Introduction
Breast cancer is the most common malignant tumor in
women worldwide and most patients with advanced breast cancer have
serious systemic metastasis, which is associated with a high death
rate (1). Chemotherapy is commonly
used in the treatment of metastatic breast cancer. However, this
treatment remains ineffective due to its toxicity to normal cells
(2). Natural medicine has been
widely used for treating malignant tumors for thousands of years
(3,4). There is great potential in identifying
drugs from natural medicine for the treatment of metastatic breast
cancer due to their wide range of biological activities and low
toxicity.
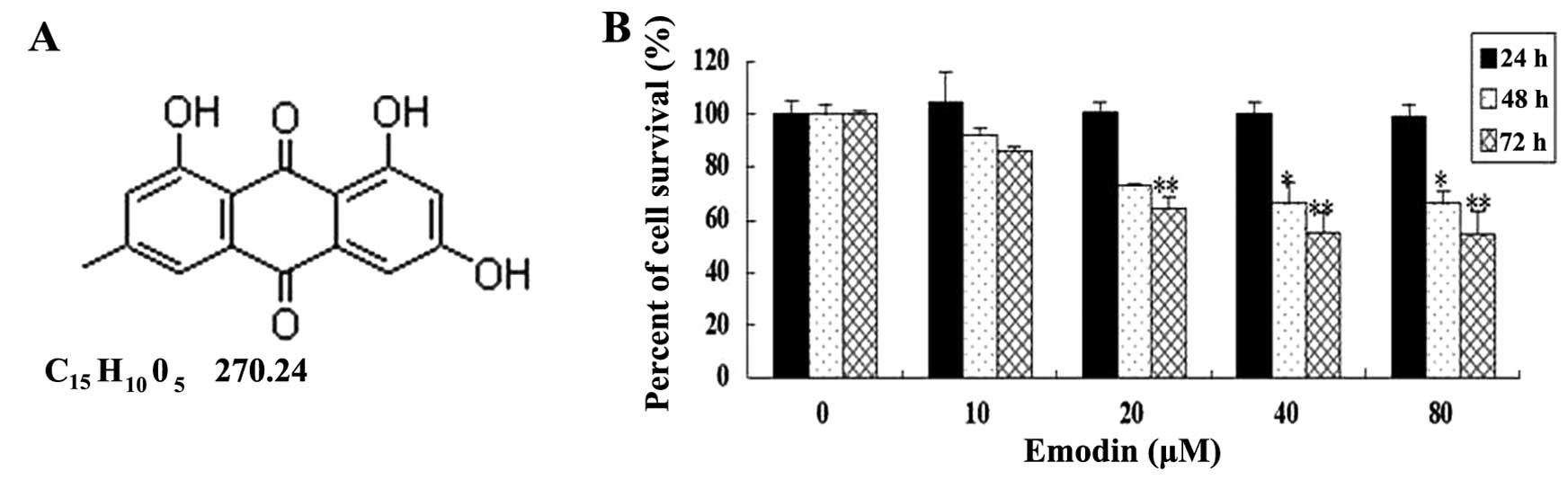
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone)
(Fig. 1A), an active monomer
extracted from Rheum, Polygonum, Buckthorn and Senna,
has been shown to be active against pancreatic (5,6), lung
(7,8), breast (9,10),
liver (11), prostate (12,13),
ovarian (14), cervical (15), colon (16), gallbladder (17) and human tongue cancer SCC-4 cells
(18,19). Moreover, it has been reported that
emodin induces apoptosis of human breast cancer MCF-7 (20) and MDA-MB-453 cells (21). However, there are no reports on the
effect of emodin on breast cancer MDA-MB-231 cells.
Proteolytic enzymes play important roles in the
process of cancer development and metastasis. Its members such as
MMP-2, MMP-9 and uPA play important roles in breast cancer
metastasis, by degrading the extracellular matrix (ECM) and
basement membrane (BM) to facilitate invasion and migration of
cancer cells (22–24).
Previous studies have shown that the
mitogen-activated protein kinase (MAPK) signaling pathway is
associated with the invasion and migration of breast cancer cells
through degradation of ECM (25,26).
In the present study, we demonstrated the efficacy of emodin
treatment on breast cancer cell migration, invasion and metastasis,
and confirmed that emodin inhibits the invasion, migration and
metastasis of MDA-MB-231 cells in vivo and in vitro.
Emodin may function as an inhibitor of p38 and ERK in the
regulation of MAPK signaling pathways through downregulation of
MMP-2, MMP-9, uPA and uPAR expression.
Materials and methods
Materials
Emodin was obtained from the Shanghai
Standardization for the Traditional Research Center and was
dissolved in dimethyl sulfoxide (DMSO). Matrigel was purchased from
Sigma (St. Louis, MO, USA) and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-
2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was purchased
from Promega (Madison, WI, USA). SB203580 and PD98059 were obtained
from Biomol (Plymouth, PA, USA). The antibodies used in the present
study were MMP-9, MMP-2, TIMP-1, TIMP-2, uPA and uPAR and were from
Santa Cruz Biotechnology (Santa Cruz, CA, USA); p38, p-p38, ERK2
and p-ERK1/2 were from Cell Signaling Technology (Boston, MA, USA).
IRDye™ fluorescence antibodies were obtained from LI-COR
Biosciences (Lincoln, NE, USA).
Cell culture
Human breast cancer MDA-MB-231 cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
and cultured in RPMI-1640 medium supplemented with 10% fetal calf
serum, 0.01 mg/ml insulin, 2 mM glutamine, 100 U/ml penicillin and
100 μg/ml streptomycin at 37°C with 5% CO2 in a
humidified atmosphere.
Cell viability assay
Cell viability was determined by MTS assay. Briefly,
MDA-MB-231 cells (5×104 cells/well) were seeded in
96-well culture plates. After overnight incubation, various
concentrations of emodin were added to the cells for varying times
followed by the addition of 20 μl MTS at 37°C for 4 h. Optical
density (OD) was measured at 490 nm using an ELISA plate reader
(BioTek, Winooski, VT, USA).
Wound healing assay
MDA-MB-231 cells were seeded at a density of
1–5×105 cells/well in 12-well culture plates and allowed
to form a confluent monolayer. The layer of cells was scraped with
a 20–200 μl micropipette tip to create a wound of ~1 mm width.
Cells were washed twice with PBS and replaced with serum-free
medium containing various concentrations of emodin. At 0, 24, 48
and 72 h, cells were washed with PBS and then fixed with 4%
paraformaldehyde followed by staining with 0.5% Coomassie brilliant
blue. Images of the wounds were monitored under a phase-contrast
microscope at ×100 magnification.
Migration and invasion assays
Cell migration was analyzed with the aid of a
Transwell (Corning Incorporated, Corning, NY, USA). To analyze cell
invasion, the upper surface of a filter membrane in the upper
compartment of a Transwell was coated with 30 μg Matrigel. Cells
(1×105) suspended in 200 μl of serum-free medium were
seeded onto the upper compartment of the Transwell and the lower
chambers were filled with medium containing 10% FCS and various
concentrations of emodin. After 24 h, the cells remaining in the
upper chamber were removed and cells on the undersurface of the
filters were fixed with 70% ethanol followed by staining with 0.5%
Coomassie brilliant blue for 10 min. The migrated or invaded cells
were visualized and counted from six randomly selected fields
(magnification, ×100) under a phase-contrast microscope.
Effects of emodin on breast cancer
MDA-MB-231 xenografts
Six-week-old female athymic nude mice were obtained
from the Laboratory Animal Center at the Shanghai University of
Traditional Chinese Medicine and were housed in a pathogen-free
condition throughout the experimental duration. All procedures
conformed to the consideration of animal welfare and were approved
by the Ethical Committee of Shanghai Traditional Chinese Medicine.
Briefly, mice were injected with 3×106 MDA-MB-231 cells
(suspended in Matrigel). One day after tumor cell inoculation, the
mice received either 1% DMSO/10% Tween-80 in PBS (sham-treated
group, n=8) or 40 mg/kg emodin (emodin-treated group, n=8) every
two days through intraperitoneal injection. Mice were closely
monitored, their bodies were weighed and tumor weights were
measured weekly. Eight weeks after tumor cell inoculation, the mice
were sacrificed and tumors were excised. Lungs were also collected
from the sacrificed animals, sectioned and stained with hematoxylin
and eosin (H&E). Representative fields for each group were
photographed, and the metastatic nodules were counted.
Function tests of the liver and
kidney
At the time of necropsy, 1 ml of blood was collected
through eye-bleeding and centrifuged at 3,000 rpm for 10 min to
obtain sera. Sera were analyzed for the levels of glutamic
oxalacetic transaminase (GOT), glutamic pyruvic transaminase (GPT),
creatinine (Cr) and urea nitrogen (BUN) using the respective
colorimeter testing kits (Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
(ELISA)
To detect the effect of emodin on the secretion of
MMP-2 and MMP-9 from MDA-MB-231 cells, the cells were treated with
20, 40 and 80 μM emodin for 24 h. Supernatants were then collected
and analyzed by ELISA using human MMP-9 (R&D Systems,
Minneapolis, MN, USA) and human MMP-2 ELISA kits (Ray Biotech,
Norcross, GA, USA) according to the manufacturer’s
instructions.
Western blot analysis
Whole cell lysates were electrophoresed on 8 or 10%
SDS-PAGE. The levels of MMP-2, MMP-9, TIMP-1, TIMP-2, uPA, uPAR,
p38, p-p38, ERK2, p-ERK1/2 and GAPDH were detected by first
incubating with the respective primary antibodies (1:1,000–5,000)
and visualized by IRDye™700DX (red) or IRDye™800DX (green)
conjugated secondary antibodies (1:10,000–20,000). Images were
generated using the Odyssey Infrared Imaging system (LI-COR
Biosciences). Quantitative analyses of western blots were performed
using the AlphaEase FC (FluorChem FC2) software. Relative protein
expression was standardized to the level of GAPDH.
Statistical analyses
All data are the means ± SD. Comparisons between
groups were analyzed by the Student’s t-test and one-way analysis
of variance (ANOVA). P<0.05 was considered to indicate a
statistically significant result.
Results
Emodin inhibits breast cancer cell
migration
To determine the general effect of emodin on breast
cancer cell survival, varying concentrations of emodin were added
to the MDA-MB-231 cell culture for up to 3 days. MTS assay showed
that cell survival was significantly decreased after cells were
treated with emodin at concentrations of 20, 40 and 80 μM for 48
and 72 h (vs. the untreated cells, P<0.05) (Fig. 1B). Subsequently, the effect of
emodin on cell migration was examined by performing a wound healing
assay with non-lethal concentrations and incubation periods (≤80
μM, ≤72 h). As shown in Fig. 2A,
the wound gaps in the 10, 20, 40 and 80 μM emodin-treated groups
were significantly wider than those of the untreated groups at 24,
48 and 72 h. The cell migration was further analyzed by Transwell
assay. Similar to the results of the wound healing assay, emodin
effectively inhibited cell migration in a dose-dependent manner,
and an ~7, 30, 60 and 75% reduction could be observed in the
MDA-MB-231 cells treated with 10, 20, 40 and 80 μM emodin,
respectively (Fig. 2B and C). The
results indicated that emodin can effectively inhibit the motility
of MDA-MB-231 cells, since emodin shows a small effect on cell
viability at these concentrations.
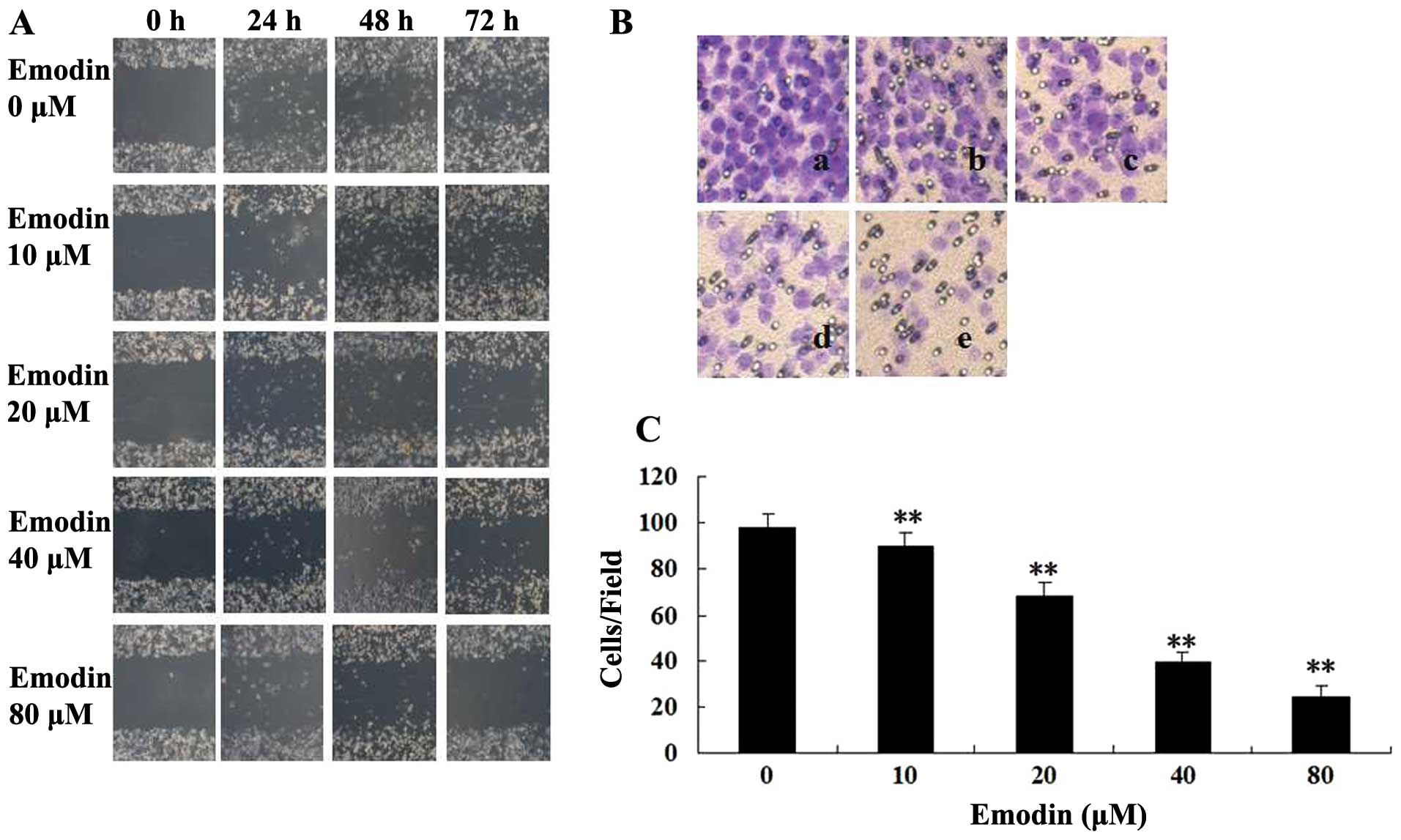 | Figure 2Effect of emodin on MDA-MB-231 cell
migration. (A) Images of wound healing assays (magnification,
×100). Cells were seeded into 12-well cell culture plates and
cultured to near confluency. The wounded monolayer was incubated in
free-FCS RPMI-1640 containing 0, 10, 20, 40 and 80 μM of emodin for
24, 48 and 72 h. (B) A Transwell chamber was used for the migration
assay (magnification, ×200). MDA-MB-231 cells were treated with (a)
0, (b) 10, (c) 20, (d) 40 or (e) 80 μM of emodin for 24 h. (a)
Blank (no added cells). (C) Percent of cell migration. Results are
presented as means ± SD of three independent experiments; SD is
denoted by the error bars. **P<0.01, vs. untreated
cells. |
Emodin inhibits breast cancer cell
invasion
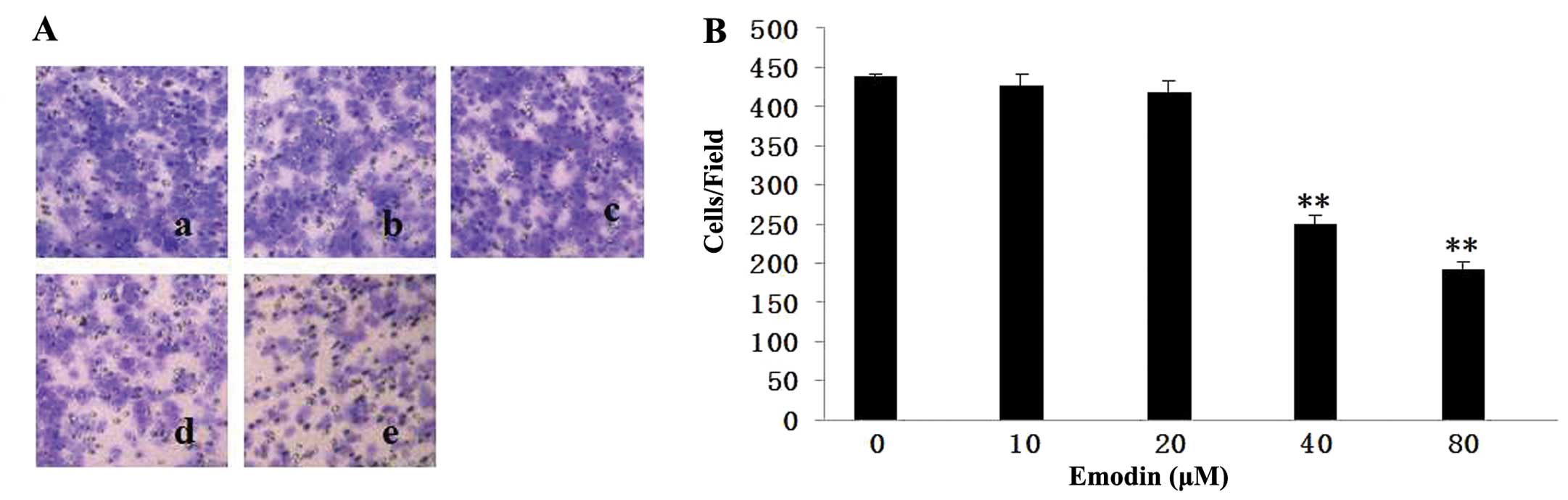
To determine the effect of emodin on cell invasion,
MDA-MB-231 cells were treated with 10, 20, 40 and 80 μM emodin
followed by allowing cells to invade in Matrigel-coated Transwells
for 24 h. The number of cells that invaded was reduced by emodin in
a dose-dependent manner (Fig. 3).
Compared to the untreated group, emodin at 40 and 80 μM suppressed
invasion by ~57 and 44%, respectively. These data clearly showed
that emodin is a strong suppressor of breast cancer cell
invasion.
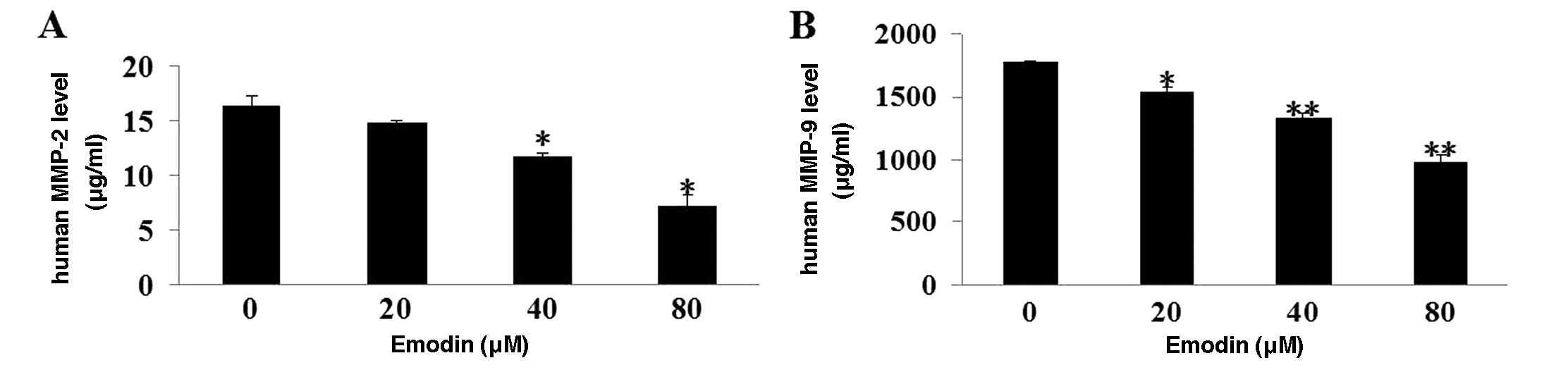
Emodin reduces MMP-2 and MMP-9 secretion
from MDA-MB-231 cells
MMP-2 and MMP-9 are known to play an essential role
in cancer cell invasion and metastasis by facilitating the
degradation of ECM and BM. Amounts of MMP-2 and MMP-9 secreted from
MDA-MB-231 cells were measured with or without emodin treatment. As
shown in Fig. 4A and B, emodin
greatly decreased the secretion of MMP-2 and MMP-9 from MDA-MB-231
cells (P<0.01).
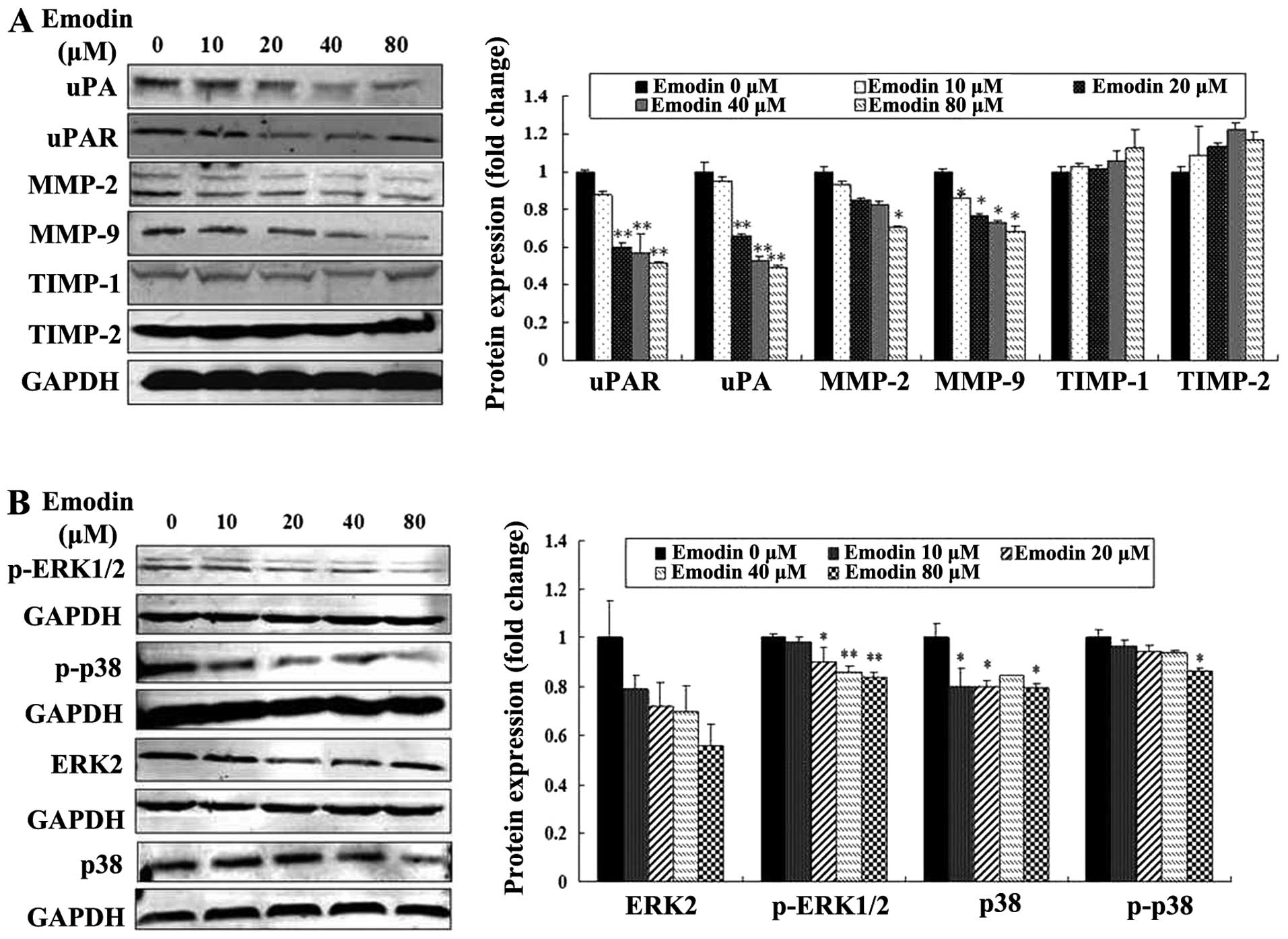
Emodin inhibits the expression of MMP-2,
MMP-9, uPA, uPAR, p38 and ERK
To determine whether the expression of proteolytic
enzymes, p38 and ERK were affected by emodin, the levels of MMP-2,
MMP-9, uPA, uPAR, TIMP-1 and TIMP-2, p38, p-p38, p-ERK1/2 and ERK2
were evaluated in MDA-MB-231 cells treated with or without emodin.
Western blotting with the respective antibodies showed that the
expression levels of uPA, uPAR, MMP-2 and MMP-9 were
concentration-dependently decreased by emodin treatment (P<0.05
or P<0.01; Fig. 5A). The levels
of TIMP-1 and TIMP-2 were increased, but they were not
significantly altered by emodin treatment (P>0.05; Fig. 5A). Moreover, emodin also reduced the
expression levels of p38, p-p38 and p-ERK1/2 without altering the
levels of ERK2 expression (P<0.05 or P<0.01; Fig. 5B). These results indicate that
emodin is most likely to inhibit MDA-MB-231 cell migration,
invasion and metastasis by downregulating the levels of uPA, uPAR,
MMP-2 and MMP-9 proteolytic enzymes, and reducing activities of p38
and ERK.
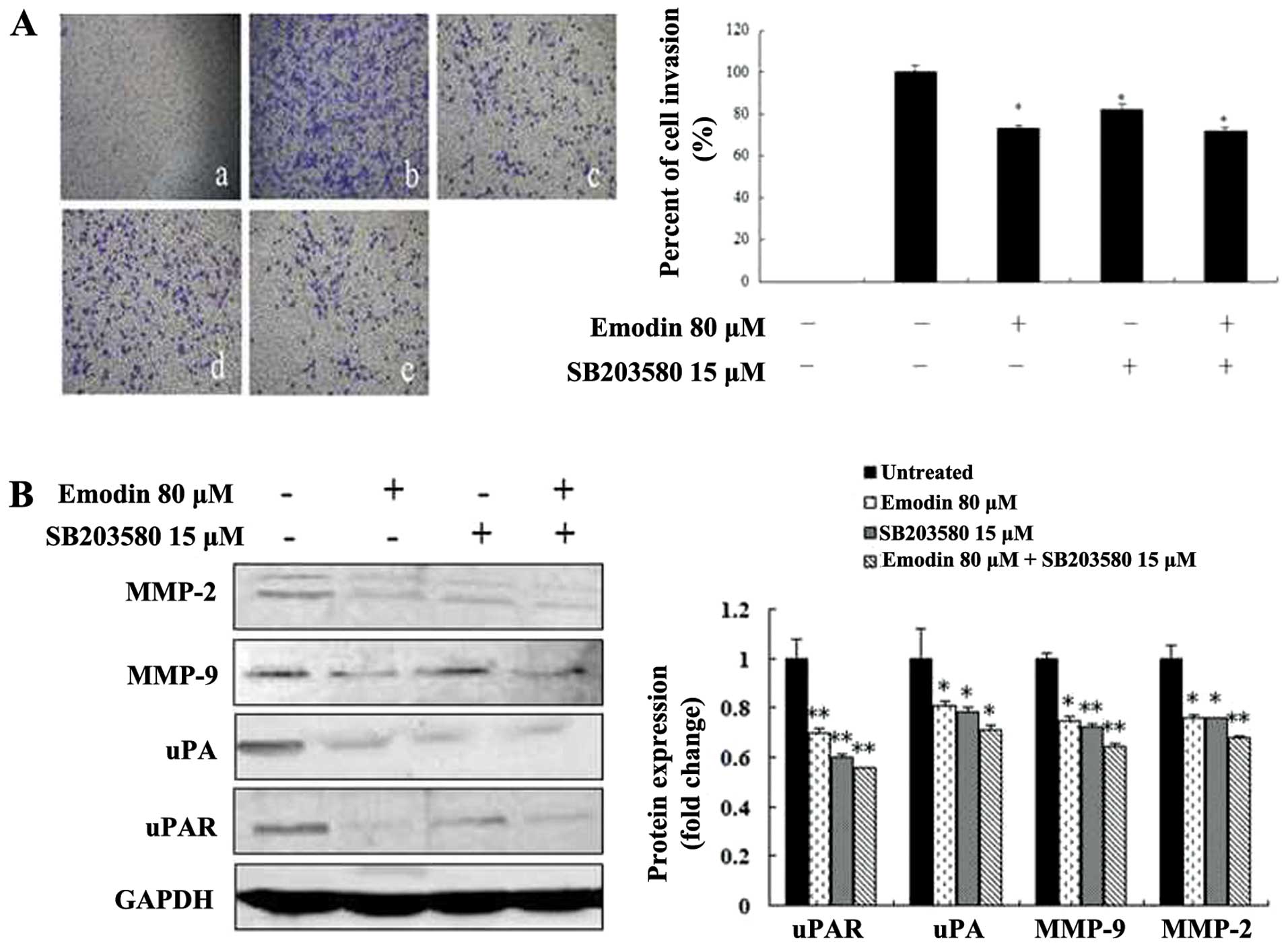
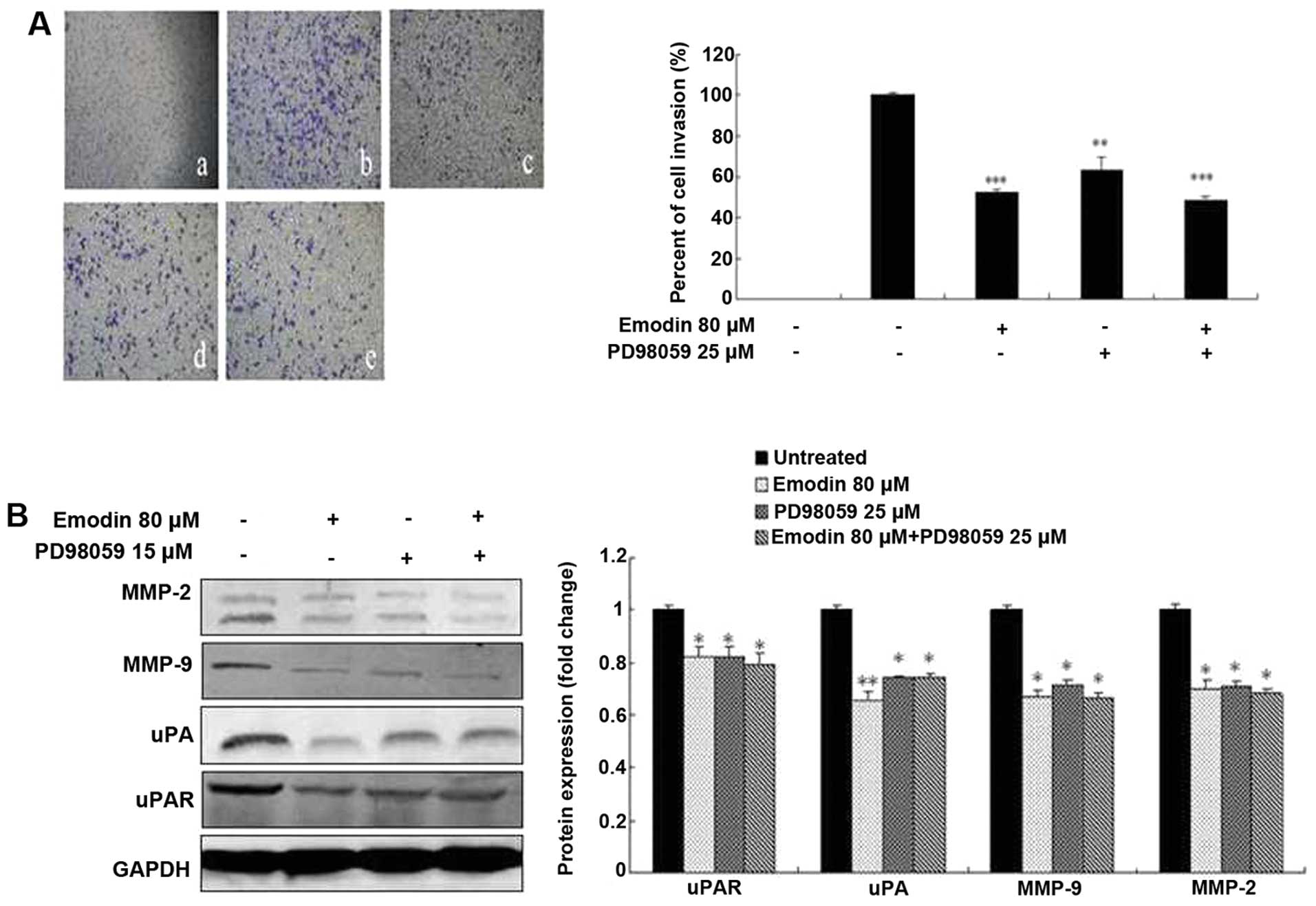
Emodin, SB203580 and PD98059 inhibit cell
invasion by regulating the expression of MMP-2, MMP-9, uPA and
uPAR
To investigate the potential functional link between
the expression of proteolytic enzymes, ERK and p38, we treated
MDA-MB-231 cells with the p38-specific inhibitor SB203580 and
ERK-specific inhibitor PD98059 in the presence or absence of emodin
followed by analysis of cell invasion and proteolytic protein
expression. Both the invasive ability of MDA-MB-231 cells and the
expression levels of MMP-2, MMP-9, uPA and uPAR were significantly
suppressed by 15 μM SB203580 (Fig.
6), 25 μM PD98059 (Fig. 7) and
80-μM emodin treatment (P<0.05 or P<0.01; Figs. 6 and 7), respectively. However, the treatment of
SB203580 or PD98059 and combination with emodin did not exhibit a
significantly greater inhibitory effect (P>0.05; Figs. 6 and 7). These results indicate that emodin is
most likely to decrease MMP-2, MMP-9, uPA and uPAR expression and
in vitro invasion as well as p38- and ERK-specific
inhibitors.
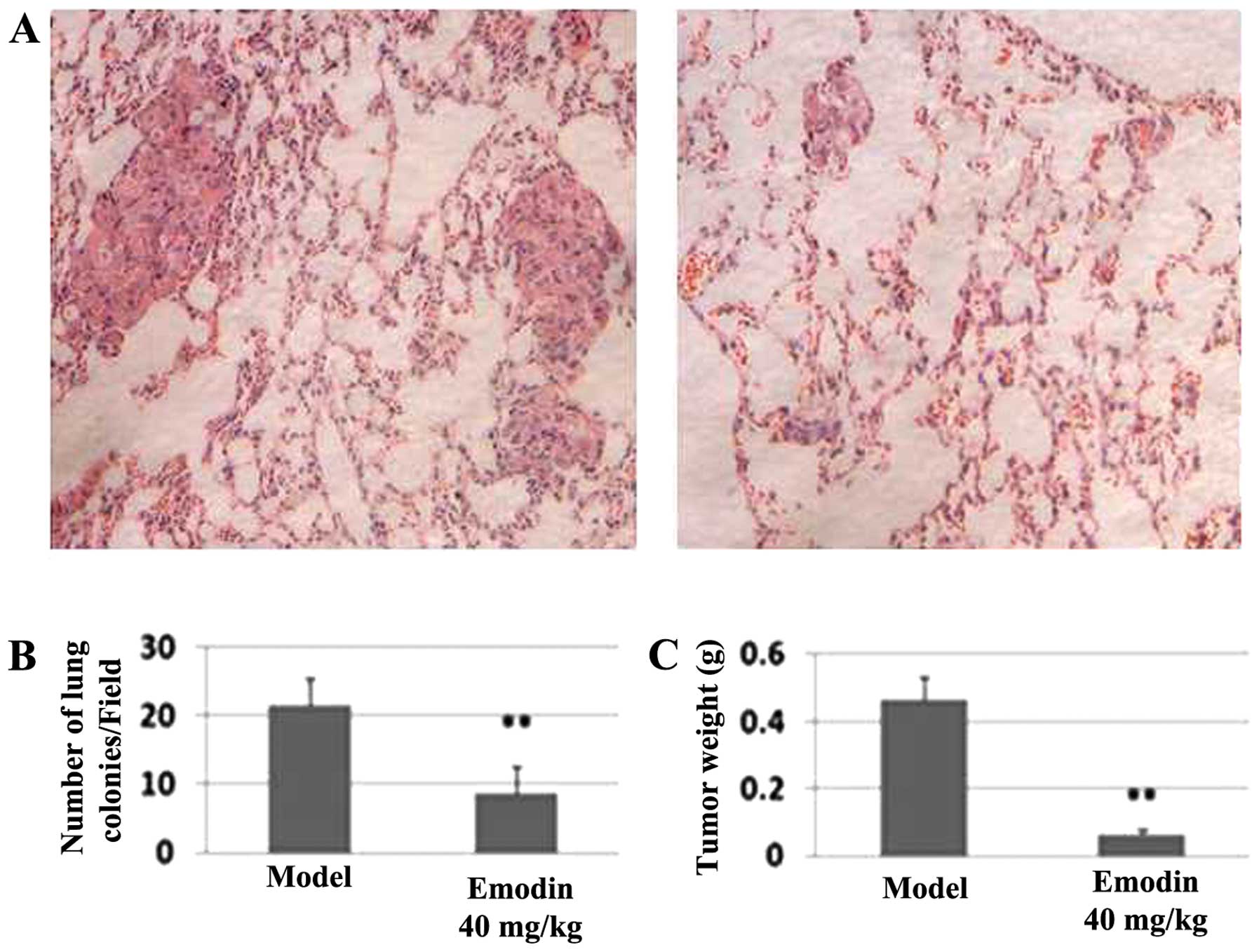
Effects of emodin on breast cancer
MDA-MB-231 cell xenografts
The ability of emodin to potently block in
vitro invasion prompted us to investigate its effectiveness to
inhibit in vivo metastasis. After athymic nude mice were
injected with MDA-MB-231 cells for one day followed by (every two
days) intraperitoneal injection of 40 mg/kg emodin and vehicle
(sham-treated) for 2 months, the metastatic nodules in lungs were
evaluated by H&E-stained sections. The average number of tumor
nodules in the sham-treated group was 21.60±3.92 while this number
was 8.6±1.51 in the emodin-treated group (P<0.01; Fig. 8B), suggesting that emodin
effectively decreases tumor cell colonization to the lung. Another
noticeable difference between the two groups was that the sizes of
these nodules were significantly larger in the sham-treated group
than those in the emodin-treated group (Fig. 8A). Moreover, the average tumor
weight was 0.46±0.07 g in the sham-treated group while the average
tumor weight was 0.10±0.05 g in the emodin-treated group (Fig. 8C), representing over 78% of
inhibition in tumor outgrowth. Together, these results suggest that
emodin can block breast tumor outgrowth and metastasis.
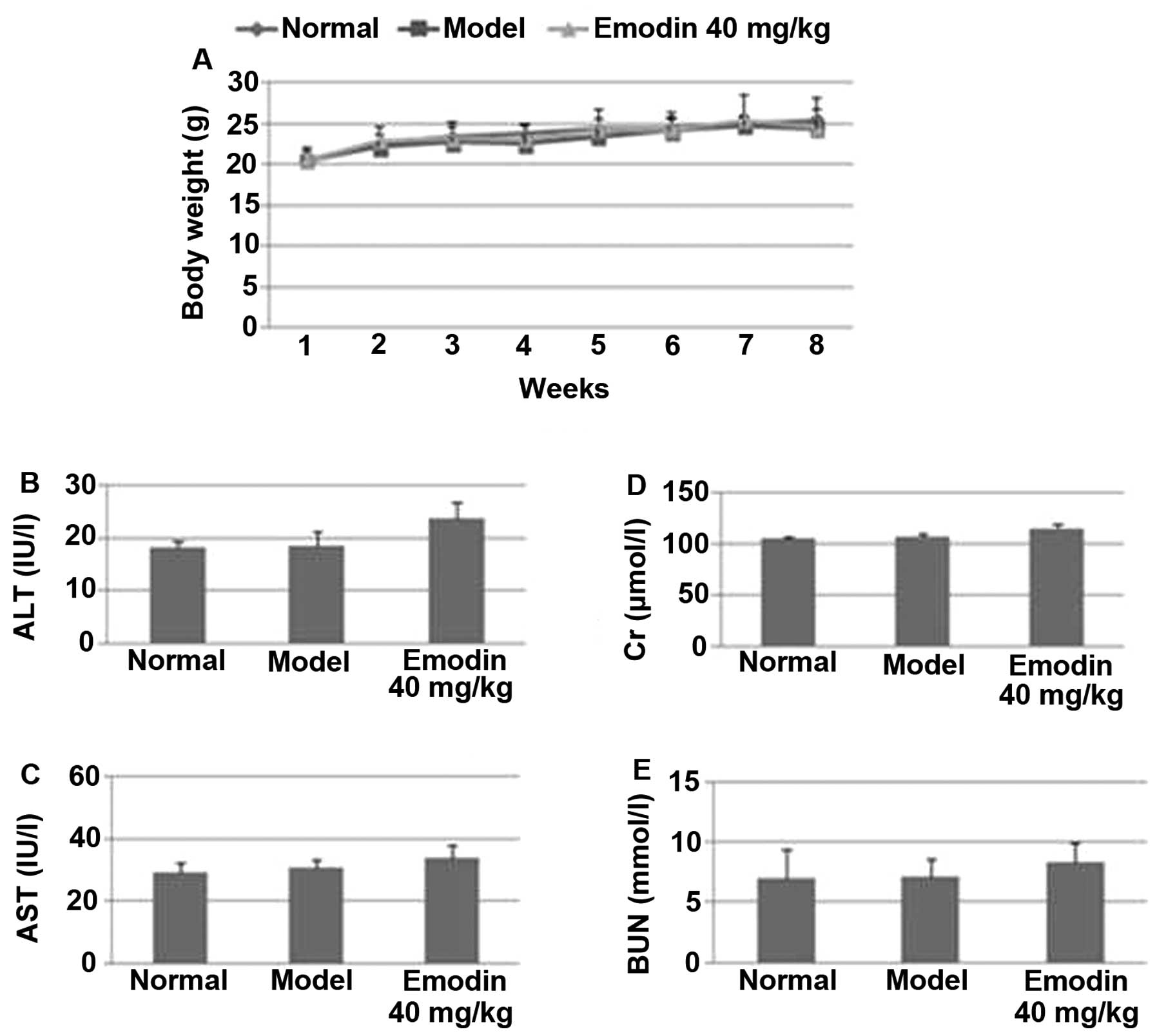
Emodin treatment does not elicit
side-effects in mice
To determine whether emodin treatment caused
side-effects, the body weights of mice were measured every week.
Among the normal, sham-treated and emodin-treated groups, there
were no significant differences in their body weights (P>0.05;
Fig. 9A). When liver and kidney
functions were further analyzed, we found that there was no
significant difference in the levels of serum GPT (Fig. 9B), GOT (Fig. 9C), Cr (Fig. 9D) and BUN (Fig. 9E) among the normal, sham- and
emodin-treated groups (P>0.05). Together, these results indicate
that emodin can be safely used in vivo for suppressing
breast tumorigenicity and metastasis.
Discussion
Throughout medical history, natural plant products
have proven to be valuable sources of novel anticancer drugs,
including vincristine, camptothecin, taxel and their derivatives
(27,28). Although these natural products have
been shown to be highly effective in clinical trials (29), some drugs such as vinblastine and
paclitaxel can produce drug resistance (30), and other drugs such as vincristine,
produce a strong cytotoxic effect (31). It has also been reported that
treatment with topotecan, a water-soluble derivative of
camptothecin, caused immunologic suppression in breast cancer
(LM2–4) xenografts (32).
Therefore, the identification of safer and more effective drugs
from natural medical plants for breast cancer metastasis is
crucial.
Emodin is a laxative ingredient derived from plants
such as Rheum, Polygonum, Buckthorn and Senna
(33). Because of its low toxicity,
it can be used for medical treatment, as well as for health care
and daily supplements such as it is used in hair care and skin care
products. It can also be added to natural pigment and laxatives
(34). Previous studies have shown
that emodin not only has anticancer activity in breast cancer
(9,10), but also did not exhibit drug
resistance and other side-effects (33). A previous study (13) reported that 40 mg/kg dosage of
emodin suppresses cancer cell growth in vivo, and we found
that emodin at this dosage inhibited both tumor outgrowth and
spontaneous lung metastasis of breast cancer (Fig. 8), without an obvious change in body
weight, liver and kidney functions in the mouse MDA-MB-231 cell
xenograft model (Fig. 9). Moreover,
emodin effectively reduced the invasion and the migration of breast
cancer cells (Figs. 2 and 3). These findings indicate that emodin may
be a potential safe and effective agent to suppress breast cancer
metastasis.
Invasion and migration of cancer cells is an
important step in cancer metastasis (35). In advanced breast cancer with poor
prognosis, proteolytic enzymes such as MMP-2, MMP-9 and uPA are
overexpressed (36), and they play
a very important role in ECM and BM degradation (37). Moreover, ECM degradation promotes
cell invasion and migration (38,39).
Previous studies have confirmed that certain natural products can
inhibit cancer metastasis by inhibition of ECM degradation through
inhibition of proteolytic enzymes (40). In the present study, we found that
emodin not only reduced the invasion and the migration of breast
cancer cells, but also significantly inhibited the expression of
MMP-2, MMP-9, uPA and uPAR, and the secretion of MMP-2 and MMP-9,
even though TIMP-1 and TIMP-2 were not obviously changed in the
breast cancer cells (Figs. 4 and
5), which indicated that emodin
inhibited the invasion and the migration of breast cancer cells
through downregulation of the expression of MMP-2, MMP-9 and uPA,
and reduction in the secretion of MMP-2 and MMP-9.
MAPK is an important signaling pathway, which is
critically involved in the process of cancer progression and
metastasis by regulating cell proliferation, differentiation and
apoptosis, angiogenesis, invasion and tumor metastasis (41,42).
Both ERK and p38 are important members of the MAPK pathways for
cancer invasion and metastasis, which are involved in the
regulation of MMP-2, MMP-9 and uPA in breast cancer (43). Previous research has found that
certain natural products can inhibit breast cancer metastasis by
downregulated the expressions of MMP-2, MMP-9 and uPA through
inhibition of the p38 signaling pathway (44). In the present study, emodin as well
as SB203580, a p38-specific inhibitor and PD98059, an ERK specific
inhibitor, decreased the activation of p38 and ERK1/2 and the
expression levels of MMP-2, MMP-9 and uPA, but the combination
treatment of emodin and SB203580 or PD98059 did not obviously alter
these inhibitory effects in MDA-MB-231 cells (Figs. 6 and 7). Together, these findings indicate that
emodin inhibits breast cancer cell invasion through the
downregulation of MMP-2, MMP-9, uPA and uPAR expression and as an
inhibitor of p38 and ERK.
Many reports suggest that, emodin, as an inhibitor,
can inhibit a variety of protein kinases such as MAPK and NF-κB and
proteolytic enzymes such as MMPs and uPA, but also inhibits cancer
cell invasion and metastasis (45).
There is previous evidence that emodin inactivated ERK1/2 in lung
cancer H1703 or A549 cells and reduced the phosphorylation of the
p38 MAPK in atherosclerosis (46).
In breast cancer, emodin suppressed HER-2/neu tyrosine kinase
activity in MDA-MB-435 cells, which overexpressed HER-2/neu and
thereby suppressing the proliferation of these cells (47). In the present study, we found that
emodin inhibited migration and invasion of MDA-MB-231 cells, and
also inhibited the expression levels of p-p38 and p-ERK1/2, MMP-2,
MMP-9 and uPA. Collectively, the present study suggests that emodin
is a non-specific protein kinase inhibitor affecting p38, ERK and
proteolytic enzymes, MMPs and uPA in inhibiting invasion of breast
cells.
In conclusion, emodin inhibited lung metastasis in
mice bearing human breast cancer MDA-MB-231 xenografts in a nude
mouse xenograft model. Emodin also inhibited the invasion and
migration of MDA-MB-231 cells by downregulating the expression of
MMP-2, MMP-9, uPA, uPAR, p-p38 and p-ERK1/2 and decreasing the
activity of p38 and ERK. Furthermore, emodin inhibited the invasion
of MDA-MB-231 cells by downregulating the expression of MMP-2,
MMP-9, uPA and uPAR as well as decreasing the activity of p38 and
ERK.
Acknowledgements
The present study was supported by the Key Program
of the National Natural Science Funds of China (no. 81330084), the
Program for Professor of Special Appointment (Eastern Scholar) at
Shanghai Institutions of Higher Learning (no. 2012-89) and the
E-Institutes of Shanghai Municipal Education Commission (no.
E03008).
References
|
1
|
Parkin DM and Fernandez LM: Use of
statistics to assess the global burden of breast cancer. Breast J.
12:S70–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Liu Q, Zhang Y, et al: Suppression
of growth, migration and invasion of highly-metastatic human breast
cancer cells by berbamine and its molecular mechanisms of action.
Mol Cancer. 8:812009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai XF, Chen Z, Li B, et al: Traditional
herbal medicine in preventing recurrence after resection of small
hepatocellular carcinoma: a multicenter randomized controlled
trial. J Integr Med. 11:90–100. 2014. View Article : Google Scholar
|
|
4
|
Jin X, Ruiz Beguerie J, Sze DM and Chan
GC: Ganoderma lucidum (Reishi mushroom) for cancer treatment.
Cochrane Database Syst Rev. 6:CD0077312012.PubMed/NCBI
|
|
5
|
Lin SZ, Wei WT, Chen H, et al: Antitumor
activity of emodin against pancreatic cancer depends on its dual
role: promotion of apoptosis and suppression of angiogenesis. PLoS
One. 7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu A, Sha L, Shen Y, Huang L, Tang X and
Lin S: Experimental study on anti-metastasis effect of emodin on
human pancreatic cancer. Zhongguo Zhong Yao Za Zhi. 36:3167–3171.
2011.(In Chinese).
|
|
7
|
He L, Bi JJ, Guo Q, Yu Y and Ye XF:
Effects of emodin extracted from Chinese herbs on proliferation of
non-small cell lung cancer and underlying mechanisms. Asian Pac J
Cancer Prev. 13:1505–1510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko JC, Su YJ, Lin ST, Jhan JY, Ciou SC,
Cheng CM and Lin YW: Suppression of ERCC1 and Rad51 expression
through ERK1/2 inactivation is essential in emodin-mediated
cytotoxicity in human non-small cell lung cancer cells. Biochem
Pharmacol. 79:655–664. 2010. View Article : Google Scholar
|
|
9
|
Guo J, Li W, Shi H, et al: Synergistic
effects of curcumin with emodin against the proliferation and
invasion of breast cancer cells through upregulation of miR-34a.
Mol Cell Biochem. 382:103–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Z, Chen G and Shi P: Effects of
emodin on the gene expression profiling of human breast carcinoma
cells. Cancer Detect Prev. 32:286–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu JQ, Bao W and Lei JC: Emodin regulates
apoptotic pathway in human liver cancer cells. Phytother Res.
27:251–257. 2013. View
Article : Google Scholar
|
|
12
|
Yu CX, Zhang XQ, Kang LD, et al: Emodin
induces apoptosis in human prostate cancer cell LNCaP. Asian J
Androl. 10:625–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cha TL, Qiu L, Chen CT, Wen Y and Hung MC:
Emodin downregulates androgen receptor and inhibits prostate cancer
cell growth. Cancer Res. 65:2287–2295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue H, Chen Y, Cai X, Zhao L, He A, Guo K
and Zheng X: The combined effect of survivin-targeted shRNA and
emodin on the proliferation and invasion of ovarian cancer cells.
Anticancer Drugs. 24:937–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yaoxian W, Hui Y, Yunyan Z, Yangqin L, Xin
G and Xiaoke W: Emodin induces apoptosis of human cervical cancer
hela cells via intrinsic mitochondrial and extrinsic death receptor
pathway. Cancer Cell Int. 13:712013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma YS, Weng SW, Lin MW, et al: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Sun Y, Li X, Li H, Chen Y, Tian Y,
Yi J and Wang J: Emodin potentiates the anticancer effect of
cisplatin on gallbladder cancer cells through the generation of
reactive oxygen species and the inhibition of survivin expression.
Oncol Rep. 26:1143–1148. 2011.PubMed/NCBI
|
|
18
|
Chen YY, Chiang SY, Lin JG, et al: Emodin,
aloe-emodin and rhein inhibit migration and invasion in human
tongue cancer SCC-4 cells through the inhibition of gene expression
of matrix metalloproteinase-9. Int J Oncol. 36:1113–1120.
2010.PubMed/NCBI
|
|
19
|
Lin SY, Lai WW, Ho CC, et al: Emodin
induces apoptosis of human tongue squamous cancer SCC-4 cells
through reactive oxygen species and mitochondria-dependent
pathways. Anticancer Res. 29:327–335. 2009.PubMed/NCBI
|
|
20
|
Li WY, Chan RY, Yu PH and Chan SW: Emodin
induces cytotoxic effect in human breast carcinoma MCF-7 cell
through modulating the expression of apoptosis-related genes. Pharm
Biol. 51:1175–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Y, Su X, Liang Y, et al: Emodin azide
methyl anthraquinone derivative triggers mitochondrial-dependent
cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol
Cancer Ther. 7:1688–1697. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eves P, Katerinaki E, Simpson C, et al:
Melanoma invasion in reconstructed human skin is influenced by skin
cells - investigation of the role of proteolytic enzymes. Clin Exp
Metastasis. 20:685–700. 2003. View Article : Google Scholar
|
|
24
|
Ahmad A, Wang Z, Kong D, et al: FoxM1
down-regulation leads to inhibition of proliferation, migration and
invasion of breast cancer cells through the modulation of
extra-cellular matrix degrading factors. Breast Cancer Res Treat.
122:337–346. 2010. View Article : Google Scholar
|
|
25
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38 signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghosh S, Bishayee K and Khuda-Bukhsh AR:
Oleanolic acid isolated from ethanolic extract of Phytolacca
decandra induces apoptosis in A375 skin melanoma cells: drug-DNA
interaction and signaling cascade. J Integr Med. 12:102–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mans DR, Rocha AB and Schwartsmann G:
Anti-cancer drug discovery and development in Brazil: targeted
plant collection as a rational strategy to acquire candidate
anti-cancer compounds. Oncologist. 5:185–198. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamagishi T, Sahni S, Sharp DM, Arvind A,
Jansson PJ and Richardson DR: P-glycoprotein mediates drug
resistance via a novel mechanism involving lysosomal sequestration.
J Biol Chem. 288:31761–31771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stanley A and Akbarsha MA: Ultrastructural
changes in the Leydig cell on treatment with vincristine. Cytobios.
79:51–58. 1994.PubMed/NCBI
|
|
32
|
Francia G, Shaked Y, Hashimoto K, et al:
Low-dose metronomic oral dosing of a prodrug of gemcitabine
(LY2334737) causes antitumor effects in the absence of inhibition
of systemic vasculogenesis. Mol Cancer Ther. 1:680–689. 2012.
View Article : Google Scholar
|
|
33
|
Srinivas G, Babykutty S, Sathiadevan PP
and Srinivas P: Molecular mechanism of emodin action: transition
from laxative ingredient to an antitumor agent. Med Res Rev.
27:591–608. 2007. View Article : Google Scholar
|
|
34
|
NTP Toxicology and Carcinogenesis Studies
of EMODIN (CAS NO. 518-82-1) Feed Studies in F344/N Rats and B6C3F1
Mice. National Toxicology Program. Natl Toxicol Program Tech Rep
Ser. 493:271–278. 2001.
|
|
35
|
Price JT and Thompson EW: Mechanisms of
tumor invasion and metastasis: emerging targets for therapy. Expert
Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang XX, Xu DP, Wang LQ and Gao LY:
Expression of MMP-2 and MMP-9 in the tissues of breast carcinoma
and its relationship with tumor invasion and metastasis. Chin J
Clin Rehabil. 8:2190–2192. 2004.
|
|
37
|
Stamenkovic I: Extracellular matrix
remodeling: the role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dung TD, Feng CC, Kuo WW, et al:
Suppression of plasminogen activators and the MMP-2/-9 pathway by a
Zanthoxylum avicennae extract to inhibit the HA22T human
hepatocellular carcinoma cell migration and invasion effects in
vitro and in vivo via phosphatase 2A activation. Biosci Biotechnol
Biochem. 77:1814–1821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer - roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang XF, Du J, Zang TL, et al: Chinese
herbal formula PC-SPESII inhibits breast cancer metastasis in vivo
and in vitro. Evid Based Complement Alternat Med.
2013:8943862013.
|
|
41
|
Yao Q, Luo JR, Chen JH, Zhang JL, Yuan SF,
Ling R and Wang L: Expression and activation of MAPK pathway
signaling molecules in human breast cancer cell lines. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 20:328–330. 2004.(In Chinese).
PubMed/NCBI
|
|
42
|
Han YC, Zeng XX, Wang R, Zhao Y, Li BL and
Song M: Correlation of p38 mitogen-activated protein kinase signal
transduction pathway to uPA expression in breast cancer. Ai Zheng.
26:48–53. 2007.(In Chinese). PubMed/NCBI
|
|
43
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: a family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du J, Wang XF, Zhou QM, et al: Evodiamine
induces apoptosis and inhibits metastasis in MDA-MB-231 human
breast cancer cells in vitro and in vivo. Oncol Rep. 30:685–694.
2013.PubMed/NCBI
|
|
45
|
Tan W, Lu J, Huang M, Li Y, et al:
Anti-cancer natural products isolated from Chinese medicinal herbs.
Chin Med. 6:272011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ueno N, Kiyokawa N and Hung M: Growth
suppression of low HER-2/neu-expressing breast cancer cell line
MDA-MB-435 by tyrosine kinase inhibitor emodin. Oncol Rep.
3:509–511. 1996.PubMed/NCBI
|
|
47
|
Shrimali D, Shanmugam MK, Kumar AP, et al:
Targeted abrogation of diverse signal transduction cascades by
emodin for the treatment of inflammatory disorders and cancer.
Cancer Lett. 341:139–149. 2013. View Article : Google Scholar : PubMed/NCBI
|