCR were as follows:
GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and GAPDH reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; ZFX forward, 5′-GGCAGTCCACAGCAAGAAC-3′
and ZFX reverse, 5′-TTGGTATCCGAGAAAGTCAGAAG-3′. ZFX expression data
were normalized to GAPDH, and the comparative Ct method was chosen
to analyze the expression changes of ZFX.
Western blotting
Western blotting was chosen to examine ZFX protein
levels to determine the knockdown efficiency of the lentivirus
expressing ZFX siRNA in the Tca-8113 and CAL-27 cells. Briefly, the
lentivirus expressing ZFX siRNA or scrambled siRNA was added to the
Tca-8113 or CAL-27 cells, and then cells were cultured for another
96 h. Cells were harvested, lysed with modified RIPA buffer, boiled
in 1X SDS loading buffer and resolved by 10% SDS-PAGE. The gel was
transferred to PVDF membranes (Amersham), and the membranes were
blocked with 5% milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) buffer for 1 h. They were then incubated overnight
at 4°C with the primary antibodies, washed three times in TBST, and
the signals were detected by HRP reaction using the SuperSignal
Chemiluminescent Substrate (Pierce).
Statistical analysis
Data analysis was performed with GraphPad Prism 6
software, and experiments in the present study were repeated at
least three times. Student’s two-tailed t-test was used to analyze
the significant differences between groups, and P<0.05 was
considered to indicate a statistically significant result. Data in
Table I were analyzed with SPSS
14.0 software and the method used was Pearson’s Chi-square
test.
Results
Aberrant high expression of ZFX protein
in tissue samples of tongue SCC patients
Aberrant expression of ZFX protein is prevalent in
multiple types of cancer, and here we evaluated its expression in
clinical specimens from tongue SCC patients using IHC staining. We
found that in normal tongue tissues, ZFX protein was mainly
expressed in the nucleus of the epithelium and its expression in
mesenchyma was quite weak (Fig. 1A and
B) while in the tumor samples from tongue SCC patients, ZFX
expression increased with advanced clinical stage (Fig. 1C–H). Furthermore, ZFX protein could
be detected in both the nucleus and cytoplasm in samples of tongue
SCC patients, in contrast to the nuclear localization in normal
tissues. In addition, we further analyzed the relationship between
ZFX expression and diverse clinicopathological parameters as shown
in Table I. Indeed, a positive ZFX
signal was detected in all (17/17) stage II–IV tongue SCC samples
while a positive ZFX signal was detected in 81.81% (26/33) of the
stage I samples (χ2=4.193, P=0.041). Furthermore, when
analyzed using the tumor grading system, a positive ZFX signal was
detected in all (19/19) grade II–III tongue SCC samples and in
77.41% (24/31) of grade I samples (χ2=4.989, P=0.026),
suggesting that ZFX protein expression was positively correlated
with tumor grade and stage (TNM). We further noted that ZFX
expression in tongue SCC patients was strongly associated with
gender, as a positive ZFX signal was observed in 96.42% (27/28) of
the tongue SCC samples from male patients while only in 72.72%
(16/22) of samples from female patients (χ2=5.748,
P=0.017). However, no significant relationship was observed between
ZFX expression and patient age (χ2=0.166, P=0.684).
Taken together, our results showed that ZFX expression was higher
in tongue SCC tumors, and increased ZFX expression was correlated
with high tumor grade and stage, suggesting that ZFX may be
essential for the development and progression of tongue SCC.
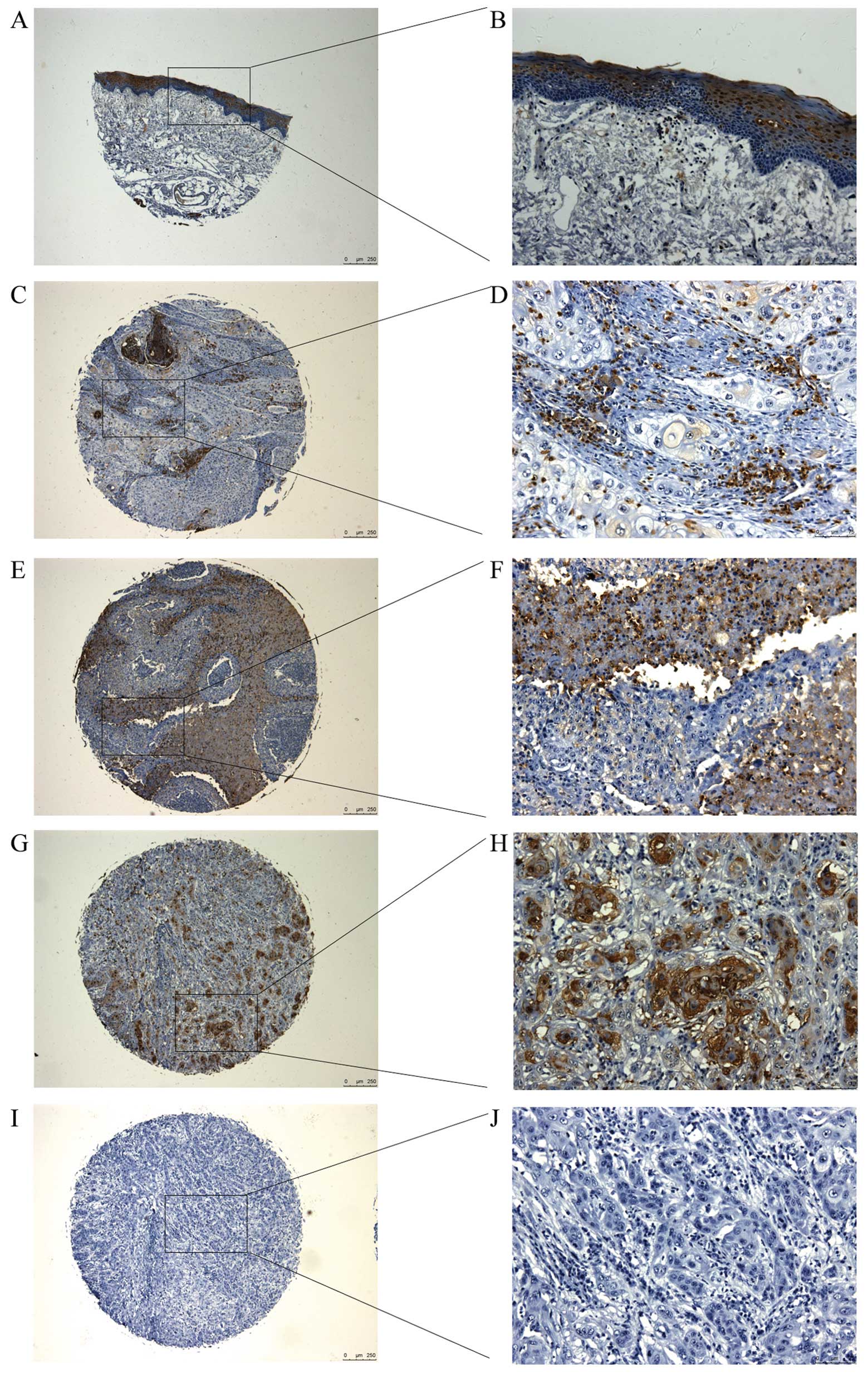 | Figure 1Expression of ZFX in normal tongue
tissues and tissue samples from tongue squamous cell carcinoma
(SCC) patients. ZFX expression in (A and B) normal tongue tissues,
and in (C and D) stage I, (E and F) stage II and (G and H) stage
III tongue SCC tissues. (I and J) Stage III tongue SCC tissues
stained with IgG were used as the negative control. (A, C, E, G and
I) magnification, ×50; (B, D, F, H and J) magnification, ×200. ZFX,
zinc finger protein, X-linked. |
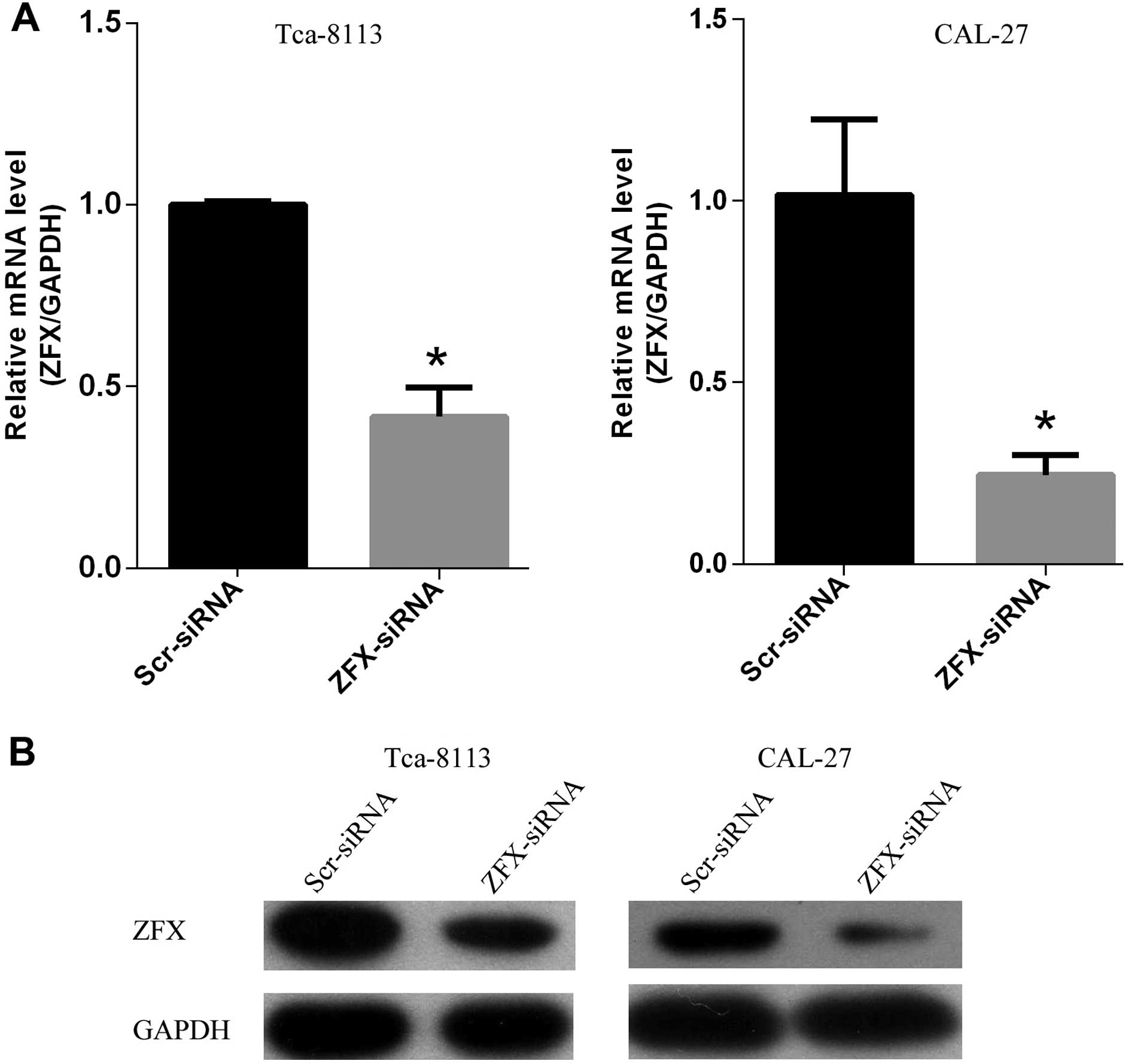
Efficient knockdown of ZFX in human
tongue SCC cell lines
To better characterize the functions of ZFX in human
tongue SCC cell lines Tca-8113 and CAL-27, we employed
lentivirus-based siRNA strategy to knock down the expression of
human ZFX in both tumor cell lines. Tca-8113 and CAL-27 cells were
first infected with the lentivirus expressing scrambled siRNA or
siRNA targeting human ZFX and cultured for another two days. Then
proteins and total RNAs were isolated to determine the knockdown
efficiency. Approximately a 61.4% reduction in ZFX mRNA was
achieved in the Tca-8113 cells and 75.4% knockdown efficiency was
achieved in the CAL-27 cells (Fig.
2A). Furthermore, western blot analysis of ZFX protein levels
confirmed the high inhibitory effect of siRNA strategy on ZFX
expression at the protein level (Fig.
2B). These results indicated that the lentivirus-based siRNA
strategy inhibited the expression of ZFX in both cell lines
efficiently and was chosen for subsequent experiments to manipulate
ZFX expression.
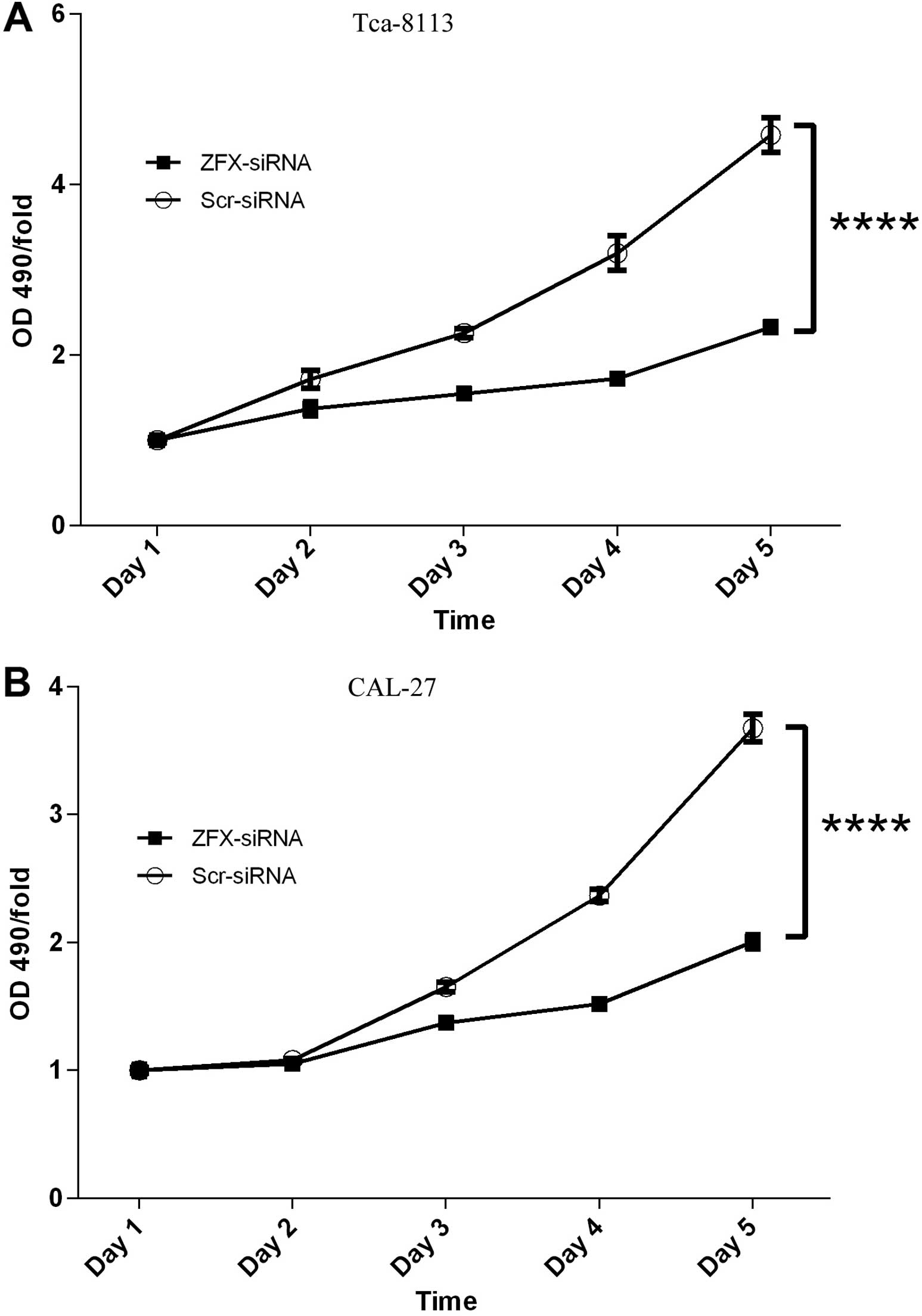
Inhibition of proliferation of human
tongue SCC cell lines by ZFX knockdown
It has been reported that ZFX is essential for the
self-renewal of stem cells, implicating the involvement of ZFX in
the cell proliferation process. It is well known that unlimited
proliferation is a hallmark of cancer, thus here we evaluated the
impact of ZFX knockdown on the proliferation of two human tongue
SCC Tca-8113 and CAL-27 cell lines. Both cell lines were infected
with the lentivirus expressing scrambled siRNA or siRNA targeting
human ZFX efficiently. Then the cell proliferation profiles were
analyzed with MTT assay every day for 5 days. In both cell lines
ZFX knockdown barely affected the proliferation in the first 3 days
after transfection. However, cell proliferation impairment became
obvious in both cell lines infected with the lentivirus expressing
the siRNA targeting human ZFX after 4 days of transfection, and the
proliferation fold decreased from 2.4 in cells expressing scrambled
siRNA to 1.5 in ZFX-knockdown cells. Furthermore, the proliferation
inhibitory effect was even more obvious after 5 days of
transfection, indicating that proliferation suppression was
positively correlated with the duration of cell culture (Fig. 3A and B).
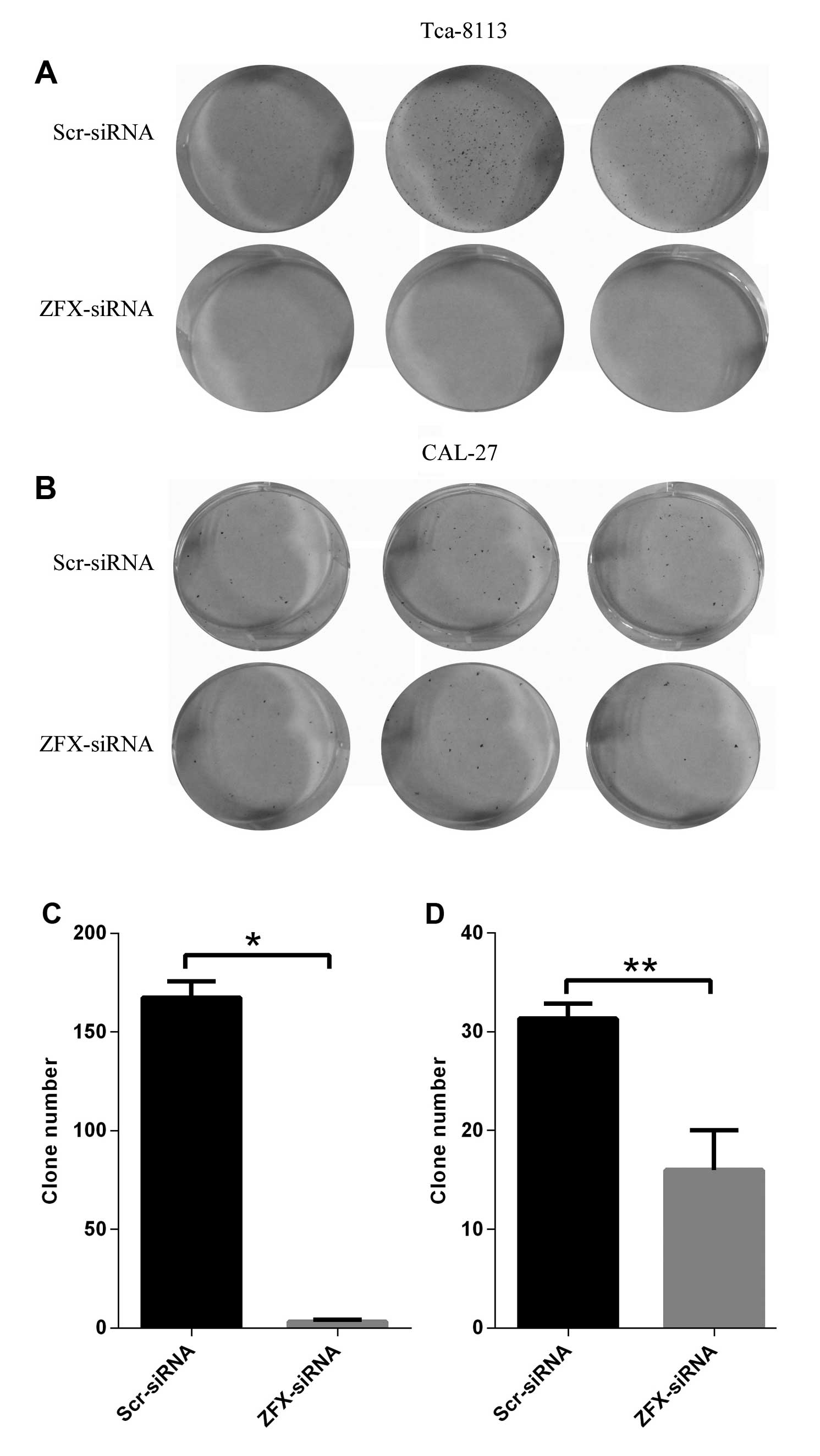
Suppression of colony formation by ZFX
depletion in human tongue SCC cell lines
Unlimited cell division is critical for
tumorigenesis, and the colony formation assay reflects the ability
of a single cell to form a colony which is composed of at least 50
cells. Thus, we carried out a colony formation assay in both CAL-27
and Tca-8113 cells to evaluate the impact of ZFX depletion on the
clonogenic capacity of the human tongue SCC cell lines. Our results
showed that ZFX depletion with the lentivirus expressing human ZFX
siRNA significantly impaired the colony formation ability of both
cell lines. An average of 31 clones formed in the CAL-27 cells
expressing scrambled siRNA while only 16 clones were detected in
the ZFX-depleted CAL-27 cells (Fig. 4B
and D). Consistent with these results, ZFX knockdown in the
Tca-8113 cells inhibited the colony formation even more
significantly, with 167 clones formed in cells expressing scrambled
siRNA and only 3 clones in the ZFX-depleted cells (Fig. 4A and C). Thus, the results from both
cell lines provided evidence that ZFX is critical for the colony
formation in human tongue squamous cell carcinoma cell lines,
implicating the involvement of ZFX in the progression of human
tongue SCC.
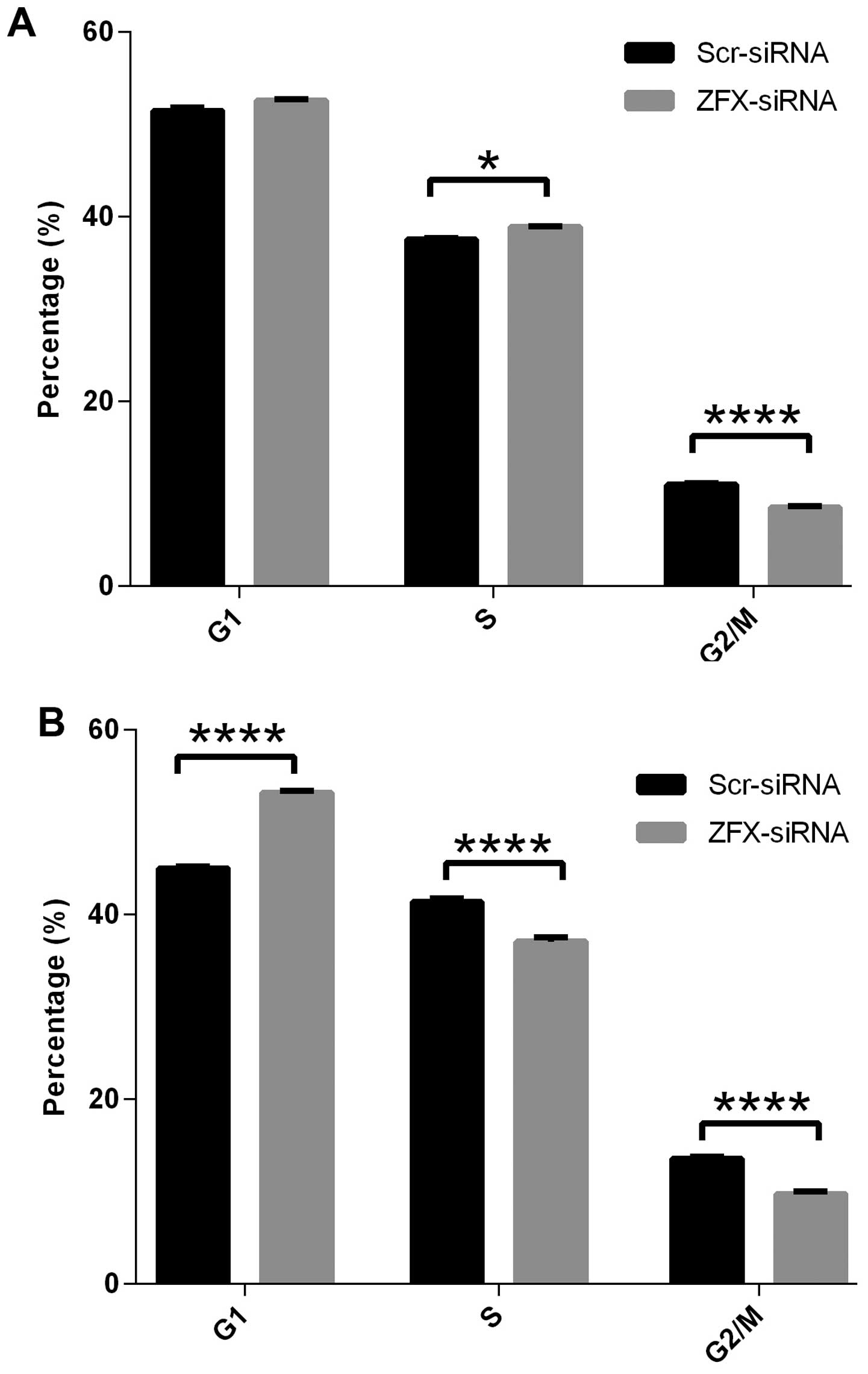
Cell cycle arrest by ZFX knockdown
It has been demonstrated that ZFX is essential for
the self-renewal of stem cells. In the present study we showed that
ZFX knockdown suppressed the proliferation of human tongue SCC
Tca-8113 and CAL-27 cell lines. To explore the underlying
mechanisms of cell proliferation impairment by ZFX knockdown, we
examined the cell cycle distribution of Tca-8113 and CAL-27 cells
infected with the lentivirus expressing specific siRNAs. Analysis
with fluorescence-activated cell sorting (FACS) showed that ZFX
knockdown led to cell cycle arrest in both cell lines. As shown in
Fig. 5, ZFX knockdown in CAL-27
cells led to a significant G1 phase arrest with a simultaneous
reduction of both S and G2/M phase cells. The cell percentage at
the G1 phase increased from 45% in cells infected with the
scrambled siRNA to 53% in the ZFX-knockdown cells while the
percentage of cells at S and G2/M phases decreased from 41 to 37%,
and from 13.6 to 9.7%, respectively (Fig. 5B). In the Tca-8113 cells, a similar
trend in cell cycle arrest was observed, yet cell cycle arrest at
the G1 phase was not obvious while the cell percentage at G2/M
phase decreased significantly, from 11% in cells infected with
scrambled siRNA to 8.5% in the ZFX-knockdown cells (Fig. 5A). Collectively, our results
indicate that ZFX promotes cell proliferation via cell cycle
regulation and ZFX knockdown impairs the cell cycle process, thus
leading to cell proliferation suppression in human tongue SCC cell
lines.
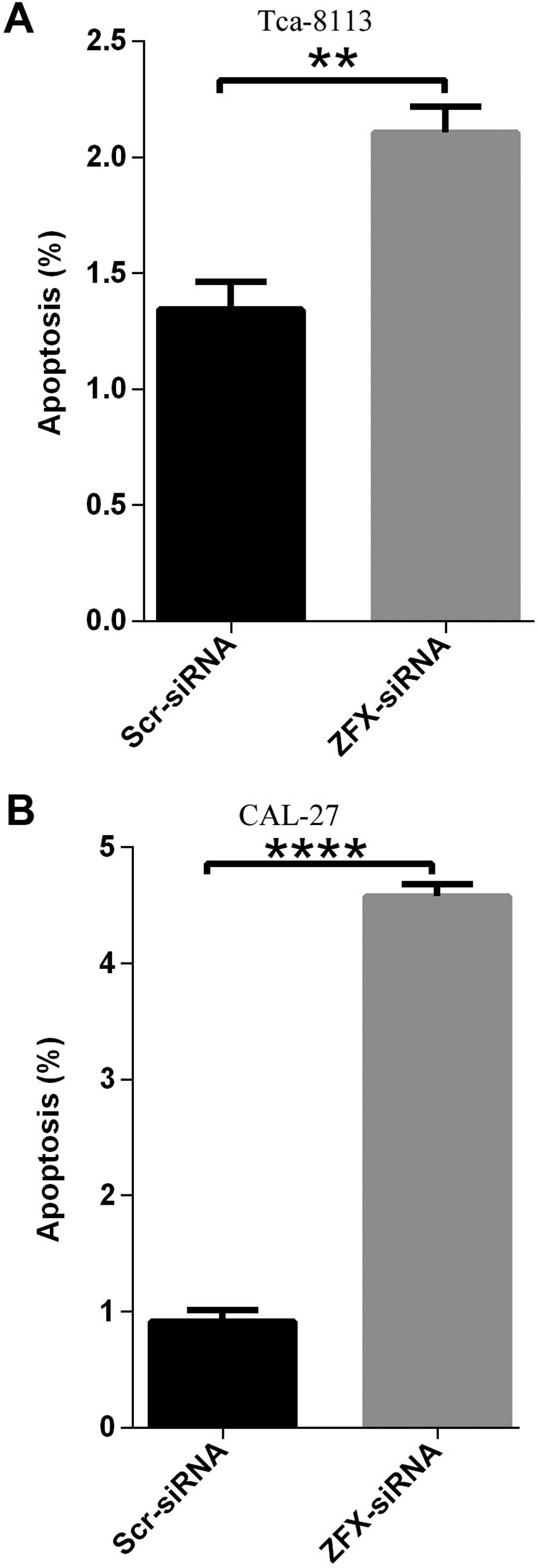
Promotion of cell apoptosis in human
tongue SCC cell lines by ZFX knockdown
Unlimited cell proliferation and enhanced colony
formation ability are critical for tumor initiation and
development. Furthermore, evasion of apoptosis is another hallmark
of cancer which is indispensable for tumorigenesis (9,10).
Thus, here, human tongue SCC Tca-8113 and CAL-27 cell lines were
infected with the lentivirus expressing scrambled siRNA or human
ZFX siRNA, and cell apoptosis was analyzed using Annexin V-APC
assay and FACS technology. In the CAL-27 cells, apoptosis was
induced in ~8.8% cells infected with the lentivirus expressing
siRNA targeting the human ZFX while the percentage of cell
apoptosis in the negative control samples was only ~1.03% (Fig. 6B). In accordance, ZFX knockdown
enhanced the apoptosis of Tca-8113 cells and the percentage of
apoptosis increased from 1.54% in the negative control cells to
4.13% in the ZFX-knockdown cells (Fig.
6A).
Discussion
Cancers of the oral cavity and oropharynx are quite
prevalent and are among the leading types of cancers worldwide with
tongue squamous cell carcinoma (SCC) accounting for nearly 90% of
cancers of the oral cavity and oropharynx. Certain lifestyle habits
such as drinking and smoking have been identified as strong risk
factors for cancers of the oral cavity and oropharynx (11). However, genetic factors underlying
the development and progression of tongue SCC remain elusive.
ZFX overexpression has been observed in variant
types of cancer including human laryngeal SCC (LSCC) (12), prostate (13) and gastric cancer (14–16),
human gliomas (17,18), non-small cell lung cancer (NSCLC)
(19) and breast cancer (20). Consistent with these studies, here
we showed that ZFX, a protein involved in survival and self-renewal
of embryonic stem cells and implicated in the development of
multiple types of cancer, was overexpressed in the tongue SCC
samples. We further revealed that ZFX expression was strongly
associated with several clinicopathological factors in the tongue
SCC specimens. Consistent with a previous study (20), we confirmed that ZFX expression was
positively correlated with tumor grade and TNM classification of
the tongue SCC, suggesting that ZFX overexpression was essential
for the progression of tongue SCC. In addition, we revealed that
ZFX overexpression was more frequent in male patients than that in
female patients, suggesting a strong association between ZFX
upregulation and gender. As ZFX is a transcriptional factor encoded
by an X-linked gene, it is possible that ZFX regulation is more
sensitive to environmental fluctuations in males than in females,
although in the future such a speculation requires further
investigation. IHC staining analysis further showed that in tongue
SCC samples, ZFX expression could be detected in both the nucleus
and cytoplasm, while in normal conditions, ZFX was mainly located
in the nucleus, suggesting that ZFX in cytoplasm may be critical
for the regulation of signal pathways involved in tumor
development. However, the mechanisms and roles behind ZFX
translocation remain to be elucidated. Taken together, the results
concerning the expression status of ZFX in tongue SCC samples
suggest that ZFX may be an efficient biomarker for tongue SCC
diagnosis and prognosis. Exploring the mechanism for ZFX
upregulation in tongue SCC and other tumors would provide more
insight into the involvement of ZFX in tumor development and
progression.
In addition to the analysis of ZFX expression in
tongue SCC patients, we further investigated the impact of ZFX
manipulation in tumor cell lines and showed that ZFX knockdown in
human tongue SCC Tca-8113 and CAL-27 cell lines led to cell cycle
arrest and reduced proliferation. Furthermore, impaired colony
formation ability and enhanced cell apoptosis were observed in the
Tca-8113 and CAL-27 cells infected with the lentivirus expressing
specific siRNA targeting human ZFX, confirming the critical role of
ZFX in tongue SCC development. These results were consistent with
previous studies which showed that ZFX knockdown impaired cancer
cell growth, proliferation and survival in vitro and in
vivo. Moreover, downstream effectors of ZFX have been
investigated extensively in other studies. The ERK-MAPK pathway is
essential for tumor growth and ZFX is critical for its activation.
It has been shown that ZFX knockdown inhibits the phosphorylation
of ERK1/2 and MEK1/2 in vitro and in vivo in gastric
cancer. Furthermore, ERK and AKT activation have been observed in
gliomas (18), NSCLC (21), LSCC (12) and breast cancers (20) following ZFX knockdown treatment.
Consistent with the results that ZFX knockdown caused cell cycle
arrest in tongue SCC cell lines, previous studies showed that ZFX
knockdown could inhibit the expression of cell cycle factors such
as cyclin D1 and B1 in multiple types of cancer (18,20,21).
Studies also found that ZFX depletion enhanced the expression of
apoptotic factors including bax, caspase 1, 3 and 9 or inhibited
the expression of anti-apoptotic factor such as bcl-2 in diverse
cancers (12–14,18,20,21),
which may account for the ZFX knockdown-induced cell apoptosis.
Thus, it is quite possible that these mechanisms also function in
tongue SCC, and further investigations are urgently needed to
elucidate the mechanisms associated with ZFX-mediated tongue SCC
development.
Here, we mainly focused on the roles of ZFX in
tongue SCC development in two Tca-8113 and CAL-27 cell lines and
explored the effect of ZFX knockdown on cell proliferation,
division, apoptosis and colony formation ability in vitro.
As a transcriptional factor, ZFX regulates the expression of
variant proteins and these targets are essential for the oncogenic
functions of ZFX which have been evaluated in diverse cancer types.
Thus, in the future, exploring the functions of ZFX in vivo
and investigating the underlining mechanisms in tongue SCC will
pave the way for the development of effective therapies to benefit
patients with tongue SCC or other ZFX-mediated tumors.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
2
|
Ganly I, Patel S and Shah J: Early stage
squamous cell cancer of the oral tongue - clinicopathologic
features affecting outcome. Cancer. 118:101–111. 2012. View Article : Google Scholar
|
|
3
|
Galan-Caridad JM, Harel S, Arenzana TL, et
al: Zfx controls the self-renewal of embryonic and hematopoietic
stem cells. Cell. 129:345–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weisberg SP, Smith-Raska MR, Esquilin JM,
et al: ZFX controls propagation and prevents differentiation of
acute T-lymphoblastic and myeloid leukemia. Cell Rep. 6:528–540.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haapa-Paananen S, Kiviluoto S, Waltari M,
et al: HES6 gene is selectively overexpressed in glioma and
represents an important transcriptional regulator of glioma
proliferation. Oncogene. 31:1299–1310. 2012. View Article : Google Scholar
|
|
6
|
Wang L, Zhou GB, Liu P, et al: Dissection
of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki K, Tsuno NH, Sunami E, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaturvedi AK, Anderson WF,
Lortet-Tieulent J, et al: Worldwide trends in incidence rates for
oral cavity and oropharyngeal cancers. J Clin Oncol. 31:4550–4559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang J, Yu Z, Lian M, et al: Knockdown of
zinc finger protein, X-linked (ZFX) inhibits cell proliferation and
induces apoptosis in human laryngeal squamous cell carcinoma. Mol
Cell Biochem. 360:301–307. 2012. View Article : Google Scholar
|
|
13
|
Jiang H, Zhang L, Liu J, et al: Knockdown
of zinc finger protein X-linked inhibits prostate cancer cell
proliferation and induces apoptosis by activating caspase-3 and
caspase-9. Cancer Gene Ther. 19:684–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu S, Lao XY, Sun TT, et al: Knockdown of
ZFX inhibits gastric cancer cell growth in vitro and in vivo via
downregulating the ERK-MAPK pathway. Cancer Lett. 337:293–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akiyoshi S, Fukagawa T, Ueo H, et al:
Clinical significance of miR-144-ZFX axis in disseminated tumour
cells in bone marrow in gastric cancer cases. Br J Cancer.
107:1345–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nikpour P, Emadi-Baygi M, Mohammad-Hashem
F, Maracy MR and Haghjooy-Javanmard S: Differential expression of
ZFX gene in gastric cancer. J Biosci. 37:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Su Z, Huang Y, et al: The Zfx gene
is expressed in human gliomas and is important in the proliferation
and apoptosis of the human malignant glioma cell line U251. J Exp
Clin Cancer Res. 30:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Z, Li K, Xu D, et al: ZFX regulates
glioma cell proliferation and survival in vitro and in vivo. J
Neurooncol. 112:17–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zha WJ, Cao L, Shen Y and Huang M: Roles
of mir-144-ZFX pathway in growth regulation of non-small-cell lung
cancer. PLoS One. 8:e741752013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Lu Y, Zheng Y, et al:
shRNA-mediated silencing of ZFX attenuated the proliferation of
breast cancer cells. Cancer Chemother Pharmacol. 73:569–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li K, Zhu ZC, Liu YJ, et al: ZFX knockdown
inhibits growth and migration of non-small cell lung carcinoma cell
line H1299. Int J Clin Exp Pathol. 6:2460–2467. 2013.PubMed/NCBI
|