Introduction
Lung cancer is one of the leading causes of cancer
mortality in developed countries, and non-small cell lung cancer
(NSCLC) accounts for 80–85% of lung cancer cases (1). The majority of NSCLC patients have
locally advanced or distant metastatic disease at the time of
presentation and thus cannot undergo surgery (2). Platinum-based doublet chemotherapy is
the mainstay of treatment for advanced NSCLC (3). However, it has significant
side-effects and a 5-year survival rate of only 20% (4). Previous findings suggest that the
chemotherapeutic treatments of NSCLC have reached a therapeutic
plateau (5,6). Thus, exploring new effective
integrated treatment methods to improve the tumor response rate and
prolong the survival time of advanced NSCLC patients is
important.
The epidermal growth factor receptor (EGFR) pathway
has been shown to be an important target in NSCLC proliferation.
The emergence of EGFR-tyrosine kinase inhibitors (EGFR-TKIs) offers
new hope to patients with advanced NSCLC patients. In 2004, it was
reported that tumors with EGFR-activating mutations had
histological characteristics of adenocarcinoma, and were highly
sensitive to EGFR-TKIs with a better prognosis as compared to the
EGFR wild-type (7–9). The efficiency of EGFR-TKIs reached
70–80% and the median survival time was 20–30 months (10,11).
EGFR-TKIs are superior to cisplatin plus paclitaxel as an initial
treatment for patients with advanced NSCLC harboring an EGFR
mutation (12). Two randomized
studies (WJTOG3405 and NEJ) showed that patients with an EGFR
mutation have a high tumor response rate and progression-free
survival (PFS) than those with EGFR-TKIs (13,14).
EGFR-TKIs provide a new option to patients since they can prolong
survival and significantly improve the quality of life (15).
The combination of EGFR-TKIs with chemotherapy is
not more beneficial than chemotherapy alone (INTACT-1 and INTACT-2,
TRIBUTE and TALENT) (16–19). An antagonistic effect exists between
EGFR-TKIs and chemotherapy drugs. The failure to achieve positive
results may be due to not being able to select EGFR-sensitizing
mutations and using inappropriate drug administration sequences,
thereby leading to cell cycle-specific antagonism (20–24).
Icotinib, an oral EGFR-TKI, has shown antitumor
activity and favorable toxicity in early phase clinical trials. To
assess the safety and tolerability of icotinib, Zhao et al
selected NSCLC patients after the failure of prior platinum-based
chemotherapy (25). Their results
showed that oral icotinib is generally well tolerated with
manageable and reversible adverse events, and shows positive
clinical antitumor activities in patients with advanced NSCLC
(25). A randomized, double-blind
phase 3 non-inferiority trial showed that icotinib is non-inferior
to gefitinib in terms of PFS, suggesting that icotinib is a new
treatment option for pretreated patients with advanced NSCLC
(26).
In the present study, we used human EGFR wild-type
and mutant NSCLC cell lines to define the differential effects of
cisplatin, paclitaxel and icotinib in different schedules on cell
growth proliferation, cell cycle distribution, apoptosis and
signaling pathways. Specifically, we tested the effects of
cisplatin plus paclitaxel combined with icotinib in different
schedules.
Materials and methods
Drugs
Icotinib, kindly provided by the Beida
Pharmaceutical Company (China), was dissolved in 20 mM dimethyl
sulfoxide (DMSO; Sigma, St. Louis, MO, USA) as stocking solution.
Cisplatin and paclitaxel were purchased from Sigma, and
respectively dissolved in 1 mM DMSO as stocking solution. The drugs
were diluted with culture medium before use.
Cell lines
HCC827, H1975, H1299 and A549 human NSCLC cell lines
were obtained from the Chinese Academy of Sciences Institute of
Life Sciences Cell Resource Center in Shanghai. The cell lines were
grown in RPMI-1640 medium supplemented with 10% fetal bovine serum
and 100 UI/ml penicillin-streptomycin at 37°C in a humidified
atmosphere with 5% CO2. Cells in the exponential growth
phase were harvested using trypsin-EDTA.
Gene sequencing
Genomic DNA was extracted from the HCC827, H1975,
H1299 and A549 cell lines. The primers of EGFR exons 19–21 were
designed using the ABI Prism™ Primer Express software (Applied
Biosystems, Foster City, CA, USA) and amplified polymerase chain
reaction of genomic DNA. The samples of positive amplified bands
were then sequenced.
Evaluation of antiproliferative
effects
Cell viability was determined using the tetrazolium
dye 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay. Cells were seeded at ~5,000/well in 96-well plates. At
24 h after seeding, the cells were exposed to the drugs. To
evaluate the single-agent treatment, the cells were exposed to
icotinib, cisplatin or paclitaxel alone for 72 h, and the half
maximal inhibitory concentration (IC50) was considered
as the concentration resulting in 50% cell growth inhibition
compared with the untreated control cells. To evaluate the
antiproliferative effects of the combined treatment, the cells were
treated with three different sequences: i) pretreated with
cisplatin/paclitaxel for 24 h and washed once with
phosphate-buffered saline (PBS), followed by icotinib for 48 h; ii)
pretreated with icotinib for 48 h and washed once, followed by
cisplatin/paclitaxel for 24 h; iii) treated concomitantly with
cisplatin/paclitaxel and icotinib for 48 h, and incubated in
drug-free medium for 24 h. At 72 h after drug treatment, the cells
were washed once with PBS and incubated with medium containing MTT
(0.5 mg/ml in medium) for 4 h at 37°C. The culture medium with MTT
was removed, and formazan crystals were reabsorbed in 200 μl of
DMSO (Sigma). Cell viability was determined by measuring the
absorbance at 570 nm. Experiments were conducted on at least three
separate occasions. Thus, we used 0.125-, 0.25-, 0.5-, 1-, 2- and
4-fold the IC50 dose in cisplatin/paclitaxel and
icotinib combination doses were used to calculate the combination
index (CI) value. The CI was calculated using CompuSyn software
(ComboSyn, Inc., Paramus, NJ, USA). The resulting CI was a
quantitative measure of the degree of interaction between different
drugs, with CI >1.0, CI=1.0 and CI <1.0, indicating
antagonistic, additive and synergistic effects, respectively.
Clonogenic survival assays
To investigate the effects of chemotherapy followed
by icotinib on the NSCLC cell lines, a standard clonogenic assay
was performed. The cells were seeded in triplicate in 6-well plates
(5×102 cells/plate) and treated with DMSO as the vehicle
control. After exposure to cisplatin/paclitaxel at IC50
levels for 24 h, the cells were washed and exposed to icotinib at
IC50 levels for 48 h. The cells were then washed and
incubated in drug-free medium for 14 days. Colonies were stained
with crystal violet and manually counted. All of ≥50 cells were
counted. The survival fraction (SF) was estimated based on the
formula: SF = number of colonies formed/number of cells seeded ×
plating efficiency of the control group. All the experiments were
performed in triplicate.
Analysis of cell cycle and apoptosis
Cells were seeded in 6-well plates at a density of
1×105/well. After 24 h, the cells were treated with
cisplatin/paclitaxel and icotinib sequentially at IC50
levels. To analyze the cell cycle, cells were trypsinized, washed
two times with PBS and harvested by centrifugation after the
treatments were completed. The cells were then fixed with 70%
ice-cold ethanol for at least 1 h, centrifuged, washed two times in
cold PBS, stained with propidium iodide (PI) solution (0.05 mg/ml
PI and 10 mg/ml RNase A) for 20 min at 37°C in the dark, and
analyzed using a flow cytometer (FACSCalibur; Becton-Dickinson
Biosciences, San Jose, CA, USA). To analyze cell apoptosis,
adherent and non-adherent cells were harvested after the drug
treatments, washed with cold PBS, stained with Annexin
V-fluorescein isothiocyanate (FITC) and PI (Joincare Medicine
Company, China) for 15 min at 37°C in the dark and analyzed using a
flow cytometer (FACSCalibur).
Western blot analysis
After the drug treatments, the cells were harvested
in ice-cold PBS and lysed with RIPA cell lysis buffer containing a
protease inhibitor cocktail. The protein concentration was
determined using the BCA protein assay reagent (both from Wolsen
Company, China). Each protein sample was resolved on sodium dodecyl
sulfate polyacrylamide gels (8%), transferred onto polyvinylidene
difluoride membranes (Millipore Corporation, Billerica, MA, USA),
blocked for 1 h at room temperature in 5% non-fat milk, and
incubated with the appropriate primary antibodies according to the
manufacturer’s instructions. The primary antibodies EGFR, p-EGFR,
AKT, p-AKT, ERK1/2, p-ERK1/2 and β-actin were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The blots were then
washed with TBST for 30–40 min, and incubated with the secondary
antibody (Wolsen Company) at room temperature for 1 h. Secondary
antibodies were also purchased from Cell Signaling Technology, Inc.
For the quantification of protein levels, films were scanned and
analyzed using Labworks software.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) of at least three experiments. Statistically significant
differences between groups were determined by the Student’s t-test
using SPSS 19.0 software. In each case, p<0.05 was considered to
indicate a statistically significant result.
Results
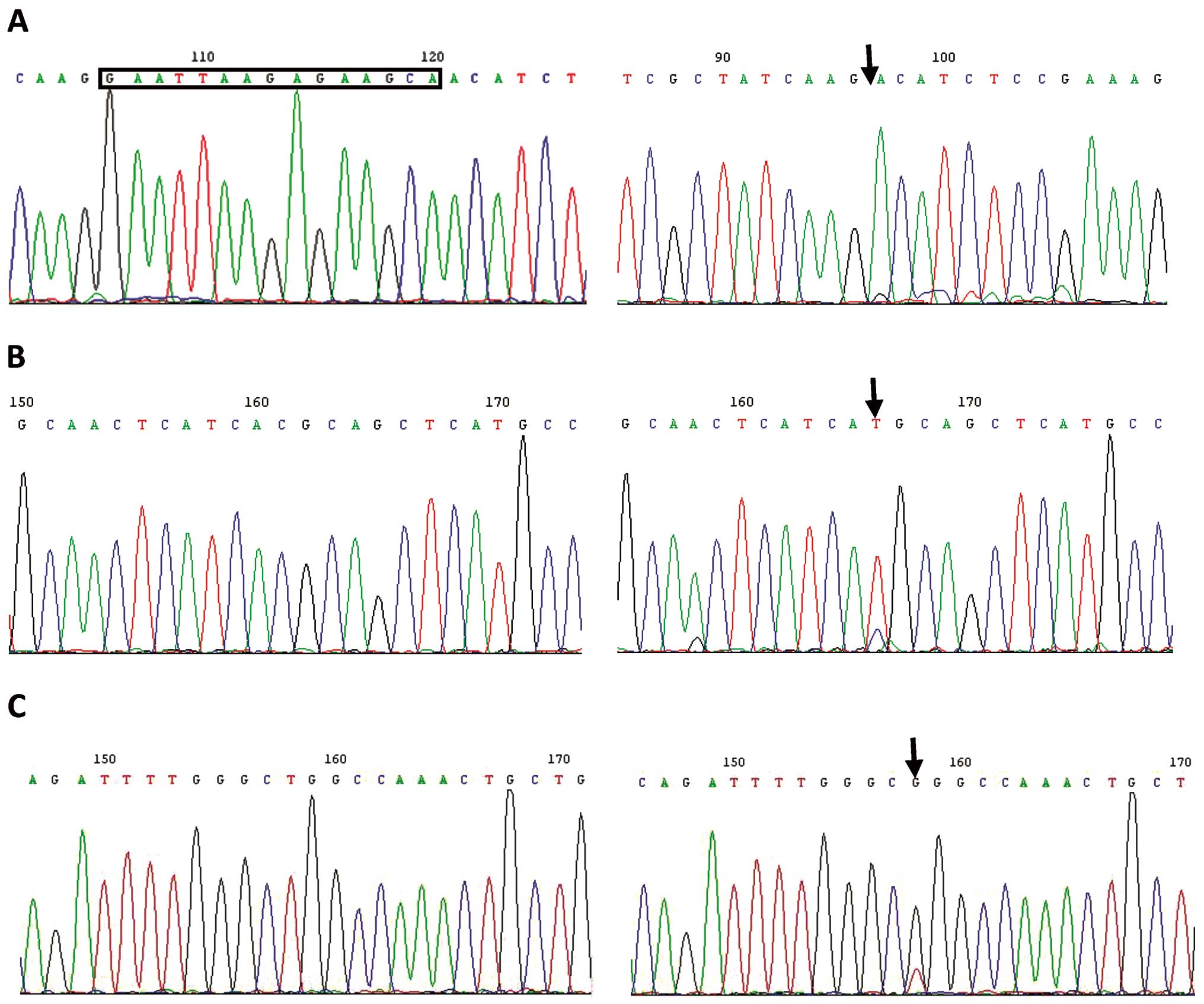
EGFR gene sequencing
The specific primers used were for amplifying the
cDNA fragments of the EGFR tyrosine kinase domain. Fig. 1 shows that the HCC827 cell line
harbors the exon 19 sequence deletion. The H1975 cell line harbors
the exon 20 sequence T790M mutation and exon 21 sequence L858R
mutation. The H1299 and A549 cell lines were wild-types of
EGFR.
Drug sensitivities of the HCC827, H1975,
H1299 and A549 cell lines
Treatment with cisplatin, paclitaxel or icotinib
alone for 72 h resulted in the dose-dependent inhibition of NSCLC
cell growth. Table I summarizes the
IC50 values of the three drugs. The four cell lines
showed sensitivities similar to cisplatin and paclitaxel. The
HCC827 cell line was highly sensitive to icotinib, whereas the
H1975, H1299 and A549 cell lines showed resistance to icotinib.
 | Table IIC50 values for each drug
were calculated by performing dose-response experiments with
cisplatin, paclitaxel and icotinib. |
Table I
IC50 values for each drug
were calculated by performing dose-response experiments with
cisplatin, paclitaxel and icotinib.
| Cell lines | Cisplatin | Paclitaxel | Icotinib |
|---|
| HCC827 | 4.9 μmol/l | 1.6 nmol/l | 290 nmol/l |
| H1975 | 11.0 μmol/l | 1.7 nmol/l | 8.8 μmol/l |
| H1299 | 7.8 μmol/l | 13.3 nmol/l | 25.9 μmol/l |
| A549 | 5.7 μmol/l | 2.3 nmol/l | 6.9 μmol/l |
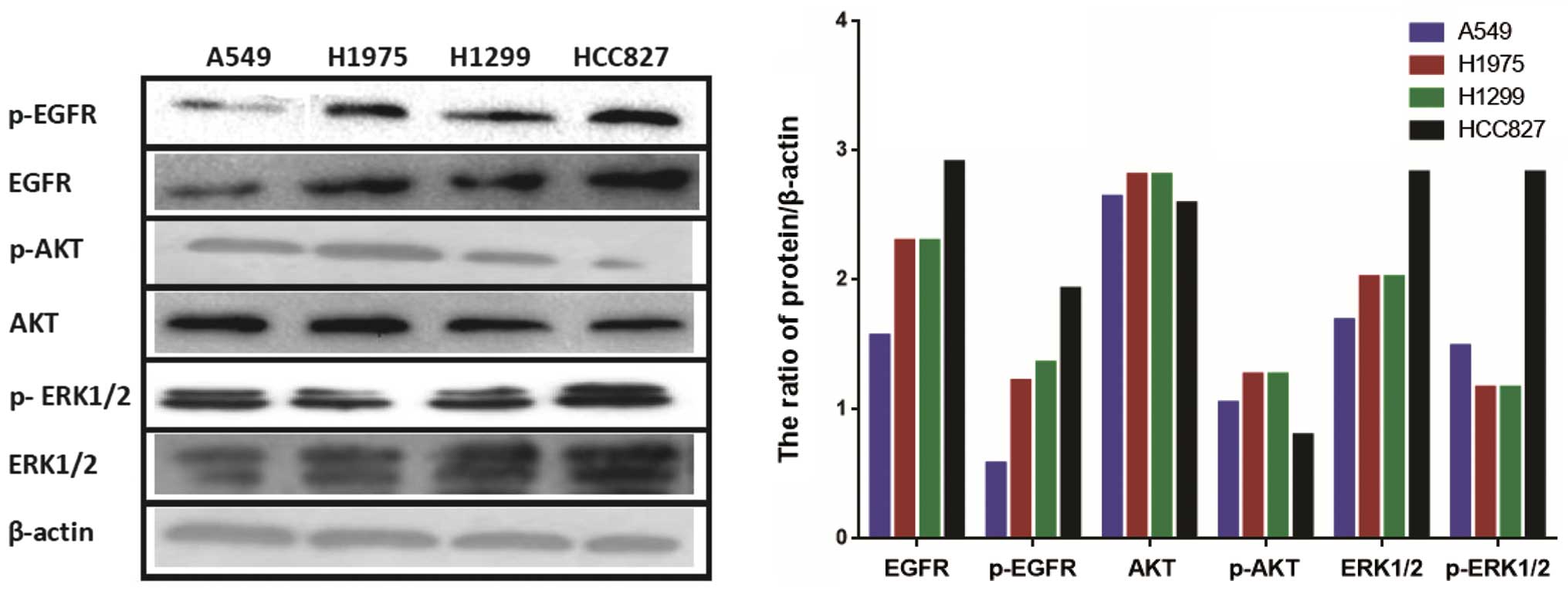
Constitutive expression levels of EGFR
and downstream signaling molecules in four NSCLC cell lines
We compared the basal EGFR expression levels of the
NSCLC cell lines by western blotting. As shown in Fig. 2, the HCC827 cell line had
significantly higher levels of EGFR and p-EGFR than A549, H1975 and
H1299 cell lines. We also observed constitutive AKT and ERK1/2
phosphorylation in the four NSCLC cell lines. The basal AKT and
p-AKT levels were similar in the four cell lines, although these
levels were slightly lower in the HCC827 cell line. The ERK1/2 and
p-ERK1/2 levels in the HCC827 cell line were higher than those in
the other cell lines.
Sequence of cisplatin/paclitaxel followed
by icotinib is more effective than other sequences in the NSCLC
cell lines
To evaluate the antiproliferative effects of
cisplatin, paclitaxel and icotinib treatments, we performed a
series of MTT cell growth assays. We evaluated the
antiproliferative effects on the HCC827, H1975, H1299 and A549 cell
lines in three different sequences. Fig. 3A shows the schema for in
vitro sequentially combined cisplatin/paclitaxel and icotinib
treatments. As shown in Fig. 3B,
the antiproliferative effects between cisplatin/paclitaxel and
icotinib were sequence-dependent. Although the differences were not
significant, the sequence of cisplatin/paclitaxel followed by
icotinib was better than other sequences in the HCC827, H1975,
H1299 and A549 cell lines.
The combined effect of cisplatin/paclitaxel and
icotinib was evaluated on the basis of the CI (Fig. 4). In the HCC827 cell line, which was
highly sensitive to EGFR-TKIs, the sequence of cisplatin followed
by icotinib resulted in a synergistic antiproliferative effect (CI
<1). By contrast, the sequence of icotinib followed by cisplatin
resulted in an antagonistic interaction (CI >1). However, three
different sequences of combined paclitaxel and icotinib resulted in
an antagonistic and synergistic interaction with the increasing
drug concentration. In the H1975 cell line harboring the T790M and
L858R mutations of EGFR, the sequence of cisplatin followed by
icotinib and concomitant administration resulted in a synergistic
effect (CI <1), whereas the sequence of icotinib followed by
cisplatin resulted in an antagonistic interaction (CI >1). Three
different sequences of combined paclitaxel and icotinib resulted in
antagonistic and synergistic interactions with the increasing drug
concentration. The H1299 and A549 cell lines were wild-types of
EGFR. In the H1299 cell line, a synergistic antiproliferative
effect was observed with the sequence of cisplatin followed by
icotinib and concomitant administration (CI <1). The sequence of
icotinib followed by cisplatin resulted in an antagonistic
interaction (CI >1). However, all three different sequences of
combined paclitaxel and icotinib resulted in an antagonistic
interaction. In the A549 cell line, the three different sequences
of combined cisplatin/paclitaxel and icotinib resulted in
synergistic and antagonistic interactions with the increasing drug
concentration.
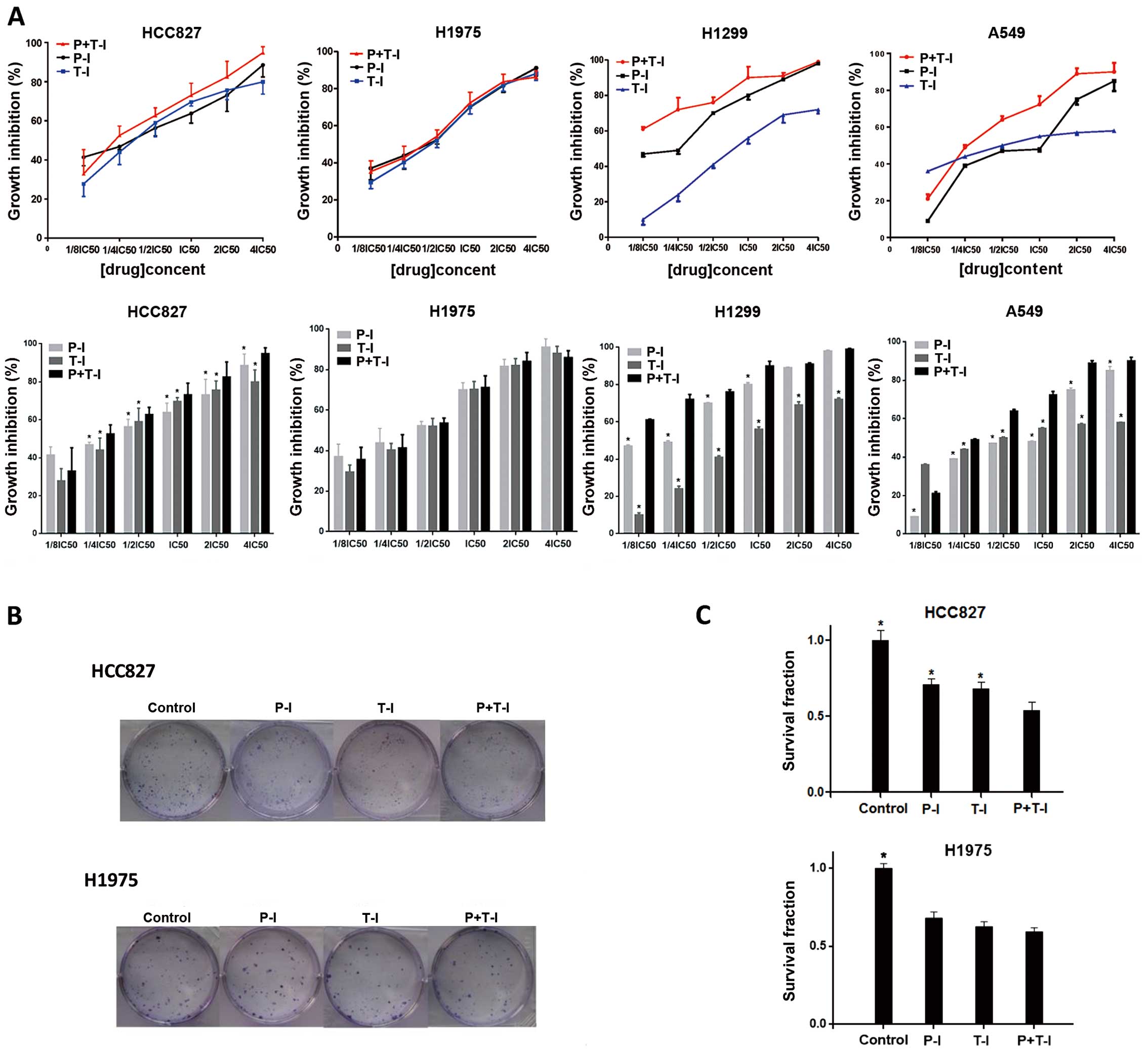
Three-drug combination is better than
two-drug combination in the HCC827, H1299 and A549 cell lines, but
not in the H1975 cell line
The antiproliferative effects of cisplatin plus
paclitaxel followed by icotinib were compared with those of
cisplatin or paclitaxel followed by icotinib. We determined that
the antiproliferative effects of cisplatin plus paclitaxel followed
by icotinib were better than those of cisplatin or paclitaxel
followed by icotinib in the HCC827, H1299 and A549 cell lines
(Fig. 5A). We also evaluated the
effects of cisplatin plus paclitaxel followed by icotinib in the
HCC827 and H1975 cell lines using a clonogenic assay (Fig. 5B and C). Cells were exposed to
cisplatin, paclitaxel and icotinib at the IC50 values.
The combinations, regardless of whether they were three-drug or
two-drug, decreased the survival rates of HCC827 and H1975 cells
compared with the control group. Clonogenic survival of cisplatin
plus paclitaxel followed by icotinib was the lowest compared with
that of cisplatin or paclitaxel followed by icotinib in the HCC827
cell line (three-drug combination vs. two-drug combination,
p<0.05), but not in the H1975 cell line (p>0.05).
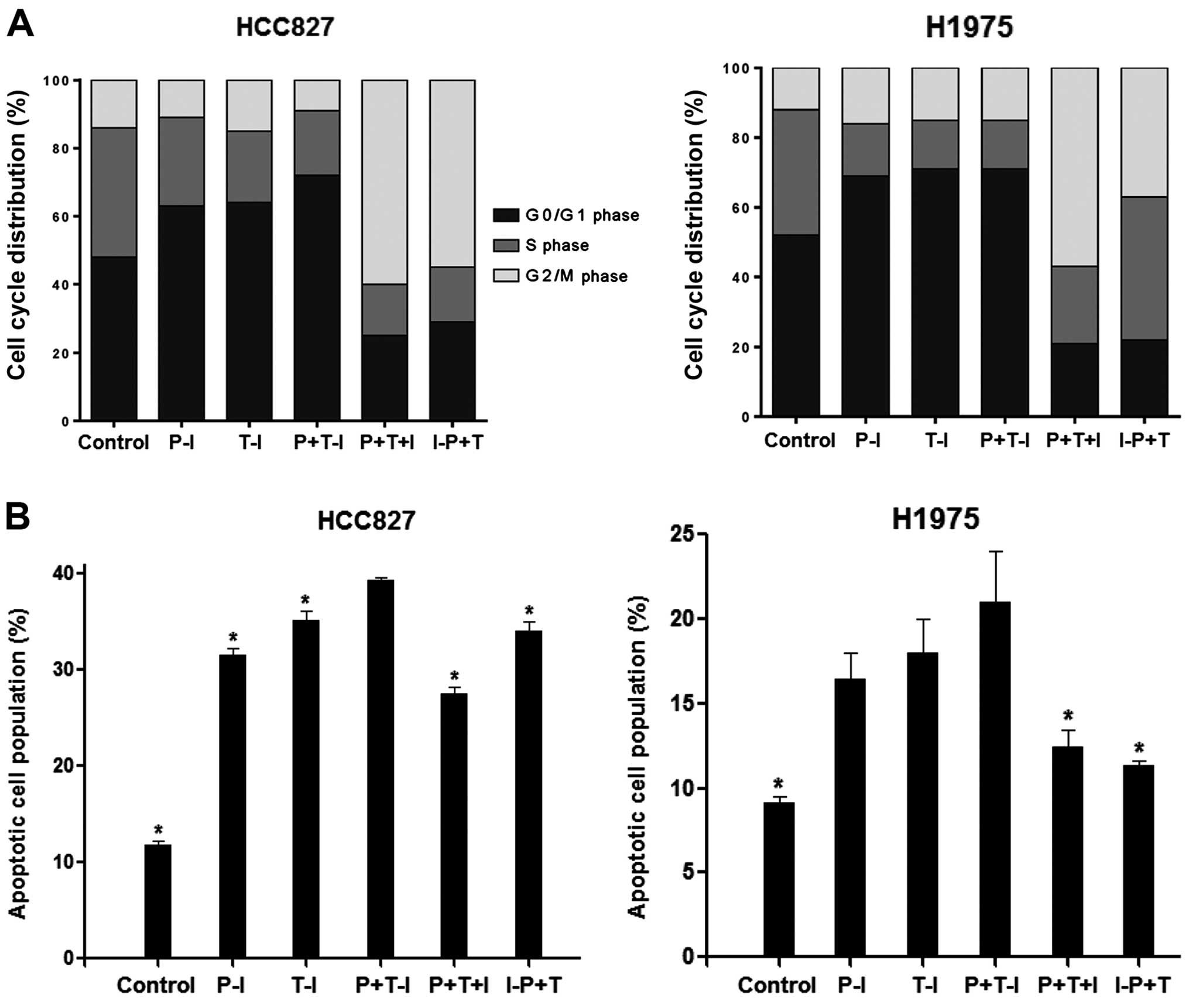
Three-drug combination induced more G0/G1
phase arrest and apoptosis than the two-drug combination in the
HCC827 cell line, but not in the H1975 cell line
DNA flow cytometry studies were performed to
evaluate the effect of drug combinations on the cell cycle
distribution. We selected the HCC827 and H1975 cell lines to
determine whether their cell cycle-modulating activity provides
evidence clues to optimize drug scheduling. The cells were exposed
to cisplatin, paclitaxel and icotinib at the IC50
values. All the agents affected the cell cycle of the HCC827 and
H1975 cell lines (Fig. 6A). In
response to the treatment of cisplatin followed by icotinib, cell
fractions in the S phase decreased (26±2.0 and 15±1.8%), whereas
those in the G0/G1 phase increased (63±1.4 and 69±1.8%) compared
with the control group in the HCC827 and H1975 cell lines,
respectively. In response to the treatment of paclitaxel followed
by icotinib, cell fractions in the S phase also decreased (21±1.5
and 14±1.3%), whereas those in the G0/G1 phase increased (64±1.9
and 71±2.0%). After the treatment with cisplatin plus paclitaxel
followed by icotinib, the proportion of the HCC827 cell line in the
G0/G1 phase significantly increased compared with that in the other
treatment groups (72±0.8%, p<0.05). However, no difference was
observed in the H1975 cell line (71±1.7%, p>0.05).
Double staining with Annexin V-FITC and PI was used
to determine whether growth inhibition was due to the induction of
apoptosis. Cells were exposed to cisplatin, paclitaxel and icotinib
at the IC50 values. As shown in Fig. 6B, the proportions of apoptotic cells
in the HCC827 and H1975 cell lines induced by cisplatin followed by
icotinib were 31.6±0.4 and 16.4±1.6%, respectively. The proportions
of apoptotic cells in the HCC827 and H1975 cell lines induced by
paclitaxel followed by icotinib were 35.2±1.0 and 18.0±2.0%,
respectively. The proportion of apoptotic cells in the HCC827 cell
line treated with cisplatin plus paclitaxel followed by icotinib
was higher than that in the HCC827 cell line treated with cisplatin
or paclitaxel followed by icotinib (39.4±0.3%, p<0.05). However,
no difference was observed in the H1975 cell line (21.0±3.8%,
p>0.05).
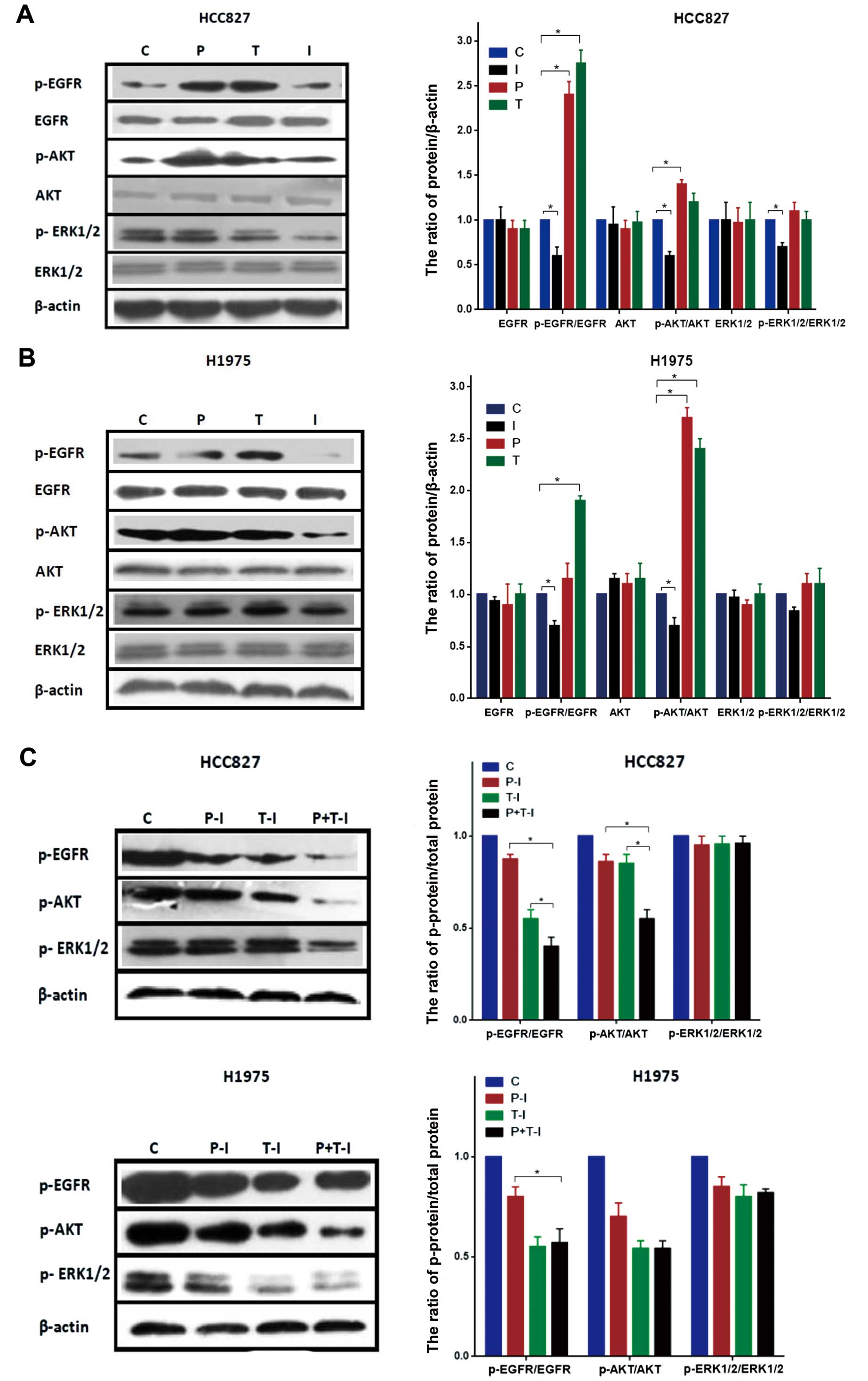
Effect of cisplatin, paclitaxel and
icotinib on EGFR and downstream signaling molecules in the HCC827
and H1975 cell lines
To gain insight into the mechanisms involved in
regulating the interaction of cisplatin, paclitaxel and icotinib,
we examined the effects on EGFR and downstream signaling molecules
in the HCC827 and H1975 cell lines (Fig. 7). The cells were exposed to the
IC50 doses of drugs. Exposure to cisplatin, paclitaxel
or icotinib alone resulted in no changes in the total proteins of
EGFR, AKT and ERK1/2 in the HCC827 and H1975 cell lines. The HCC827
cell line exhibited increases in p-EGFR and p-AKT in response to
cisplatin alone, although p-ERK1/2 was unchanged. When the HCC827
cell line was exposed to paclitaxel alone, the p-EGFR levels
significantly increased, whereas the p-AKT and p-ERK1/2 levels were
unchanged. We observed that icotinib significantly inhibited
p-EGFR, p-AKT and p-ERK1/2 in the HCC827 cell line (Fig. 7A). In the H1975 cell line, we
observed an increase in the p-AKT level after cisplatin. In
addition, the paclitaxel-treated H1975 cell line showed an increase
in the p-EGFR and p-AKT levels. Icotinib-treated H1975 cells showed
a decrease in the p-EGFR and p-AKT levels, although the p-ERK1/2
level was unchanged (Fig. 7B).
We also examined the effect of cisplatin/paclitaxel
followed by icotinib on EGFR and downstream signaling molecules in
the HCC827 and H1975 cell lines (Fig.
7C). In the HCC827 and H1975 cell lines, the combinations of
cisplatin/paclitaxel followed by icotinib affected the expression
of p-EGFR and p-AKT, but not p-ERK1/2. In the HCC827 cell line, the
expression levels of p-EGFR and p-AKT with cisplatin plus
paclitaxel followed by icotinib were significantly lower than those
of cisplatin or paclitaxel followed by icotinib (p<0.05),
although not in the H1975 cell line.
Discussion
The present study aimed to investigate the optimal
schedule of combined treatment with cisplatin/paclitaxel and
icotinib in NSCLC cell lines, and gain insight into the molecular
mechanisms underlying the interaction of these drugs in
vitro. In the present study, we observed that the sequence of
cisplatin followed by icotinib resulted in a synergistic effect on
the HCC827, H1975 and H1299 cell lines. In addition, paclitaxel
followed by icotinib showed a synergistic effect in the HCC827 and
H1975 cell lines at high concentrations. However, the sequences of
cisplatin followed by icotinib and paclitaxel followed by icotinib
resulted in synergistic effects only in low concentrations in the
A549 cell line. The antiproliferative effect of cisplatin plus
paclitaxel followed by icotinib was superior to that of cisplatin
or paclitaxel followed by icotinib in the HCC827, H1299 and A549
cell lines, although not in the H1975 cell line harboring the T790M
and L858R mutations. This antiproliferative effect seemed to have
no correlation with the constitutive expression levels of EGFR and
downstream signaling molecules in the four NSCLC cell lines. We
also determined that the potentiation of the antiproliferative
activity of EGFR-TKI and chemotherapy in combination was
sequence-dependent.
In previous studies sequence-dependent interactions
between EGFR-TKIs and chemotherapy in human cancer cell lines were
shown (27). Cheng et al
observed that the sequence of paclitaxel followed by gefitinib is
an appropriate treatment combination that is superior to other
sequences in treating the NSCLC cell lines (28,29).
Tsai et al determined that the concomitant
gefitinib/cisplatin combination shows antagonism in the majority of
sensitizing mutations of EGFR wild-type or NSCLC cells, and the
three-drug combination is not better than the two-drug combination
(30). The present study was
innovative since we determined that cisplatin plus paclitaxel
followed by icotinib was superior to cisplatin or paclitaxel
followed by icotinib in some of the NSCLC cell lines. In addition,
the EGFR signaling pathway may have a function in the
sequence-dependent interaction in the NSCLC cell lines.
Given that the INTACT-1, INTACT-2, TALENT and
TRIBUTE clinical trials were unsuccessful, Gandara et al
(21,31) suggested two hypotheses that likely
explain these negative results. Firstly, patients were not selected
based on a predictive response marker. Secondly, the potentiation
of the antagonistic interaction between concurrent EGFR-TKI and
chemotherapy may have had a function. Davies et al (20) suggested that EGFR-TKIs primarily
cause cell cycle arrest and accumulation of cells in G1, and can
interfere with cell cycle-specific cytotoxicity when administered
concurrently with chemotherapy. Other studies also showed that
pretreatment with EGFR-TKIs causes G1 arrest and effectively
abrogates the activity of chemotherapy, resulting in decreased
cytotoxicity and apoptosis (32–34).
Paclitaxel is an M-phase-specific drug, and these studies may
explain the reason of the sequence of paclitaxel followed by
icotinib being better than other sequences. Cisplatin is a cell
cycle non-specific drug, and the sequence of icotinib followed by
cisplatin also caused antagonism. The negative interaction between
cisplatin and icotinib may be associated with various cisplatin
resistance mechanisms (35–38). Firstly, icotinib may act to decrease
cisplatin uptake and/or increase efflux. Secondly, icotinib may
change the susceptibility to DNA damage from cisplatin.
Anti-apoptosis may also be induced, changing the efficacy of
cisplatin.
The present study determined that cisplatin plus
paclitaxel followed by icotinib caused more antiproliferative
effects, apoptosis and G1 arrest than cisplatin or paclitaxel
followed by icotinib in the HCC827 cell line, although not in the
H1975 cell line. Improper activation of EGFR results in increased
malignant cell survival, proliferation, invasion and metastasis
(39,40). The present study and several studies
have determined that paclitaxel can increase the expression of EGFR
phosphorylation and activate the EGFR signaling pathway (28,29,41).
We also determined that cisplatin significantly increased the
expression of EGFR phosphorylation in the HCC827 cell line, but not
that in the H1975 cell line. However, the exact mechanisms
underlying the increased EGFR phosphorylation level remain unknown.
In the HCC827 cell line, cisplatin and paclitaxel increased the
expression of EGFR phosphorylation, and icotinib decreased the
expression of EGFR phosphorylation. This significant increase in
EGFR phosphorylation was inhibited by subsequent exposure to
icotinib, which may explain the reason for the effect of cisplatin
plus paclitaxel followed by icotinib being better than that of
cisplatin or paclitaxel followed by icotinib in the HCC827 cell
line, but not in the H1975 cell line. The present study has shown
that cisplatin/paclitaxel followed by icotinib influenced the
expression of p-EGFR and p-AKT, although the expression of p-ERK1/2
was unchanged. Whether the antiproliferative effect of EGFR-TKI and
chemotherapy is related to the PI3K/AKT pathway remains to be
determined.
In conclusion, the present study has demonstrated
that the most advantageous schedule to treat NSCLC in vitro
was the sequence of cisplatin/paclitaxel followed by icotinib. We
also characterized the molecular mechanisms involved in the
synergistic effect between cisplatin/paclitaxel and icotinib
against the NSCLC cell lines. Although the extrapolation of in
vitro data to the clinical setting should be considered with
caution, these results may provide a rationale for the ongoing
clinical investigation of the sequential treatment of NSCLC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81101777), and
the social development research project of Shaanxi Provincial
Department of Science and Technology (no. 2013K12-08-3).
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
EGFR-TKIs
|
epidermal growth factor receptor
tyrosine kinase inhibitors
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Jemal A, Thun MJ, Ries LA, et al: Annual
report to the nation on the status of cancer, 1975–2005, featuring
trends in lung cancer, tobacco use, and tobacco control. J Natl
Cancer Inst. 100:1672–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar
|
|
3
|
Salama JK and Vokes EE: New radiotherapy
and chemoradiotherapy approaches for non-small-cell lung cancer. J
Clin Oncol. 31:1029–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Zhang Y and Ma S: EGFR inhibitors
with concurrent thoracic radiation therapy for locally advanced
non-small cell lung cancer. Lung Cancer. 73:249–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. New Engl J Med. 346:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smit EF, van Meerbeeck JP, Lianes P, et
al: Three-arm randomized study of two cisplatin-based regimens and
paclitaxel plus gemcitabine in advanced non-small-cell lung cancer:
a phase III trial of the European Organization for Research and
Treatment of Cancer Lung Cancer Group - EORTC 08975. J Clin Oncol.
21:3909–3917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. New Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pao W, Miller V, Zakowski M, et al: EGF
receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar
|
|
10
|
Brandao GD, Brega EF and Spatz A: The role
of molecular pathology in non-small-cell lung carcinoma-now and in
the future. Curr Oncol. 19(Suppl 1): S24–S32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsudomi T and Yatabe Y: Epidermal growth
factor receptor in relation to tumor development: EGFR gene and
cancer. FEBS J. 277:301–308. 2010. View Article : Google Scholar
|
|
12
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.
New Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
14
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. New Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small-cell lung
cancer. New Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mok TS, Wu YL, Yu CJ, et al: Randomized,
placebo-controlled, phase II study of sequential erlotinib and
chemotherapy as first-line treatment for advanced non-small-cell
lung cancer. J Clin Oncol. 27:5080–5087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giaccone G, Herbst RS, Manegold C, et al:
Gefitinib in combination with gemcitabine and cisplatin in advanced
non-small-cell lung cancer: a phase III trial - INTACT 1. J Clin
Oncol. 22:777–784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herbst RS, Giaccone G, Schiller JH, et al:
Gefitinib in combination with paclitaxel and carboplatin in
advanced non-small-cell lung cancer: a phase III trial - INTACT 2.
J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davies AM, Ho C, Lara PN Jr, Mack P,
Gumerlock PH and Gandara DR: Pharmacodynamic separation of
epidermal growth factor receptor tyrosine kinase inhibitors and
chemotherapy in non-small-cell lung cancer. Clin Lung Cancer.
7:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gandara DR and Gumerlock PH: Epidermal
growth factor receptor tyrosine kinase inhibitors plus
chemotherapy: case closed or is the jury still out? J Clin Oncol.
23:5856–5858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Normanno N: Gefitinib and cisplatin-based
chemotherapy in non-small-cell lung cancer: simply a bad
combination? J Clin Oncol. 23:928–930. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baselga J: Combining the anti-EGFR agent
gefitinib with chemotherapy in non-small-cell lung cancer: how do
we go from INTACT to impact? J Clin Oncol. 22:759–761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milano G, Spano JP and Leyland-Jones B:
EGFR-targeting drugs in combination with cytotoxic agents: from
bench to bedside, a contrasted reality. Br J Cancer. 99:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Q, Shentu J, Xu N, et al: Phase I
study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine
kinase inhibitor, in patients with advanced NSCLC and other solid
tumors. Lung Cancer. 73:195–202. 2011. View Article : Google Scholar
|
|
26
|
Shi Y, Zhang L, Liu X, et al: Icotinib
versus gefitinib in previously treated advanced non-small-cell lung
cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority
trial. Lancet Oncol. 14:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu JM, Azzariti A, Colucci G and Paradiso
A: The effect of gefitinib (Iressa, ZD1839) in combination with
oxaliplatin is schedule-dependent in colon cancer cell lines.
Cancer Chemother Pharmacol. 52:442–448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng H, An SJ, Dong S, et al: Molecular
mechanism of the schedule-dependent synergistic interaction in
EGFR-mutant non-small cell lung cancer cell lines treated with
paclitaxel and gefitinib. J Hematol Oncol. 4:52011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng H, An SJ, Zhang XC, et al: In vitro
sequence-dependent synergism between paclitaxel and gefitinib in
human lung cancer cell lines. Cancer Chemother Pharmacol.
67:637–646. 2011. View Article : Google Scholar
|
|
30
|
Tsai CM, Chen JT, Stewart DJ, et al:
Antagonism between gefitinib and cisplatin in non-small cell lung
cancer cells: why randomized trials failed? J Thorac Oncol.
6:559–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gandara D, Narayan S, Lara PN Jr, et al:
Integration of novel therapeutics into combined modality therapy of
locally advanced non-small cell lung cancer. Clin Cancer Res.
11:5057s–5062s. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Ling YH, Goldman ID and Perez-Soler
R: Schedule-dependent cytotoxic synergism of pemetrexed and
erlotinib in human non-small cell lung cancer cells. Clin Cancer
Res. 13:3413–3422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gumerlock PH, Pryde BJ, Kimura T, et al:
Enhanced cytotoxicity of docetaxel OSI-774 combination in non-small
cell lung carcinoma (NSCLC). J Clin Oncol. 22:26612003.
|
|
34
|
Mahaffey CM, Davies AM, Lara PN Jr, et al:
Schedule-dependent apoptosis in K-ras mutant non-small-cell lung
cancer cell lines treated with docetaxel and erlotinib: rationale
for pharmacodynamic separation. Clin Lung Cancer. 8:548–553. 2007.
View Article : Google Scholar
|
|
35
|
Stewart DJ, Raaphorst GP, Yau J and
Beaubien AR: Active vs. passive resistance, dose-response
relationships, high dose chemotherapy, and resistance modulation: a
hypothesis. Invest New Drugs. 14:115–130. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall MD, Okabe M, Shen DW, Liang XJ and
Gottesman MM: The role of cellular accumulation in determining
sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol
Toxicol. 48:495–535. 2008. View Article : Google Scholar
|
|
38
|
Stewart DJ: Tumor and host factors that
may limit efficacy of chemotherapy in non-small cell and small cell
lung cancer. Crit Rev Oncol Hematol. 75:173–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Citri A and Yarden Y: EGF-ERBB signalling:
towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. New Engl J Med. 358:1160–1174.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Van Schaeybroeck S, Kyula J, Kelly DM, et
al: Chemotherapy-induced epidermal growth factor receptor
activation determines response to combined gefitinib/chemotherapy
treatment in non-small cell lung cancer cells. Mol Cancer Ther.
5:1154–1165. 2006. View Article : Google Scholar : PubMed/NCBI
|