Introduction
Change to people's lifestyle and dietary habits have
led to an increase in the incidence of colon cancer (1–3).
Although there have been advances in the study of this type of
cancer, colon cancer is a common gastrointestinal malignancy with a
high mortality rate (4), owing to
metastasis. Therefore, the main challenge is identification of
methods to inhibit the metastasis of colon cancer effectively in
the clinic. Metastasis occurs due to the interaction of multiple
genes and is a complex system (5).
However, the exact mechanism underlying metastasis of the disease
remains to be elucidated.
Tumor invasion and metastasis are closely associated
with epithelial-mesenchymal transition (EMT). EMT refers to
epithelial cells under physiological and pathological conditions
that are specific to mesenchymal transition (6). EMT has been associated with the
initial stage of tumor metastasis, whereby tumor cells have lost
the characteristics of epithelial cells and cell polarity, and
attained the characteristics of mesothelial and invade adjacent
tissues (7). This process is
associated with change at the molecular level and the occurrence of
cell morphology (8). In the
evolution process of tumor growth, EMT includes alteration of cell
polarity, reconstruction of the cytoskeleton, loss of intercellular
adhesion and destruction of the tumor basement membrane and
extracellular matrix between the cells (9), and mesenchymal phenotypes, such as
high migration, invasion, resistance apoptosis and degradation of
extracellular matrix. Therefore, EMT provides optimal conditions
for invasion and metastasis, and tumor cell growth (10,11).
Previous findings showed that the EMT phenomenon is prevalent in
tumor invasion and metastasis, such as in situ colon,
breast, lung, and liver cancer, and plays an important role in
tumor invasion and metastasis (12–14).
Chemokine receptor 4 (CXCR4) is a member of the
super-family of the seven-transmembrane G-protein coupled receptors
and is the only receptor of CXCL12. CXCR4 is involved in a variety
of physiological and pathological processes. The CXCL12/CXCR4
biological axis structure participates in the infiltration of
inflammatory cells and lymphocytes, as well as migration and homing
(15,16). It also plays an important role in
mediating tumor invasion and metastasis (17–20).
Thus, CXCR4 is a common chemokine receptor in tumor cells, and its
expression is increased significantly in gastric, lung, and breast
cancer, as well as soft tissue sarcoma tumor cells.
In recent years, significant progress has been made
in the study of the anti-tumor effects of traditional Chinese
medicine, particularly with regard to natural plant-derived
anticancer drugs that have become a global hot spot. Curcumin is a
plant polyphenol that is extracted from the zingiberaceae plant,
Curcuma longa root turmeric. Curcumin has various effects
including antioxidant, anti-inflammatory, anti-atherosclerotic, and
anti-aging, and also eliminates free radicals (21). Numerous studies have been conducted
on the anti-tumor effects of curcumin and its mechanism worldwide.
The findings have shown that curcumin obviously inhibits tumor
invasion and metastasis in different tumor tissues (22,23).
At present, some studies have reported that curcumin
inhibits EMT in tumors (24). The
Wnt signaling pathway is a conservative EMT-related signaling
pathway that plays an important role in the development of a
variety of tumors. The β-catenin is the hub of the molecule in the
Wnt signaling pathway, which mediates the membrane and facilitates
the transfer of molecules from the cytoplasm into the nucleus in
the Wnt pathway (25,26). Curcumin also reduced the level of
β-catenin gene expression significantly; thus, it has anti-tumor
effects through the inhibition of the Wnt signaling pathway
(18,27,28).
Therefore, the mechanism of action of curcumin with regard to tumor
inhibition remains to be determined. Using gene expression
profiles, we analyzed the changes of tumor cell expression profiles
of curcumin-treated cells, and identified inhibitors of the Wnt
pathway naked cuticle homolog 2 (NKD2) (29). NKD2, as the Wnt signaling pathway
regulation of gene suppression, significantly delayed the mitosis
of HeLa cells (30). Thus, curcumin
may inhibit the Wnt signaling pathway by regulation of the
expression of NKD2. Previous findings showed that curcumin can also
reduce the expression of CXCR4 in tumor cells (31,32).
Thus, according to the preliminary experiment, the mechanism of
action of curcumin in colon cancer cells is likely to inhibit the
Wnt signaling pathway by affecting NKD2 gene expression, EMT and
the expression of CXCR4 in tumor cells and eventually inhibiting
tumor invasion and metastasis.
Materials and methods
Reagents
Antibodies purchased for the present study included:
axin and TCF4 (Cell Signaling Technology, Boston, MA, USA),
β-catenin (Epitomics, San Francisco, CA, USA), NKD2 (Novus
International, St. Charles, MO, USA), CXCR4 antibody (Thermo Fisher
Scientific, Waltham, MA, USA), and β-actin (BD Biosciences, New
York, NY, USA). Curcumin was purchased from Sigma (Beijing, China),
and NKD2 small-interfering RNA (siRNA) from Shanghai GenePharma
Co., Ltd. (Shanghai, China). The following two pairs of siRNA were
designed: NKD2-homo-962 sense, 5′-CAGAUACACAUGCCGUACATT-3′ and
antisense, 5′-UGUACGGCAUGUGUAUCUGTT-3′; NKD2-homo-480 sense,
5′-CACGCUCUAUGACUUUGACTT-3′ and antisense,
5′-GUCAAAGUCAUAGAGCGUGTT-3′. NKD2 and CXCR4 primers were purchased
from Biosune Biotechnology Co., Ltd. (Shanghai, China). NKD2
primers used were: 5′-ATGCCTCGGTCAACCACTCC-3′and
5′-TCTGCCAGTTCACCCTCCATC-3′ and the length of the product was 151
bp. CXCR4 primers used were: 5′-CCGTGGCAAACTGGTACTTT-3′ and 5′-GAC
GCCAACATAGACCACCT-3′ and the length of the product was 188 bp.
The CXCR4 expression plasmid (CXCR4 NM_001008540)
was purchased from Shanghai GenePharma Co., Ltd. The Wnt pathway
activator (WAY 262611) was purchased from Abcam (Shanghai,
China).
SW620 human colon cancer cells were provided by the
Zhejiang Provincial Key Laboratory of Gastroenterology (Zhejiang,
China). The instruments and equipment employed were provided by the
Zhejiang Key Laboratory of Biotherapy (Zhejiang, China).
Cell cultures and transfection
SW620 human colon cancer cells were routinely
cultivated in RPMI-1640 medium containing 10% FBS, at 37°C with 5%
CO2. The cells were passaged at 80% confluency, using 1
mmol/l EDTA with 0.025% trypsin for 3–5 min, and then sub-cultured
at a ratio of 1:3–1:5. Cells at the logarithmic growth phase were
collected for experiments. Once the cell density had increased to
30–50%, Lipofectamine™ 2000 liposome transfection kit (Invitrogen
Life Technologies, Carlsbad, CA, USA) was used to transfect the
plasmid into the cells according to the manufacturer's
instructions. Following cultivation for 2 days, the original
culture medium was discarded and screened using RPMI-1640 culture
medium.
MTS assay
SW620 cells in the logarithmic growth phase were
collected and the cell concentration was adjusted to
5×104/ml. Subsequently, 200 µl of the
abovementioned cell suspension was added into each well of several
96-well plates. When the cells adhered to the wall, curcumin (5–40
µmol/l) was added to the cells and these were cultured for
24 h. Five parallel walls were established for each drug
concentration. The supernatant was aspirated and 100 µl MTS
was added into each well. A multi-mode microplate reader (Infinite
M200; Tecan, Geneva, Switzerland) was used to determine the
absorbance, with a detection wavelength of 490 nm. The experiments
were repeated 3 times. The cell viability was calculated using the
formula: viability (%) = [(treate d-blank)/(control-blank)] × 100%.
The experiments were performed in triplicate.
Western blot analysis
For biochemical analysis, the cells were washed with
ice-cold phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Jiangsu, China) and lysed in
radioimmunoprecipitation assay lysis buffer [50 mM Tris, pH 7.4;
150 mM NaCl; 1% Triton X-100; 1% sodium deoxycho-late; 0.1% sodium
dodecyl sulfate (SDS); Beyotime Institute of Biotechnology)]. The
lysates were kept on ice for 30 min, followed by centrifugation at
12,000 × g for 25 min at 4°C. The clear lysate was then collected
and β-catenin, axin, TCF4, E-cadherin, vimentin, NKD2, CXCR4 and
β-actin proteins were separated by 12% SDS-polyacrylamide gel
electrophoresis (30–50 µg protein/lane) and transferred to a
polyvinylidine fluoride membrane (Beyotime Institute of
Biotechnology). The membranes were incubated in 5% milk for 1.5 h,
and then with β-catenin (1:1000), axin (1:1000), TCF4 (1:2000),
E-cadherin (1:2000), vimentin (1:1000), NKD2 (1:1000), CXCR4
(1:1500) and β-actin (1:2000) antibodies diluted in non-fat milk
overnight. The membranes were then washed with Tris-buffered saline
with Tween-20 (Beyotime Institute of Biotechnology) and incubated
with the HRP-conjugated secondary antibodies for 2 h.
Immunoreactive proteins were visualized using a BeyoECL Plus kit
(Beyotime Institute of Biotechnology).
Reverse transcription-qPCR (RT-qPCR)
analysis
The cells were washed with ice-cold PBS and total
RNA was extracted from the SW620 human colon cancer cells using
TRIzol reagent (Invitrogen-Technologies, Carlsbad, CA, USA) and
quantified by UV spectrophotometer (Lengguang, Shanghai, China). In
addition, the purity and RNA concentration were measured by the UV
spectrophoto meter. cDNA was obtained by reverse transcription. The
PCR conditions were: 10 min at 25°C, 2 h at 37°C, 5 min at 85°C and
maintained at 4°C. cDNA was used as a template for PCR
amplification of the target genes and GAPDH was used as the
standard control. Amplification conditions were: Denaturation for 2
min at 94°C, 30 sec at 94°C, 30 sec at 52°C, 5 sec at 72°C and,
following 40 cycles, a total extension of 4 min at 72°C (33).
Statistical analysis
Statistical analysis was conducted using SPSS,
version 18.0 (SPSS, Inc., Chicago, IL, USA). Each experiment was
performed ≥3 times. Data are indicated as mean values ± standard
deviation and any differences were analyzed using a Student's
t-test. P<0.05 was considered to indicate statistically
significant results.
Results
Effect of curcumin on the viability of
SW620 cells
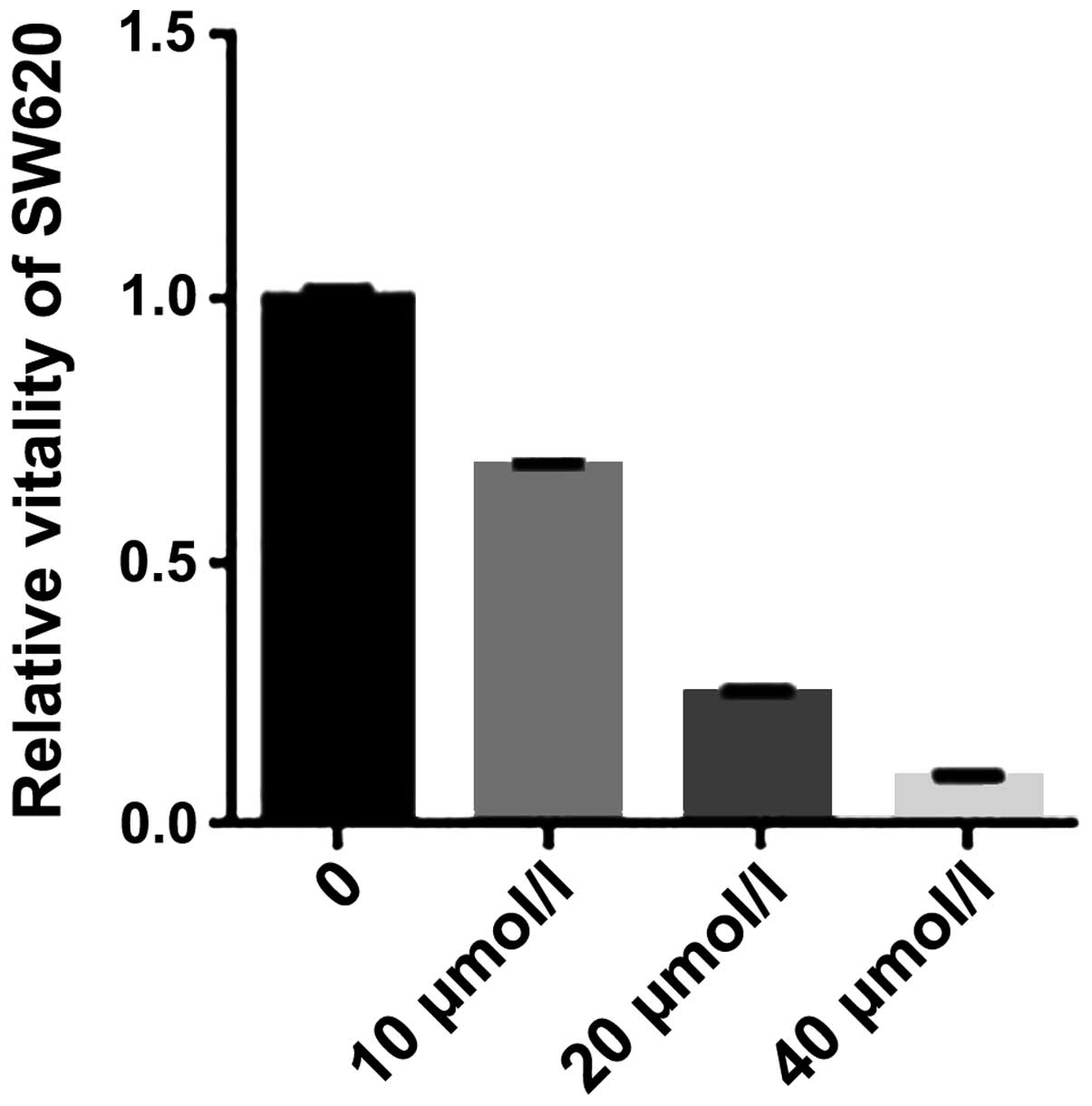
We initially investigated the effect of curcumin on
the proliferation of SW620 cells. SW620 cells were treated for 24 h
with graded concentrations of curcumin (0–40 µmol/l) and the
cell viability was measured using an MTS assay. The results showed
the cell viability s was inhibited by curcumin (Fig. 1). An increase in the concentration
of curcumin led to a significant change in the rate of inhibition.
The results suggested that curcumin effectively inhibited SW620
cell viability.
Curcumin inhibits Wnt signaling and
expression of markers of EMT in SW620 cells
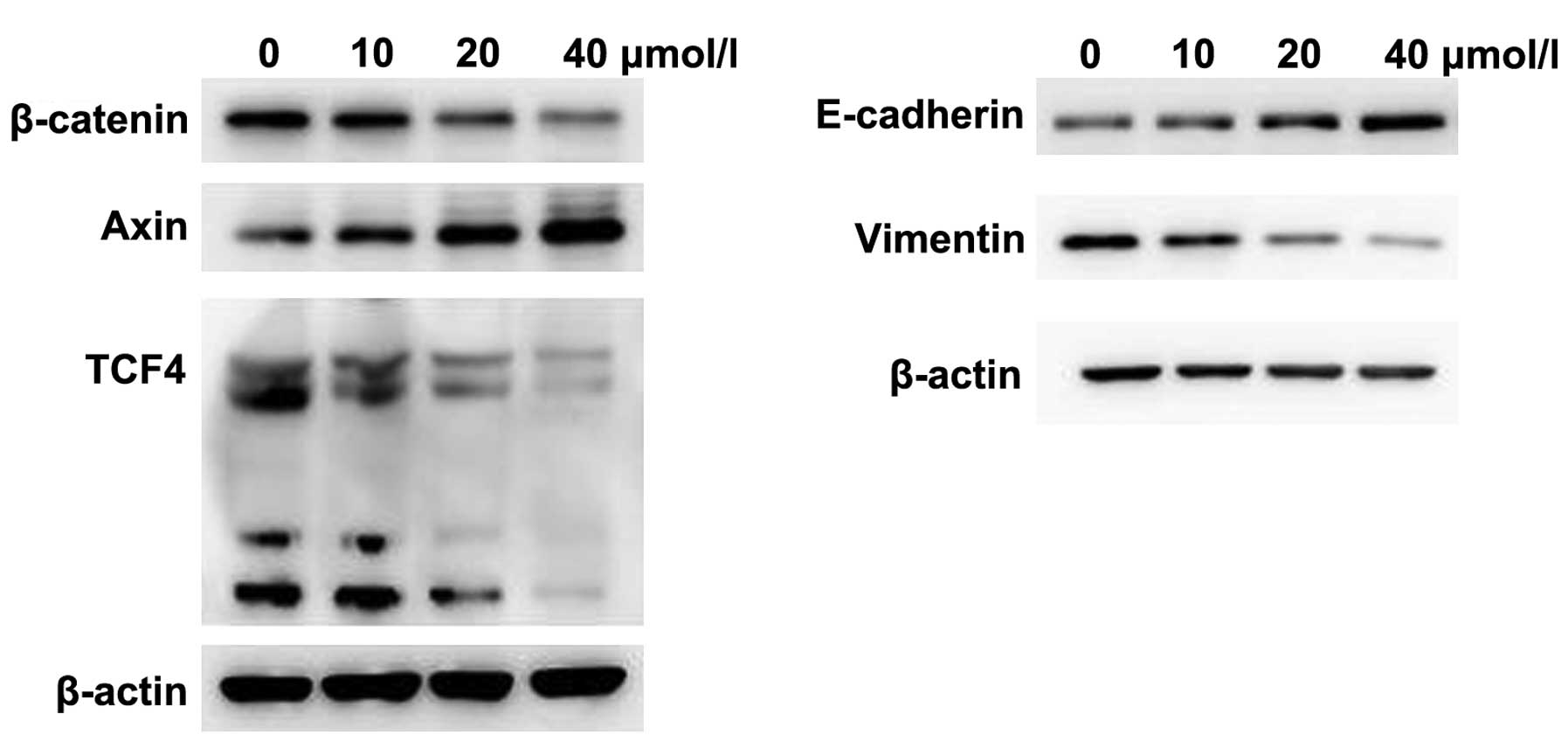
The phenotypic alterations occurring suggested that
SW620 cells had undergone EMT. Thus, there were two expressions of
specific EMT markers, including the epithelial marker E-cadherin
and mesenchymal marker vimentin (34). Due to the conservative EMT-related
signaling pathway, the β-catenin, axin, and
TCF4 genes, which were associated with the Wnt signaling
pathway, were selected. The β-catenin is the hub of the molecule in
the Wnt signaling pathway and axin a negative regulator of the Wnt
signaling pathway (35,36). TCF4 is a Wnt pathway transcription
factor and is highly expressed in colorectal cancer. To determine
the effect of curcumin on the Wnt signaling pathway and EMT, we
used different concentrations of curcumin to treat SW620 cells for
24 h, and measured the protein expression using western blot
analysis. We found that β-catenin, TCF4 and vimentin protein
expression were significantly reduced, while the axin and
E-cadherin protein expression were increased in the curcumin group
(Fig. 2). By increasing the
concentration of curcumin, the protein expression was more
significantly altered. Thus, curcumin is capable of inhibiting the
Wnt signaling pathway and EMT in SW620 cells.
Curcumin increases the expression of NKD2
and inhibits the expression of CXCR4 in SW620 cells
NKD2 acts as an inhibitor of the Wnt signaling
pathway, playing an important role in tumor of EMT (37). Chemokine receptor CXCR4 acts as an
α-chemokine receptor specific for stromal-derived-factor-1. CXCR4
is highly expressed in various tumors and promotes tumor growth and
metastasis (38). Therefore, to
clarify whether curcumin significantly affected the genes, we
carried out a biological analysis. We used different concentrations
of curcumin to treat SW620 cells for 24 h and measured the protein
expression of the genes using western blot analysis and mRNA
expression RT-qPCR analysis. The NKD2 protein expression was
significantly increased, while the CXCR4 protein expression was
reduced in the curcumin group (Fig.
3A). The CXCR4 mRNA expression was reduced in the curcumin
group (Fig. 3B) while the NKD2 mRNA
expression was significantly increased in the curcumin group
(Fig. 3C). Thus, curcumin increases
the expression of NKD2 in the WNT signaling pathway and inhibits
that of CXCR4 in the SW620 cells.
NKD2 siRNA transfection and Wnt pathway
activator promotes the Wnt signaling pathway
We demonstrated whether the NKD2 siRNA and Wnt
pathway activator affects the Wnt signaling pathway. SW620 cells
were transfected with NKD2 siRNA for 48 h and the Wnt pathway
activator for 24 h, respectively, and the Wnt signaling pathway
protein expression levels were detected. Western blot analysis
(Fig. 4A–B) revealed that the
protein expression of β-catenin and TCF4 was significantly
upregulated in the NKD2 siRNA and Wnt pathway activator group
compared to the control group. The results suggested that NKD2
siRNA can promote the Wnt signaling pathway by silencing the
NKD2 gene.
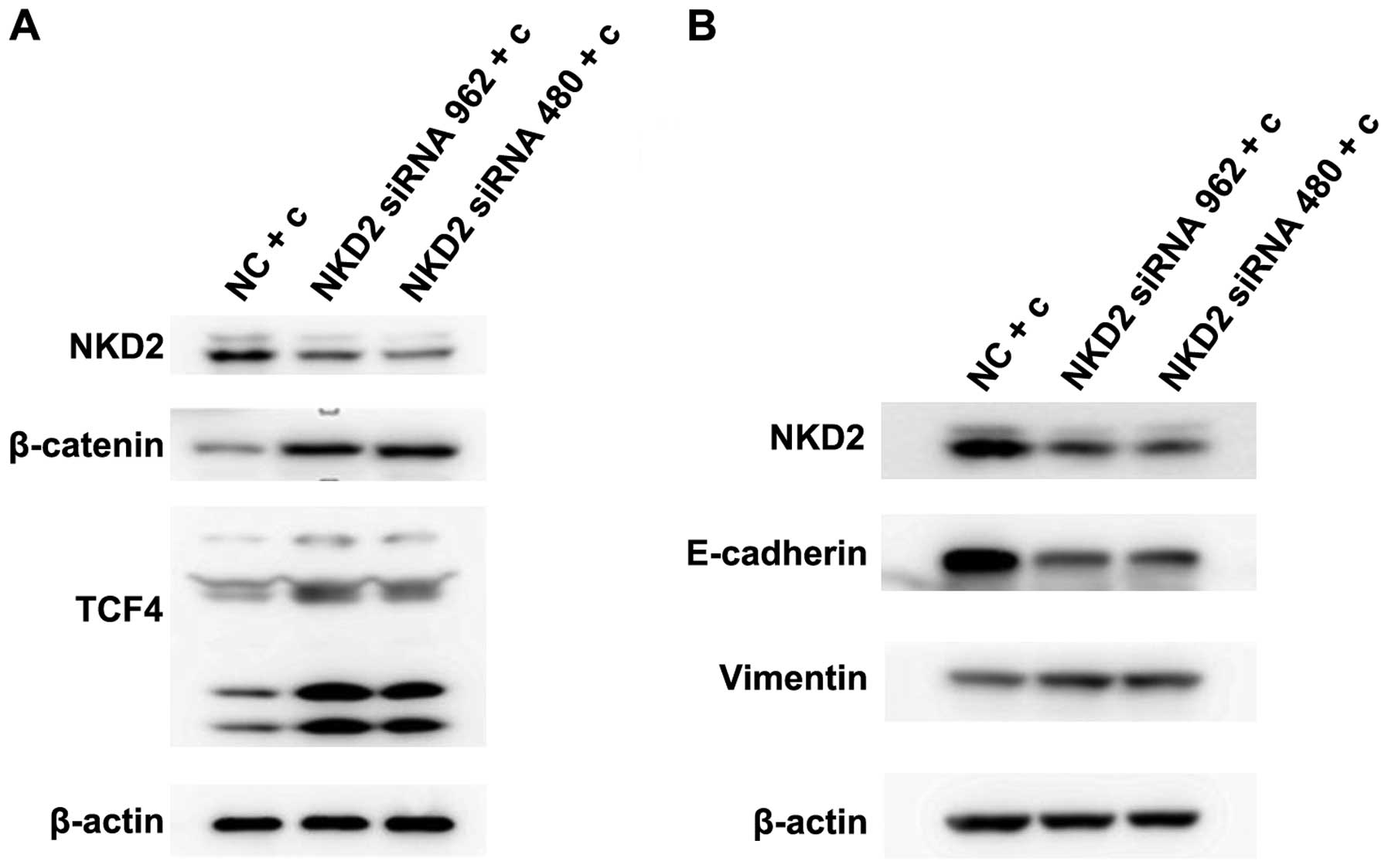
NKD2 siRNA transfection reverses curcumin
inhibition of the Wnt signaling pathway and EMT in the SW620
cells
Curcumin has been previously found to inhibit Wnt
signaling pathways and EMT in 95D cells (39). Therefore, we examined whether
curcumin inhibit this pathway by influencing the expression of
NKD2. Initially, we transfected the SW620 cells for 48 h with NKD2
siRNA and then treated the cells with curcumin (10 µmol/l)
for 24 h. β-catenin and TCF4 protein expression was significantly
increased (Fig. 5A). The E-cadherin
protein expression was reduced while the vimentin protein
expression was increased in the NKD2 siRNA transfection group
(Fig. 5B). Thus, previous results
(39) together with those of the
present study demonstrated that curcumin inhibits the Wnt signaling
pathway and EMT by increasing the expression of NKD2.
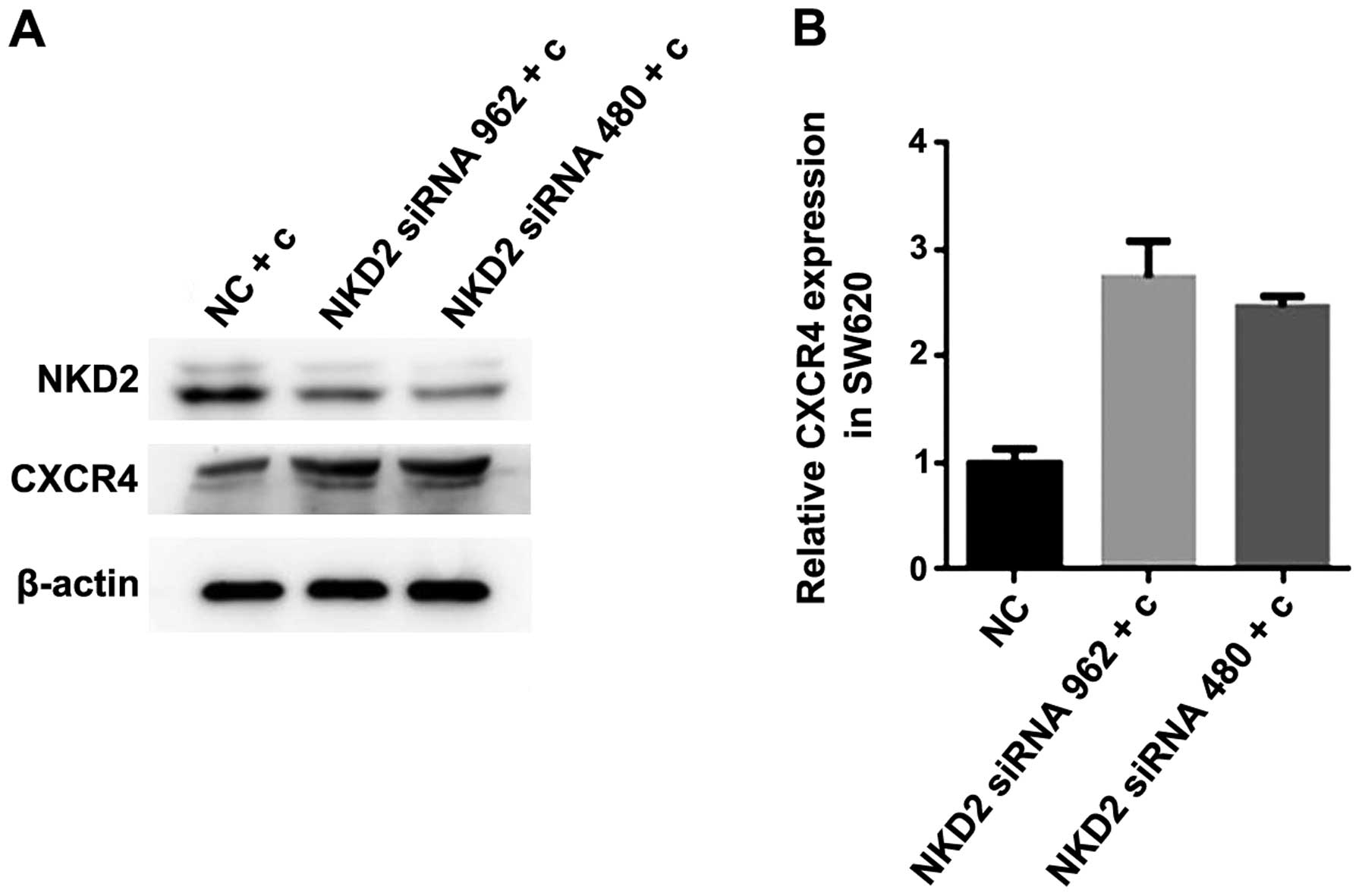
NKD2 siRNA transfection increases the
expression of CXCR4 in the SW620 curcumin-treated cells
CXCR4 is closely associated with EMT and the two
induce and promote each other. To confirm whether curcumin
inhibited the expression of CXCR4 by influencing the expression of
NDK2, we transfected NKD2 siRNA for 48 h, followed by treatment
with curcumin (10 µmol/l) for 24 h in SW620 cells. We
measured the protein expression using western blot analysis and the
mRNA expression by RT-qPCR analysis. The CXCR4 protein expression
was significantly increased in the NKD2 siRNA transfection group
(Fig. 6A). The CXCR4 mRNA
expression was increased in the NKD2 siRNA transfection group
(Fig. 6B). Thus, our results
demonstrated that curcumin inhibited the expression of CXCR4 by
increasing the expression of NDK2 in the SW620 cells.
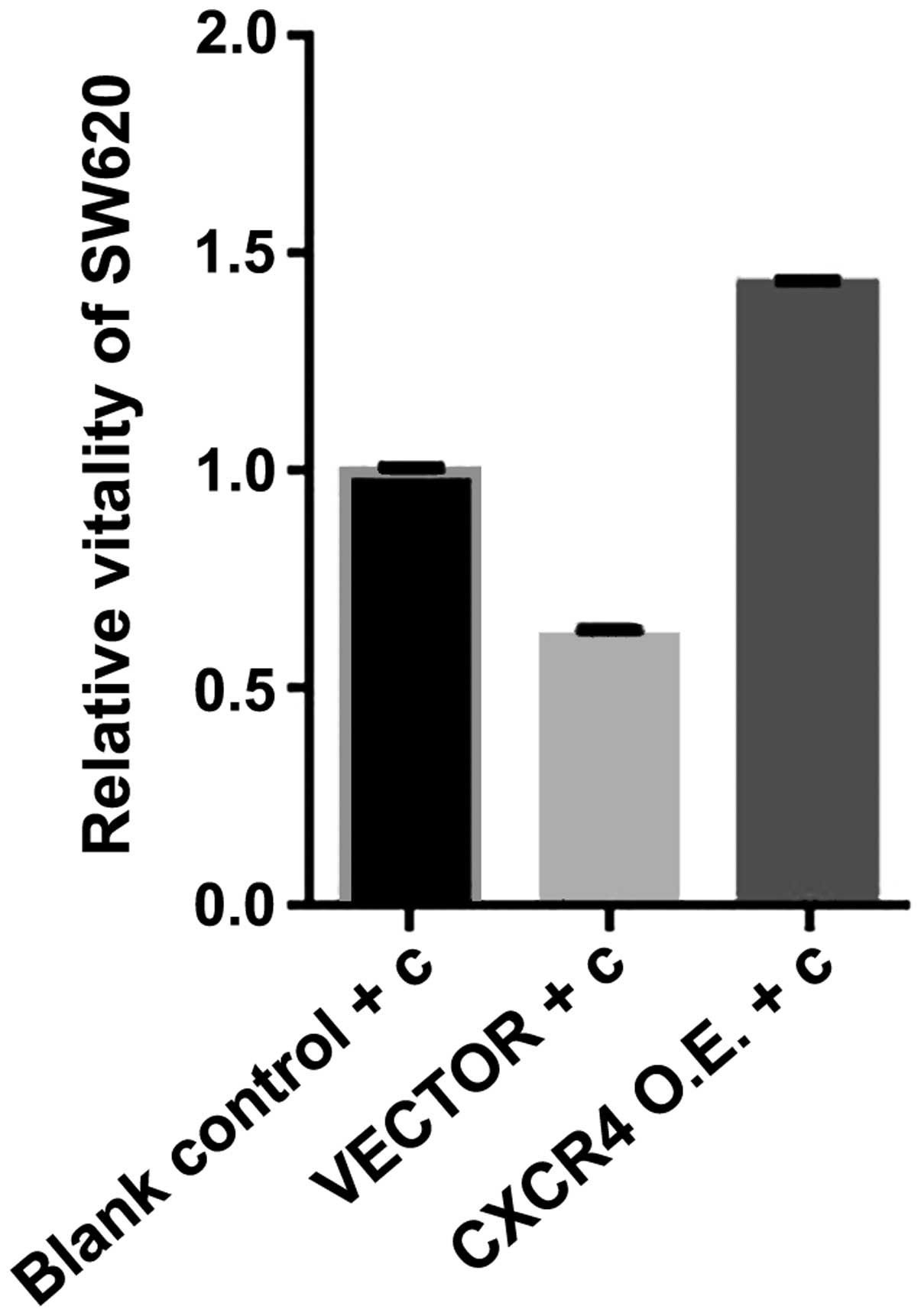
CXCR4 expression plasmid transfection
increases the viability of SW620 curcumin-treated cells
As a chemokine receptor, CXCR4 reflects the invasion
and metastasis of tumor cells directly. Therefore, we investigated
whether CXCR4 expression plasmid affected the viability of SW620
cells treated with curcumin in vitro. We transfected CXCR4
expression plasmid for 72 h, followed by treatment with the SW620
cells for 24 h with curcumin (10 µmol/l). Cell viability was
measured using an MTS assay. As shown in Fig. 7, cell viability was increased by
CXCR4 expression plasmid. The results suggested that CXCR4
expression plasmid effectively increased cell viability. Thus,
curcumin inhibits the viability of SW620 in vitro by
reducing CXCR4 gene expression.
CXCR4 expression plasmid transfection
reverses curcumin inhibition of EMT and promotes the expression of
CXCR4 in the SW620 cells
In the process of tumor invasion and metastasis,
CXCR4 is highly expressed in metastatic tissues, and tumor invasion
and metastasis are closely associatied with EMT. Previous
experiments (40) confirmed that
curcumin is capable of inhibiting EMT in HCT116 cells. Thus, we
transfected CXCR4 expression plasmid to observe whether it is able
to reverse curcumin inhibition of EMT. We transfected CXCR4
expression plasmid for 72 h in SW620 cells, followed by treatment
with curcumin (10 µmol/l) for 24 h. We measured the protein
expression of EMT and CXCR4 expression using western blot analysis
and RT-qPCR analysis. E-cadherin protein expression was reduced
while the protein expression of vimentin and CXCR4 was increased in
the CXCR4 expression plasmid transfection group (Fig. 8A). The CXCR4 mRNA expression was
significantly increased in the CXCR4 expression plasmid
transfection group (Fig. 8B). Thus,
CXCR4 expression plasmid is capable of inducing EMT and promote the
expression of CXCR4 in SW620 cells.
Discussion
Colon cancer, the second most frequent cancer, is a
common gastrointestinal malignancy that occurs in the junction of
the rectum and sigmoid colon. The highest incidence of colon cancer
occurs in middle-aged and elderly individuals (41). Patients with advanced colon cancer
are prone to distant metastases, particularly in the liver, lungs,
and peritoneal metastasis(40). The
present study indicate that EMT plays a key role in tumor
metastasis. EMT refers to epithelial cells that acquire
mesenchymal, fibroblast-like phenotypes with reduced cell-to-cell
adhesion, and loss of cell polarity, with increased migration and
invasiveness (42). Furthermore,
the Wnt signaling pathway, one of the important pathways in EMT, is
important in embryogenesis and human diseases including various
types of cancer. Wnt signals can be transduced to the canonical Wnt
pathway for cell-fate determination or to the non-canonical Wnt
pathway for the regulation of tissue polarity and cell movement
(43). In a previous study, Deng
et al reported that celecoxib inhibited the Wnt signaling
pathway in colon cancer (44).
Curcumin is an antioxidant polyphenol derived from
several curcuma species, commonly known as turmeric (Curcuma
longa), which has been shown to inhibit carcinogen activation
and angiogenesis, modulate cell survival and apoptosis, with
anti-invasive and anti-metastatic effects on lung, breast and
prostate cancer (45,46). Curcumin induces anti-migratory
activity, which functions via the Wnt signaling pathway (28,47).
In the current study, different concentrations of curcumin were
used for 24 h in SW620 cells. We found that curcumin has a strong
inhibitory effect on the Wnt signaling pathway and EMT. However,
these anti-metastatic effects remain to be elucidated.
Nevertheless, to the best of our knowledge, the present is the
first study to identify that curcumin upregulates the expression of
NKD2. NKD2, a secreted-type Wnt signaling inhibitor (48), suppressed the Wnt signaling pathway
significantly. Hu et al reported that myristoylated NKD2
antagonizes the Wnt pathway by degrading dishevelled-1 at the
plasma membrane (49). Therefore,
it can activate the Wnt signaling pathway following transfection of
NKD2 siRNA into SW620 cells. We found curcumin inhibited the Wnt
pathway and EMT by increasing the expression of NKD2.
The chemokine superfamily plays multifaceted roles
in the regulation of tumor development and progression. Chemokines
induce leukocyte infiltration to tumors and regulate immune
functions, direct the homing of tumor cells to specific metastatic
sites, and regulate antigenicity at the tumor milieu (50). Thus, CXCR4 was considered an
important factor in tumor cell metastasis and is a potential
therapeutic target for the treatment of cancer. Recent findings
have indicated that CXCR4 expression is closely associated with the
Wnt signaling pathway (51,52). Hu et al identified that CXCR4
promoted CRC progression and EMT was regulated by the Wnt/β-catenin
signaling pathway in colorectal cancer (33). Additionally, Wang et al
identified that CXCR4 is crucial in the metastasis of human ovarian
cancer possibly by modulating the Wnt/β-catenin pathway (53). To the best of our knowledge, this is
the first study to demonstrate that the anti-metastatic effects of
curcumin are associated with NKD2, the Wnt signaling pathway and
CXCR4 in colon cancer cells. Curcumin can also inhibit the Wnt
signaling pathway through the upregulation of NKD2 gene
expression, suppression of EMT and the expression of CXCR4, and
inhibition of tumor invasion and metastasis. Results of the present
study therefore offer a new perspective on the role of curcumin in
preventing the progression of cancer.
Acknowledgments
The present study was supported by a grant from the
State Key Laboratory of Molecular Oncology, the Chinese Academic of
Medical Sciences, Cancer Institute (Beijing, China). We would like
to thank the Laboratory of Cancer Epigenetics, Biomedical Research
Center, Sir Runrun Shaw Hospital, School of Medicine, Zhejiang
University (Hangzhou, China) and Zhejiang Provincial Key Laboratory
of Gastroenterology (Zhejiang, China) for providing the
experimental facilities, instruments and guidance.
References
|
1
|
Noreen F, Röösli M, Gaj P, Pietrzak J,
Weis S, Urfer P, Regula J, Schär P and Truninger K: Modulation of
age- and cancer-associated DNA methylation change in the healthy
colon by aspirin and lifestyle. J Natl Cancer Inst. 106:1062014.
View Article : Google Scholar
|
|
2
|
Pelser C, Arem H, Pfeiffer RM, Elena JW,
Alfano CM, Hollenbeck AR and Park Y: Prediagnostic lifestyle
factors and survival after colon and rectal cancer diagnosis in the
National Institutes of Health (NIH)-AARP Diet and Health Study.
Cancer. 120:1540–1547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winkels RM, Heine-Bröring RC, van Zutphen
M, van Harten-Gerritsen S, Kok DE, van Duijnhoven FJ and Kampman E:
The COLON study: Colorectal cancer: Longitudinal, Observational
study on Nutritional and lifestyle factors that may influence
colorectal tumour recurrence, survival and quality of life. BMC
Cancer. 14:3742014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sunkara V and Hébert JR: The colorectal
cancer mortality-to- incidence ratio as an indicator of global
cancer screening and care. Cancer. 121:1563–1569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang W, Liu Z, Zhou G, Tian A and Sun N:
Magnetic gold nanoparticle-mediated small interference RNA
silencing Bag-1 gene for colon cancer therapy. Oncol Rep.
35:978–984. 2016.PubMed/NCBI
|
|
6
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kabashima A, Higuchi H, Takaishi H,
Matsuzaki Y, Suzuki S, Izumiya M, Iizuka H, Sakai G, Hozawa S,
Azuma T, et al: Side population of pancreatic cancer cells
predominates in TGF-beta-mediated epithelial to mesenchymal
transition and invasion. Int J Cancer. 124:2771–2779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
12
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y and Zhou BP: Epithelial-mesenchymal
Transition-A Hall mark of Breast Cancer Metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar
|
|
15
|
Gonzalez EJ, Arms L and Vizzard MA: The
role(s) of cytokines/chemokines in urinary bladder inflammation and
dysfunction. BioMed Res Int. 2014:1205252014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sallusto F and Baggiolini M: Chemokines
and leukocyte traffic. Nat Immunol. 9:949–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T,
Chen LP and Shen W: CXCL12/CXCR4 chemokine signaling in spinal glia
induces pain hypersensitivity through MAPKs-mediated
neuro-inflammation in bone cancer rats. J Neurochem. 132:452–463.
2015. View Article : Google Scholar
|
|
18
|
Batsi O, Giannopoulou I, Nesseris I,
Valavanis C, Gakiopoulou H, Patsouris ES, Arapandoni-Dadioti P and
Lazaris AC: Immunohistochemical evaluation of CXCL12-CXCR4 axis and
VEGFR3 expression in primary urothelial cancer and its recurrence.
Anticancer Res. 34:3537–3542. 2014.PubMed/NCBI
|
|
19
|
Cojoc M, Peitzsch C, Trautmann F,
Polishchuk L, Telegeev GD and Dubrovska A: Emerging targets in
cancer management: Role of the CXCL12/CXCR4 axis. Onco Targets
Ther. 6:1347–1361. 2013.PubMed/NCBI
|
|
20
|
Zhang Z, Ni C, Chen W, Wu P, Wang Z, Yin
J, Huang J and Qiu F: Expression of CXCR4 and breast cancer
prognosis: A systematic review and meta-analysis. BMC Cancer.
14:492014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sahebkar A: Dual effect of curcumin in
preventing atherosclerosis: The potential role of
pro-oxidant-antioxidant mechanisms. Nat Prod Res. 29:491–492. 2015.
View Article : Google Scholar
|
|
22
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Chen Q, Wang Y, Peng W and Cai H:
Effects of curcumin on ion channels and transporters. Front
Physiol. 5:942014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang GM, Xie WY, Wang HS, Du J, Wu BP, Xu
W, Liu HF, Xiao P, Liu ZG, Li HY, Liu SQ, Yin WJ, Zhang QG, Liang
JP and Huang HJ: Curcumin combined with FAPalphac vaccine elicits
effective antitumor response by targeting
indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-alpha
in melanoma. Oncotarget. 6:25932–25942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherwood V: WNT signaling: An emerging
mediator of cancer cell metabolism? Mol Cell Biol. 35:2–10. 2015.
View Article : Google Scholar :
|
|
26
|
Tan CW, Gardiner BS, Hirokawa Y, Smith DW
and Burgess AW: Analysis of Wnt signaling β-catenin spatial
dynamics in HEK293T cells. BMC Syst Biol. 8:442014. View Article : Google Scholar
|
|
27
|
Chen J, Xu L, Hu X, Zhang J, Xu C, Li G
and Jiang H: Curcumin regulates VSMC phenotype transition via
modulation of Notch and Wnt signaling pathways. Drug Dev Res.
74:252–258. 2013. View Article : Google Scholar
|
|
28
|
Kim HJ, Park SY, Park OJ and Kim YM:
Curcumin suppresses migration and proliferation of Hep3B
hepatocarcinoma cells through inhibition of the Wnt signaling
pathway. Mol Med Rep. 8:282–286. 2013.PubMed/NCBI
|
|
29
|
Van Raay TJ, Coffey RJ and Solnica-Krezel
L: Zebrafish Naked1 and Naked2 antagonize both canonical and
non-canonical Wnt signaling. Dev Biol. 309:151–168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi YJ and Huo K: Nkd2, a negative
regulator of Wnt pathway, delays mitotic exit in Hela cell. Genes
Genomics. 35:569–573. 2013. View Article : Google Scholar
|
|
31
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skommer J, Wlodkowic D and Pelkonen J:
Gene-expression profiling during curcumin-induced apoptosis reveals
downregulation of CXCR4. Exp Hematol. 35:84–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo
H, Tian T, Ruan ZP, Kang XM, Wang J, et al: SDF-1/CXCR4 promotes
epithelial-mesenchymal transition and progression of colorectal
cancer by activation of the Wnt/β-catenin signaling pathway. Cancer
Lett. 354:417–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaw SY, Majeed AA, Dalley AJ, Chan A,
Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tacchelly-Benites O, Wang Z, Yang E, Lee E
and Ahmed Y: Toggling a conformational switch in Wnt/β-catenin
signaling: Regulation of Axin phosphorylation. The phosphorylation
state of Axin controls its scaffold function in two Wnt pathway
protein complexes. Bioessays. 35:1063–1070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta A, Verma A, Mishra AK, Wadhwa G,
Sharma SK and Jain CK: The Wnt pathway: Emerging anticancer
strategies. Recent Pat Endocr Metab Immune Drug Discov. 7:138–147.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong Y, Cao B, Zhang M, Han W, Herman JG,
Fuks F, Zhao Y and Guo M: Epigenetic silencing of NKD2, a major
component of Wnt signaling, promotes breast cancer growth.
Oncotarget. 6:22126–22138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu C, Zhao H, Chen H and Yao Q: CXCR4 in
breast cancer: Oncogenic role and therapeutic targeting. Drug Des
Devel Ther. 9:4953–4964. 2015.PubMed/NCBI
|
|
39
|
Xu JH, Yang HP, Zhou XD, Wang HJ, Gong L
and Tang CL: Role of wnt inhibitory factor-1 in inhibition of
bisdemethoxycurcumin mediated Epithelial-to-Mesenchymal transition
in highly metastatic lung cancer 95D cells. Chin Med J (Engl).
128:1376–1383. 2015. View Article : Google Scholar
|
|
40
|
Buhrmann C, Kraehe P, Lueders C, Shayan P,
Goel A and Shakibaei M: Curcumin suppresses crosstalk between colon
cancer stem cells and stromal fibroblasts in the tumor micro-
environment: potential role of EMT. PLoS One. 9:e1075142014.
View Article : Google Scholar
|
|
41
|
Braendegaard WS, Baatrup G, Pfeiffer P and
Qvortrup C: Trends in colorectal cancer in the elderly in Denmark,
1980–2012. Acta Oncol. 55(Suppl 1): 29–39. 2016. View Article : Google Scholar
|
|
42
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar
|
|
44
|
Deng Y, Su Q, Mo J, Fu X, Zhang Y and Lin
EH: Celecoxib downregulates CD133 expression through inhibition of
the Wnt signaling pathway in colon cancer cells. Cancer Invest.
31:97–102. 2013. View Article : Google Scholar
|
|
45
|
Jagtap S, Meganathan K, Wagh V, Winkler J,
Hescheler J and Sachinidis A: Chemoprotective mechanism of the
natural compounds, epigallocatechin-3-O-gallate, quercetin and
curcumin against cancer and cardiovascular diseases. Curr Med Chem.
16:1451–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen QY, Jiao DM, Wang LF, Wang L, Hu HZ,
Song J, Yan J, Wu LJ and Shi JG: Curcumin inhibits
proliferation-migration of NSCLC by steering crosstalk between a
Wnt signaling pathway and an adherens junction via EGR-1. Mol
Biosyst. 11:859–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leow PC, Bahety P, Boon CP, Lee CY, Tan
KL, Yang T and Ee PL: Functionalized curcumin analogs as potent
modulators of the Wnt/β-catenin signaling pathway. Eur J Med Chem.
71:67–80. 2014. View Article : Google Scholar
|
|
48
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu T, Li C, Cao Z, Van Raay TJ, Smith JG,
Willert K, Solnica-Krezel L and Coffey RJ: Myristoylated Naked2
anta-go nizes Wnt-beta-catenin activity by degrading Dishevelled-1
at the plasma membrane. J Biol Chem. 285:13561–13568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ben-Baruch A: The multifaceted roles of
chemokines in malignancy. Cancer Metastasis Rev. 25:357–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choe Y and Pleasure SJ: Wnt signaling
regulates intermediate precursor production in the postnatal
dentate gyrus by regulating CXCR4 expression. Dev Neurosci.
34:502–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao S, Wang J and Qin C: Blockade of
CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma
progression and metastasis via inactivation of canonical Wnt
pathway. J Exp Clin Cancer Res. 33:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang J, Cai J, Han F, Yang C, Tong Q, Cao
T, Wu L and Wang Z: Silencing of CXCR4 blocks progression of
ovarian cancer and depresses canonical Wnt signaling pathway. Int J
Gynecol Cancer. 21:981–987. 2011. View Article : Google Scholar : PubMed/NCBI
|