Introduction
Tongue squamous cell carcinoma (TSCC) is the most
prevalent type of oral malignant tumor. Despite advances in recent
decades in diagnosis, as a result of improved imaging modalities
and surgical techniques, the survival of TSCC patients has remained
unchanged (1-3). This is mainly due to the high
recurrence rate and the high risk of cervical lymph node metastasis
or distant metastasis (1-3).
There is substantial evidence indicating that
interactions between cancer cells and the cancer microenvironment
(CME) are of great importance to the development and progression of
cancer. Cancer cells alter the surrounding cancer stroma. Cancer
stromal cells and cytokines, in turn, promote cancer progression
and the acquisition of invasive properties (4,5).
Recently, it has become apparent that carcinomas recruit benign
microenvironment-supporting cells to facilitate invasion and
metastasis (6,7). Cancer-associated fibroblasts (CAFs)
are tumor-associated fibroblasts with myofibroblast-like phenotypes
that can be observed within the CME, particularly close to the
cancer nests (7-9). It has been reported that CAFs are
derived from normal cancer stromal fibroblasts under the direct
impact of cancer cell-derived cytokines, and they further
facilitate local and distant migration, as well as aiding in the
suppression of the host immune response (9-13).
CAFs exhibit changes in protein expression levels that represent an
activated myofibroblastic phenotype, which typically involves the
upregulation of α-smooth muscle actin (αSMA) (14,15).
They also promote cancer progression via the stimulation of
epithelial cell growth, migration and invasion by imitating
activated wound repair fibroblasts (5,16-18).
Experiments in vitro have also revealed that CAFs can
stimulate the proliferation and invasion of TSCC cells (19,20).
The most striking role of CAFs in the CME is the
stimulation of the epithelial-mesenchymal transition (EMT), which
is a necessary step toward invasion, metastasis and apoptosis
resistance (7,8,9,21).
Although the definition of EMT is broad, it is generally agreed
that it involves loss of E-cadherin expression resulting in loss of
cell-cell contact between cancer epithelial cells, cytoskeleton
reorganization via switching from cytokeratin (CK) to vimentin
intermediate filaments, loss of apical-basal polarity, acquisition
of a fibroblast-like cell shape and increased cellular motility
(22,23). In the EMT process, cancer cells
with decreased E-cadherin expression are primarily located at the
cancer periphery and directly contact with CAFs, indicating that
EMT may be modulated by CAFs (8).
To achieve EMT, the coordinated expression of several sets of genes
and signalling pathways is required, a number of which have been
demonstrated to regulate specific aspects of malignant
transformation and cancer progression (24,25).
One pathway involves the activation of transcription factors,
including Snail, Slug and Twist, which cause transcriptional
repression of E-cadherin, a defining step in EMT (22,23,26).
In our clinical experience, there have been several
cases of early-stage TSCC with local recurrence in the early
postoperative period, despite tumor resection with adequate safety
and negative margins. We hypothesised that such aggressive tumors
may be accompanied by the process of EMT stimulation within the
CME; therefore, evaluation of the CME may provide clues that enable
the detection of prognostic factors for the local recurrence of
TSCC. The present study used multiple immunofluorescent analysis
(MIA), which enables detailed and concurrent examination of
multiple markers, to evaluate the distribution of CAFs in the CME
using tissue specimens obtained from the early-stage TSCC patients
with local recurrence who underwent glossectomy with clear margins.
The results demonstrated that localised distribution of CAFs in CME
was significantly different between cases with local recurrence and
those without. It was demonstrated that the cancer cells
neighbouring CAFs exhibited simultaneous expression of E-cadherin
and vimentin, in keeping with a study by Wang et al
(27); however, all
vimentin-positive cancer cells continued to express the epithelial
marker, CK, and even certain vimentin-positive cells continued to
express E-cadherin. To date, the expression of vimentin and the
loss of E-cadherin in epithelial cancer cells have been used to
diagnose EMT, direct conversion of epithelial cells to mesenchymal
cells. However, such a diagnosis does not take into account the
possibility that epithelial cancer cells are able to transform back
into epiblasts, which are undifferentiated CK-positive cells
expressing vimentin.
In the present study, we propose a novel concept
referred to as anaplastic transition (APT), in which epithelial
cancer cells dedifferentiate into primitive states. This proposal
was based on the observation that the loss of E-cadherin expression
does not necessarily indicate a loss of CK, and that these cells
retain epithelial and mesenchymal CKs.
Materials and methods
Patients and samples
A total of 10 male and 4 female patients with
early-stage TSCC were included in the present study. The mean age
of the patients at the time of surgery was 61.4 years (range, 39-79
years) in the LR group and 64.1 years (range, 52-86 years) in the
control group. All samples in the present study were obtained from
14 patients who were clinically diagnosed with early-stage (cT1-2)
squamous cell carcinoma and who underwent glossectomy with adequate
safety and negative margins without preoperative neoadjuvant
chemotherapy and/or radiation at the department of Clinical Oral
Oncology in Nagasaki University Hospital between April 2006 and
December 2015. Tumors in all patients were classified according to
the World Health Organization (WHO) classification by a pathologist
who was blinded to the clinical findings. The pathological
Tumor-Node-Metastasis (TNM) classification was established using
the International System for Staging adopted by the American Joint
Committee on Cancer and the Union for International Cancer Control
(8th edition) (28,29). This retrospective study includes 7
cases of TSCC who experienced local recurrence (LR group) following
appropriate resection with clear safety margins (>10 mm) and 7
randomly selected cases of TSCC without local recurrence who
underwent glossectomy over the same period (control group). Sex,
age, TNM classification, clinical growth pattern, differentiation,
vascular/lymphatic perineural invasion, and outcomes were
evaluated.
Postoperative follow-up was performed using
contrast-enhanced computed tomography (CT) and examination by
ultrasound of the neck for at least 3 months. LR was defined as the
detection of a mass on CT images and/or clinical inspection.
Ethical approval
The present study was approved by the Institutional
Review Board of Nagasaki University (registration no. 17082111) and
was performed in line with the Declaration of Helsinki.
Multiple immunofluorescence
Surgical specimens were fixed in 10% formaldehyde at
room temperature (RT) overnight and embedded in paraffin according
to a standard protocol. Serial 4-µm histological sections
were deparaffinised in xylene and hydrated in descending dilutions
of ethanol. All samples were stained with haematoxylin and eosin
(H&E) at RT for 4 and 2 min, respectively, and histological
subtypes and pathological stage were reconfirmed. For antigen
retrieval, slides were placed in a water bath at 95°C for 40 min in
the citrate buffer (pH 6.0; S2031; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA). Endogenous peroxidase activity was
blocked by incubation with 0.3% hydrogen peroxide (catalog no.
081-04215; Wako Pure Chemical Industries, Ltd., Osaka, Japan) in
99% methanol (catalog no. 136-01837; Fujifilm; Wako Pure Chemical
Industries) for 30 min at RT. The slides were immersed in blocking
buffer dispensed in 5% skimmed milk (catalog no. 232100; BD
Biosciences, Franklin Lakes, NJ, USA) and Triton(R) X-100 (catalog
no. 35501-15; Nacalai Tesque, Inc., Kyoto, Japan) at RT for 5 min.
The blocking buffer included the following antibiotics: 0.1%
streptomycin (catalog no. 85886) and 0.1% amphotericin (catalog no.
A2942) (both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
The slides were incubated at room temperature for 2 h with the
following primary antibodies: Mouse anti-human CK14 monoclonal
antibody (catalog no. ab181595; dilution, 1:100) to detect cancer
epithelium, mouse anti-human PCNA monoclonal antibody (catalog no.
ab201672; dilution, 1:100) (both from Abcam, Cambridge, UK) to
evaluate the proliferative activity of the tumor; chicken
anti-human vimentin monoclonal antibody (catalog no. 919101;
dilution, 1:100; BioLegend, Inc., San Diego, CA, USA) and rabbit
anti-human αSMA (catalog no. ab32575; dilution, 1:100; Abcam) to
detect CAFs (5,7,30);
and mouse anti-human E-cadherin (catalog no. 610182; dilution,
1:100; BD Biosciences) to evaluate the progression of EMT. Slides
were then incubated for 1 h with the following secondary
antibodies: Alexa Fluor 488-conjugated goat anti-mouse IgG
antibody, Alexa Fluor 546-conjugated goat anti-rabbit IgG antibody
or Alexa Fluor 647-conjugated goat anti-chicken antibody (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Slides were washed
using PBS with 10% glycerol and mounted in PBS containing DAPI.
Finally, slides were sealed with a cover glass.
Images were captured using a fluorescent microscope
(DM-6000B) and a digital camera (DFC-350 FX) (both from Leica
Microsystems GmbH, Wetzlar, Germany) attached to the fluorescent
microscope, and 3 randomly selected areas were selected to generate
the optical density plots using the Histogram tool in the Image
menu of Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA).
The average fluorescent intensities within the 3 randomly selected
areas were recorded in arbitrary units and the relative fluorescent
intensities were calculated to obtain relative levels.
Fig. 1 indicates
the specificity of the anti-vimentin antibody. In the mucosal
region, vimentin signals could not be detected in the epithelial
cells that were positive for CK14.
Statistical analysis
All statistical analyses were conducted using SPSS
for Windows Version 24.0 (IBM Corp., Armonk, NY, USA). The
distributions of CAFs and expression levels of vimentin and
E-cadherin in LR patients and control patients were analysed using
the Mann-Whitney U test. Significance was evaluated using Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
Based on the cancer differentiation pattern defined
by the WHO, there was one case of moderately-differentiated TSCC in
the LR group, while the remaining 13 patients had
well-differentiated cancer. The median tumor thickness in the LR
group was 5.85 mm (range, 4.5–11.0 mm), and was 4.5 mm (range,
3.0–12.0 mm) in the control group. The depth of invasion was <10
mm. No significant difference was detected between the two groups.
Vascular, lymphatic and perineural invasion were more likely to be
observed in the LR group than in the control group. In the LR
group, all TSCCs recurred from the deep and/or posterior margin of
the primary lesion. The mean period between surgery and the
detection of LR was 8.7 months (range, 0.2–27.8 months). All LR
patients underwent an additional glossectomy. However, 4 patients
in the LR group (4/7 cases, 57.1%) succumbed due to uncontrollable
LR (1 cases), controllable LR (1 case), late lymph node metastasis
(1 case) and lung metastases (1 case). Although one patient
exhibited controllable LR, the patient made non-treatment decision.
The mean follow-up period was 49.2 months for the whole series
(range, 5.1–134.0 months). Table I
shows a summary of all 14 patients.
 | Table IPatient characteristics and
outcomes. |
Table I
Patient characteristics and
outcomes.
| Sex | Age | TNM | Clinical growth
pattern pattern |
Differentiation | Vascular, lymphatic
and perineural invasion | Outcome |
|---|
| LR group |
| Case 1 | M | 39 | T2N0M0 | Invasive | Well | – | DFS |
| Case 2 | M | 75 | T1N0M0 | Invasive | Moderate | v | N mortality |
| Case 3 | M | 57 | T2N0M0 | Invasive | Well | v | Other death |
| Case 4 | F | 62 | T2N0M0 | Invasive | Well | ly, v, pn | DFS |
| Case 5 | M | 79 | T2N0M0 | Invasive | Well | v, pn | T mortality |
| Case 6 | M | 72 | T2N1M0 | Unknown | Well | ly, v, pn | T mortality |
| Case 7 | F | 46 | T2N0M0 | Expansive | Well | pn | DFS |
| Control group |
| Case 1 | M | 63 | T2N0M0 | Superficial | Well | v | DFS |
| Case 2 | M | 52 | T2N0M0 | Invasive | Well | – | DFS |
| Case 3 | M | 61 | T1N0M0 | Superficial | Well | – | DFS |
| Case 4 | F | 64 | T2N0M0 | Invasive | Well | v | DFS |
| Case 5 | F | 61 | T2N0M0 | Superficial | well | v, pn | DFS |
| Case 6 | M | 62 | T2N0M0 | Invasive | well | ly, v | DFS |
| Case 7 | M | 86 | T2N0M0 | Invasive | well | v | DFS |
Evaluation of the cancer microenvironment
using multiple immunofluorescent analysis
A representative image of the LR case obtained by
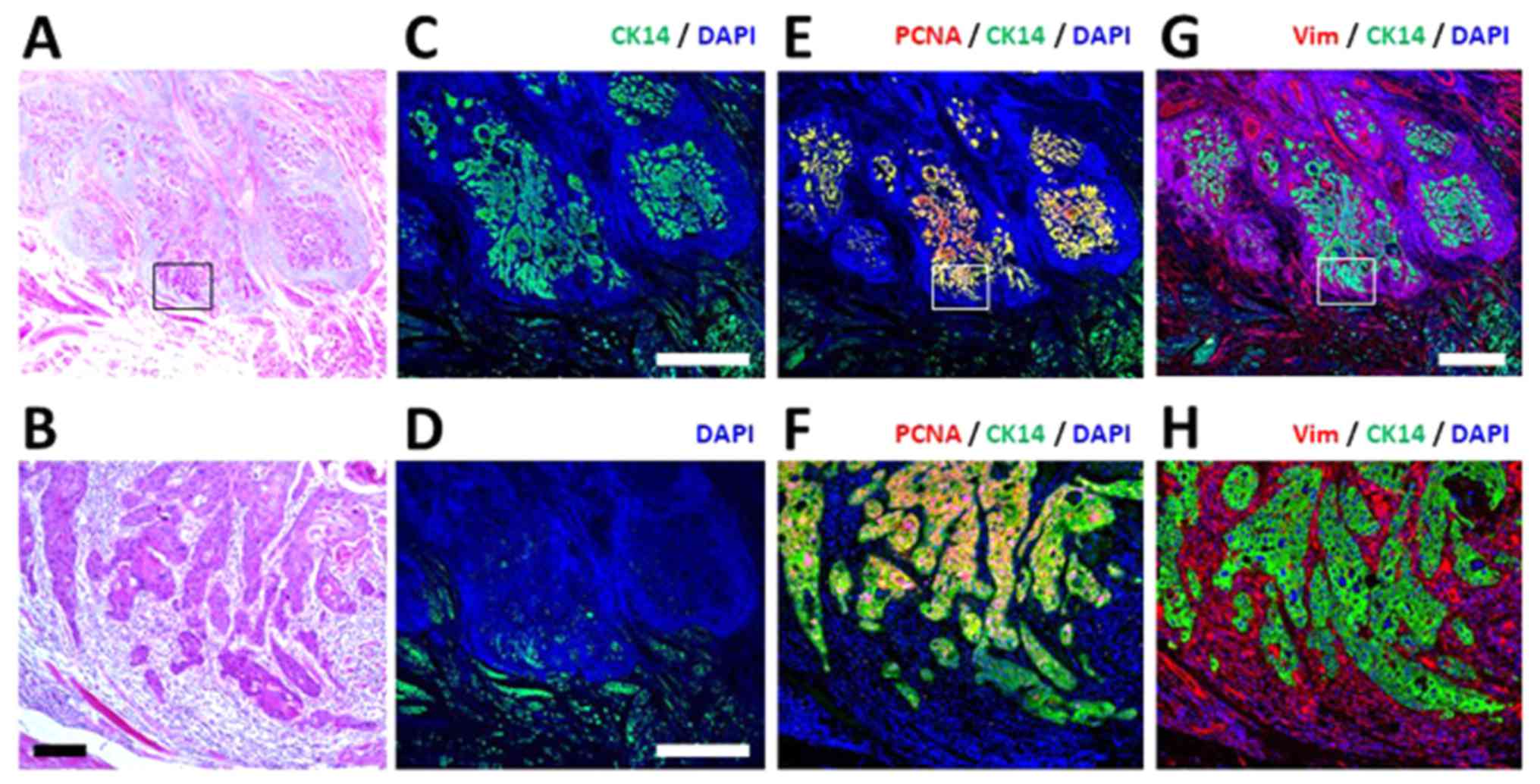
MIA is presented in Fig. 2. In the
tumor region determined by H&E staining (Fig. 2A and B), CK14-positive cells, which
are clearly discriminated from the green auto-fluorescence
(Fig. 2C and D), were identified
as independent clusters. They were PCNA-positive (Fig. 2E and F) and were embedded in CME,
which consists of vimentin-positive stromal cells (Fig. 2G and H). Comparison of the
distribution between CK14-positive cancer cells (Fig. 2E and F) and vimentin-positive
stromal cells indicated that stromal components appear to be
present prior to the invasive propagation of cancer cells (Fig. 2G). High-magnification MIA analysis
revealed that cancer clusters with high PCNA positivity were
surrounded by vimentin-positive stromal cells (Fig. 2H). The authors considered spindled
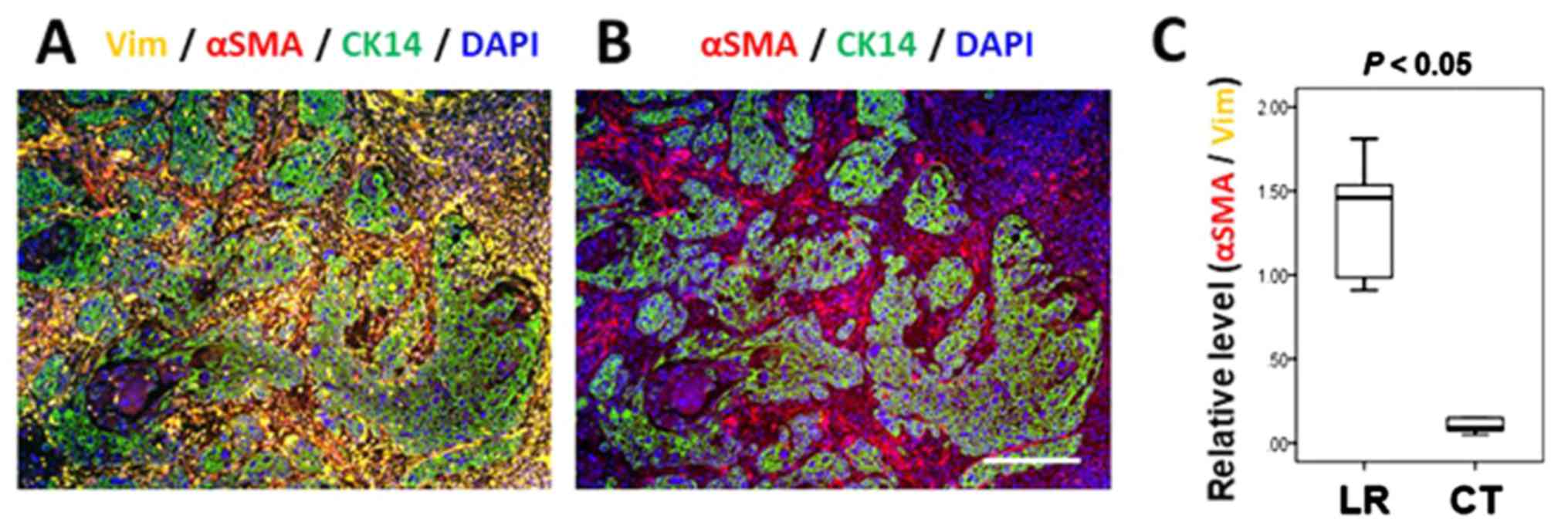
cells that were positive for αSMA and vimentin as CAFs (5,7,30).
CAFs were detected around the invasion front of CK14-positive
cancer tissues. It should be noted that vimentin-positive stromal
cells frequently express αSMA, indicating that they are CAFs
(Fig 3A and B). Quantitative
analysis of αSMA expression versus vimentin expression in the
stromal cells revealed that activation of stromal cells was
significantly accelerated in the LR group compared with the control
group (P=0.001; Mann-Whitney U test; Fig. 3C). The present study also compared
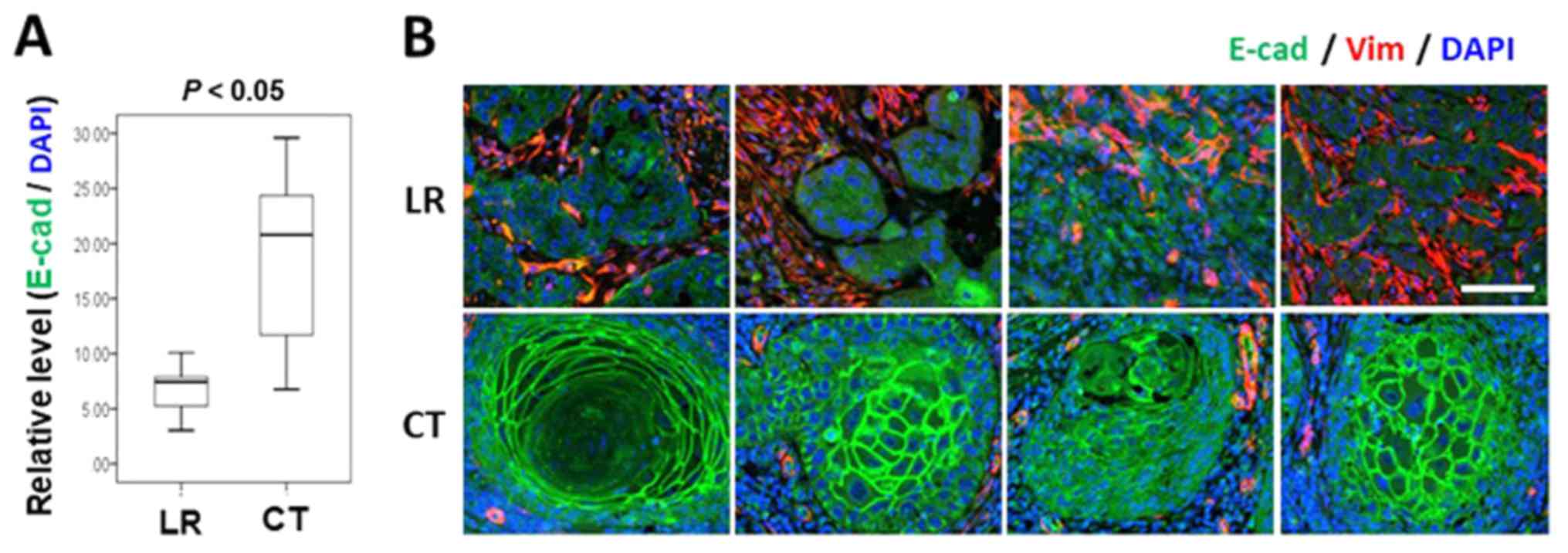
E-cadherin expression in CK14-positive cancer epithelium between
the LR and control groups. As demonstrated in Fig. 4, characteristic membrane
organization of E-cadherin was lost in PCNA-positive cancer
clusters in the LR group. The expression of E-cadherin in the
CK14-positive cancer epithelia also differed significantly between
the two groups (P=0.007; Mann-Whitney U test; Fig. 4A), and the expression levels of
E-cadherin, as well as the organization pattern was apparently
compromised in the LR cases (Fig.
4B).
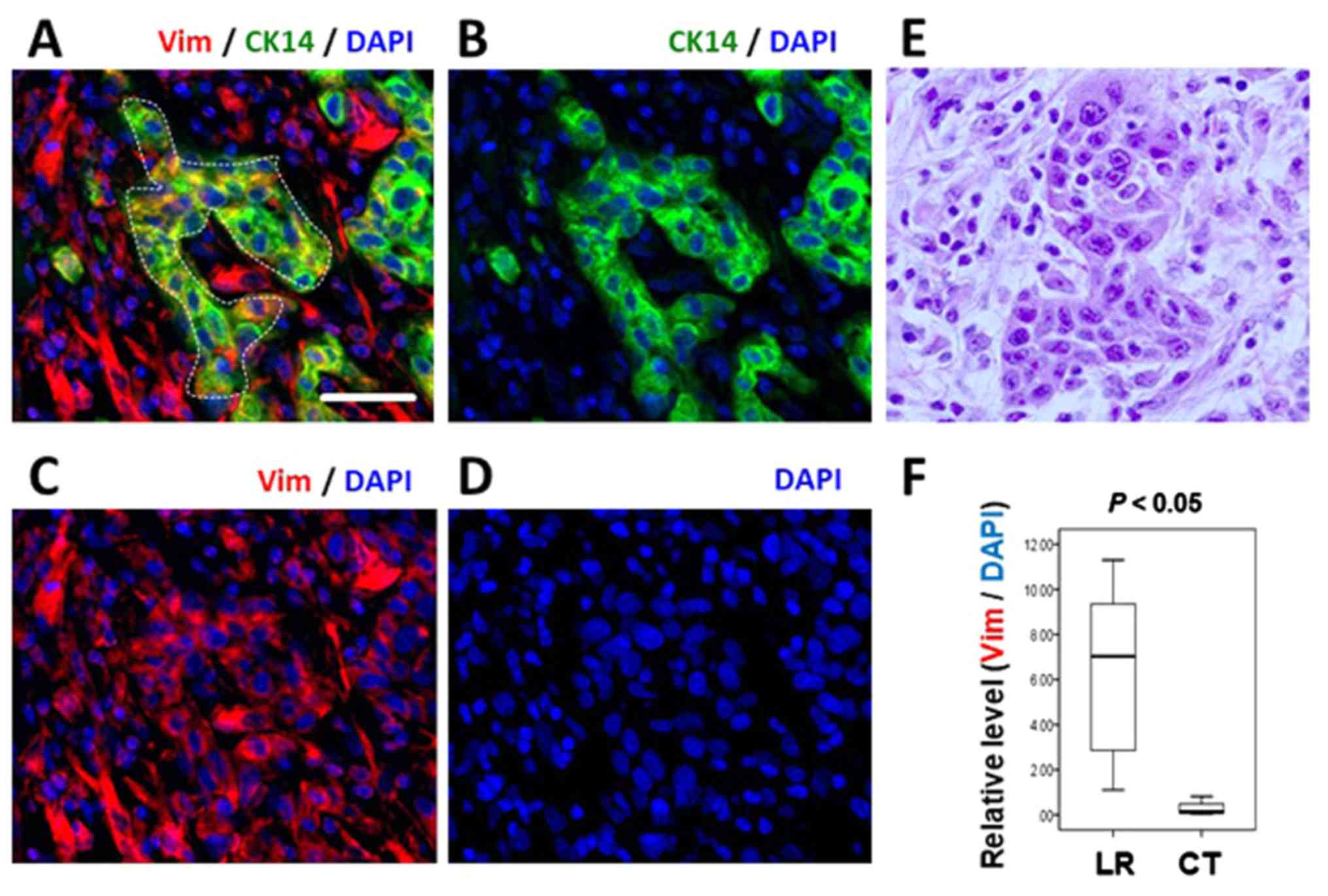
While normal CK14-positive epithelial cells did not
express vimentin (Fig. 1),
CK14-positive cancer cell clusters in numerous LR cases exhibited
concurrent vimentin expression (Fig.
5A–E and Table II). For
example, the cancer cells in the dotted area in Figure 5A, which are positive for CK14,
also express vimentin. The cells exhibit epithelial morphology and
CK14 expression levels equivalent to that in the normal epithelium
(data not shown). Next, expression levels of vimentin within the
CK14-positive cancer cells were quantified, and the Mann-Whitney U
test revealed that the two groups were significantly different
(P=0.004; Fig. 5F). It should also
be emphasized that such vimentin-positive cancer cells continue to
exhibit normal amounts of CK14 expression, indicating that they are
CK14-positive epithelial cells but that they concurrently express
vimentin. The MIA also revealed various intermediate phenotypes, in
which vimentin-positive cancer cells retain E-cadherin membrane
distribution (Fig. 6A–C).
 | Table IIRelative expression of αSMA, Vimentin and E-cadherin. |
Table II
Relative expression of αSMA, Vimentin and E-cadherin.
| αSMA (αSMA/Vimentin) | Vimentin
(Vimentin/DAPI) | E-cadherin
(E-cadherin/DAPI) |
|---|
| LR group |
| Case 1 | 0.94±0.12 | 2.93±0.74 | 7.46±0.99 |
| Case 2 | 1.56±0.42 | 2.80±0.68 | 8.15±1.52 |
| Case 3 | 1.46±0.21 | 8.31±1.20 | 3.0±1.34 |
| Case 4 | 1.81±0.27 | 7.03±1.43 | 5.83±1.30 |
| Case 5 | 1.51±0.44 | 11.3±2.86 | 4.67±1.90 |
| Case 6 | 1.01±0.18 | 10.4±1.83 | 7.60±1.16 |
| Case 7 | 0.93±0.20 | 5.59±1.06 | 6.75±1.72 |
| Control group |
| Case 1 | 0.06±0.01 | 0.14±0.07 | 29.6±3.46 |
| Case 2 | 0.09±0.03 | 0.13±0.08 | 25.3±3.12 |
| Case 3 | 0.15±0.07 | 0.02±0.01 | 12.9±1.32 |
| Case 4 | 0.09±0.06 | 0.15±0.09 | 20.8±2.86 |
| Case 5 | 0.91±0.19 | 1.11±0.51 | 10.1±2.11 |
| Case 6 | 0.05±0.03 | 0.82±0.29 | 10.5±2.10 |
| Case 7 | 0.15±0.06 | 0.02±0.01 | 23.4±6.31 |
Discussion
The present study applied MIA to visualize the
spatial distribution of CAFs in the TSCC cases with local
recurrence, despite the glossectomy with adequate safety margins
having been performed. The results of the present study clearly
demonstrated that the distribution of CAFs was significantly
associated with LR. While the distribution of vimentin-positive
stromal cells was equivalent between the LR and control cases,
vimentin-positive cells frequently co-expressed αSMA in the LR
cases.
It has recently been reported that CAFs occurring in
high frequency in oral SCCs are associated with cancer recurrence
(8,31). Furthermore, CAFs are key factors in
the collective invasion of cancer cells as they are capable of
remodelling the extracellular matrix and providing the mechanical
propulsive forces required to facilitate tumor invasion (8). Epithelial cancer cells are able to
activate CAFs, an interaction that is mediated by ligands,
including tumor necrosis factor-α (TNF-α) and interleukin-1α/β
(IL-1α/β). CAFs secrete molecules, including TNF-α, IL-1α/β, IL-33,
CCL7, SDF-1, MDNF, type 1 collagen, HGF, IGF2, BMP4, MMPs, PGE2,
KGF, activin A and PDGF, and microRNAs (32). In this way, pre-cancerous cell
growth (33), cancer cell
migration and invasion, metastasis, angiogenesis, immune system
evasion (34) and chemotherapy
resistance (35) are promoted.
Additionally, it has been reported that a CAF-rich reactive stroma
is associated with high-grade malignancies and a poor prognosis in
oral cancer (36). Therefore,
these reports support the results of the present study, in which
activation of stromal cells are associated with local
recurrence.
In the present study, 6/7 patients in the LR group
exhibited perineural and/or vascular invasion and a poor prognosis.
Certain studies have reported that perineural and vascular invasion
are prognostic factors for regional metastasis and poor outcomes in
patients with TSCC, although they are not significant associated
with local recurrence (37).
To date, a diagnosis of EMT has been given when
epithelial cancer cells express vimentin and lose E-cadherin.
However, the expression of epithelial markers, including CKs, in
epithelial cancer cells was not taken into consideration, since
general tissue staining methods do not permit the examination of
simultaneous expression of multiple markers in the same cell.
Therefore, MIA was introduced and it was demonstrated that
vimentin-positive cancer cells retained CK14 expression. CK14
expression was confirmed to be equivalent to that observed in the
normal epithelial cells. It is therefore not adequate to utilize
EMT or partial EMT to describe the results of the present study.
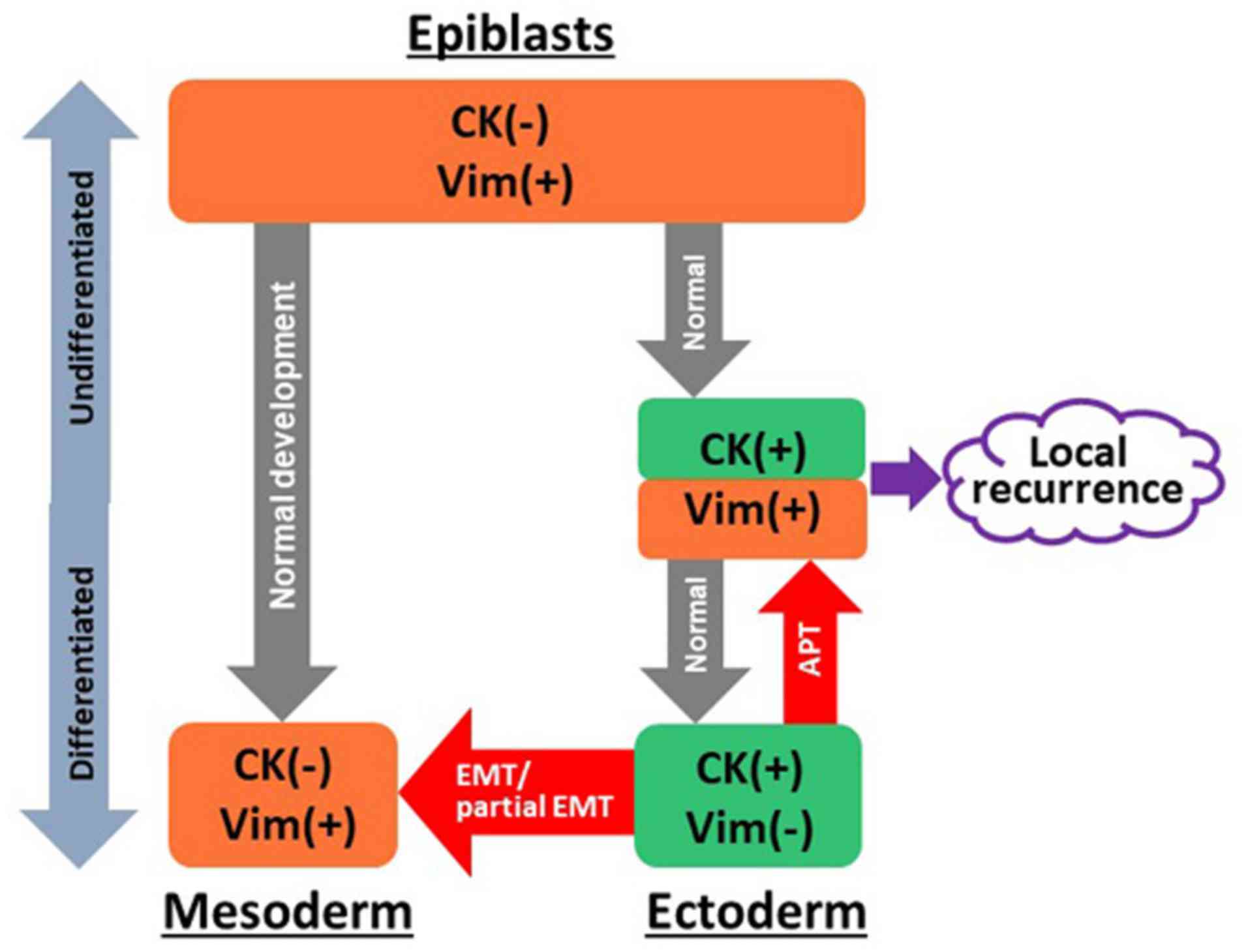
Thus, the concept of APT was proposed, in which epithelial cancer
cells dedifferentiate into more primitive states (Fig. 7). During embryonic development, it
is well described that vimentin is co-expressed with CKs in the
mesoderm, since the mesoderm originates from vimentin-positive
epiblasts. Next, specification of epithelial cells limit the
expression of vimentin, and therefore, epithelial cells do not
express any vimentin protein. The present study observed that
CK14-positive cells co-express vimentin, indicating that epithelial
cells obtained more primitive features, including epiblasts, which
should be separated from concepts of EMT and partial EMT. Notably,
it has been reported that vimentin co-exists with CK14 in the
migrating epithelial cells (38).
There are also studies demonstrating that vimentin and CKs are
co-expressed in epithelial cancer cells (27,39,40).
This result was also observed in the LR group; therefore, we
hypothesized that APT may be a prognostic marker of LR and aid in
improving the understanding of LR in patients with early-stage
TSCC. Clinically, patients with APT should undergo frequent
examinations using contrast enhanced-CT, ultrasound and
positron-emission tomography if required, in addition to inspection
during postoperative follow-up; if clinician can perform an early
diagnosis, they will be able to begin treatment of the LR tumor
sooner.
It was also noted that one major limitation of the
present study was the small number of early-staged TSCC patients
with LR (n=7), which may have affected the statistical results. To
begin with, the number of these cases was relatively small. Since
almost all cases (98.5%) postoperatively elapsed without LR,
further accumulation of LR cases to hone accuracy of statistics is
required in the future.
MIA also enabled the detection of pleiotropic
phenotypes of cancer cells. For example, as demonstrated in
Figure 6, vimentin and E-cadherin
are co-expressed in the same cells, indicating that EMT is not an
all-or-none phenomena. Such intermediate phenotypes have been
referred to as partial EMT. Grigore et al (41) discussed the concept of partial EMT
in associated with tumor budding at the invasion front, which was
associated with decreased E-cadherin and increased vimentin levels,
suggesting that it may be a morphological feature of EMT. Wang
et al (27) performed
immunohistochemical analysis with pan-CK staining and revealed that
budding cells scattered in the stroma were also positive for
vimentin. The results of the present study not only confirmed those
of previous reports, but also provided the concept of APT, which
may aid in dissolving controversial reports on partial EMT.
In current clinical practice, clinicians tend to
diagnose EMT when epithelial cancer cells loose E-cadherin and
express vimentin. However, the diagnosis does not include an
evaluation of CK expression, the specific nature of epithelia. A
more detailed diagnosis of the degree of malignancy includes the
evaluation of the histopathological features with HE staining, as
well as MIA using CK and vimentin. The analysis of expression of
transcriptional factors, including Snail, Slug, ZEB1, Twist and
Bmi1, has also been used to evaluate EMT progression and the degree
of malignancy (42), and the
analysis of changes in expression of these genes will also provide
us with a more detailed mechanism through which APT is
achieved.
In conclusion, a novel concept of APT was proposed,
in which epithelial cancer cells concurrently obtain mesenchymal
features. MIA first enabled us to evaluate the residual expression
of CK in epithelial cancer cells that have lost E-cadherin and
begun to express vimentin. Since APT is significantly associated
with the LR, it can be used as a predictive marker for LR in
patients with early-stage TSCC.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
KO and KS contributed toward conception and design
of the study, data acquisition, analysis and interpretation, and
drafting of the manuscript. KO and HT prepared sample slides. KO,
SY, TN and MU contributed toward data analysis. KS, MU and SY
critically revised the manuscript. All authors approved the final
manuscript and agree to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki on medical protocol and ethics and was
approved by the regional Ethical Review Board of Nagasaki
University (Nagasaki, Japan; registration no. 17082111).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin NN, Wang P, Zhao D, Zhang FJ, Yang K
and Chen R: Significance of oral cancer-associated fibroblasts in
angiogenesis, lymphangiogenesis, and tumor invasion in oral
squamous cell carcinoma. J Oral Pathol Med. 46:21–30. 2017.
View Article : Google Scholar
|
|
2
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Döbrossy L: Epidemiology of head and neck
cancer: Magnitude of the problem. Cancer Metastasis Rev. 24:9–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodrigues-Lisoni FC, Peitl P Jr, Vidotto
A, Polachini GM, Maniglia JV, Carmona-Raphe J, Cunha BR, Henrique
T, Souza CF, Teixeira RA, et al Head and Neck Genome Project
GENCAPO: Genomics and proteomics approaches to the study of
cancer-stroma interactions. BMC Med Genomics. 3:142010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanna E, Quick J and Libutti SK: The
tumour microenvironment: A novel target for cancer therapy. Oral
Dis. 15:8–17. 2009. View Article : Google Scholar
|
|
7
|
Zhou B, Chen WL, Wang YY, Lin ZY, Zhang
DM, Fan S and Li JS: A role for cancer-associated fibroblasts in
inducing the epithelial-to-mesenchymal transition in human tongue
squamous cell carcinoma. J Oral Pathol Med. 43:585–592. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vered M, Dayan D, Yahalom R, Dobriyan A,
Barshack I, Bello IO, Kantola S and Salo T: Cancer-associated
fibroblasts and epithelial-mesenchymal transition in metastatic
oral tongue squamous cell carcinoma. Int J Cancer. 127:1356–1362.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Zhang J, Chen SW, Liu LL, Li L, Gao
F, Zhuang SM, Wang LP, Li Y and Song M: Cancer-associated
fibroblasts provide a suitable microenvironment for tumor
development and progression in oral tongue squamous cancer. J
Transl Med. 13:1982015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jewett A, Head C and Cacalano NA: Emerging
mechanisms of immunosuppression in oral cancers. J Dent Res.
85:1061–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wheeler SE, Shi H, Lin F, Dasari S,
Bednash J, Thorne S, Watkins S, Joshi R and Thomas SM: Enhancement
of head and neck squamous cell carcinoma proliferation, invasion,
and metastasis by tumor-associated fibroblasts in preclinical
models. Head Neck. 36:385–392. 2014. View Article : Google Scholar
|
|
14
|
Sappino AP, Skalli O, Jackson B, Schürch W
and Gabbiani G: Smooth-muscle differentiation in stromal cells of
malignant and non-malignant breast tissues. Int J Cancer.
41:707–712. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lazard D, Sastre X, Frid MG, Glukhova MA,
Thiery JP and Koteliansky VE: Expression of smooth muscle-specific
proteins in myoepithelium and stromal myofibroblasts of normal and
malignant human breast tissue. Proc Natl Acad Sci USA. 90:999–1003.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mueller MM and Fusenig NE: Friends or foes
- bipolar effects of the tumour stroma in cancer. Nat Rev Cancer.
4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rønnov-Jessen L, Petersen OW and Bissell
MJ: Cellular changes involved in conversion of normal to malignant
breast: Importance of the stromal reaction. Physiol Rev. 76:69–125.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
el-Naggar AK, Lai S, Luna MA, Zhou XD,
Weber RS, Goepfert H and Batsakis JG: Sequential p53 mutation
analysis of pre-invasive and invasive head and neck squamous
carcinoma. Int J Cancer. 64:196–201. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kellermann MG, Sobral LM, da Silva SD,
Zecchin KG, Graner E, Lopes MA, Kowalski LP and Coletta RD: Mutual
paracrine effects of oral squamous cell carcinoma cells and normal
oral fibroblasts: Induction of fibroblast to myofibroblast
transdifferentiation and modulation of tumor cell proliferation.
Oral Oncol. 44:509–517. 2008. View Article : Google Scholar
|
|
20
|
Daly AJ, McIlreavey L and Irwin CR:
Regulation of HGF and SDF-1 expression by oral
fibroblasts–implications for invasion of oral cancer. Oral Oncol.
44:646–651. 2008. View Article : Google Scholar
|
|
21
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Potenta S, Zeisberg E and Kalluri R: The
role of endothelial-to-mesenchymal transition in cancer
progression. Br J Cancer. 99:1375–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Zimmermann M, Tinhofer I, Kaufmann
AM and Albers AE: Epithelial-to-mesenchymal transition and cancer
stem(-like) cells in head and neck squamous cell carcinoma. Cancer
Lett. 338:47–56. 2013. View Article : Google Scholar
|
|
25
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Huang H, Huang Z, Wang A, Chen X,
Huang L, Zhou X and Liu X: Tumor budding correlates with poor
prognosis and epithelial-mesenchymal transition in tongue squamous
cell carcinoma. J Oral Pathol Med. 40:545–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and neck cancers-major changes in the American Joint Committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
Wiley-Blackwell; Chichester: 2017
|
|
30
|
Lim KP, Cirillo N, Hassona Y, Wei W,
Thurlow JK, Cheong SC, Pitiyage G, Parkinson EK and Prime SS:
Fibroblast gene expression profile reflects the stage of tumour
progression in oral squamous cell carcinoma. J Pathol. 223:459–469.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawashiri S, Tanaka A, Noguchi N, Hase T,
Nakaya H, Ohara T, Kato K and Yamamoto E: Significance of stromal
desmoplasia and myofibroblast appearance at the invasive front in
squamous cell carcinoma of the oral cavity. Head Neck.
31:1346–1353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Min A, Gao S and Tang Z: Genetic
regulation and potentially therapeutic application of
cancer-associated fibroblasts in oral cancer. J Oral Pathol Med.
43:323–334. 2014. View Article : Google Scholar
|
|
33
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
34
|
Takahashi H, Sakakura K, Kudo T, Toyoda M,
Kaira K, Oyama T and Chikamatsu K: Cancer-associated fibroblasts
promote an immunosuppressive microenvironment through the induction
and accumulation of protumoral macrophages. Oncotarget.
8:8633–8647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johansson AC, Ansell A, Jerhammar F, Lindh
MB, Grénman R, Munck-Wikland E, Östman A and Roberg K:
Cancer-associated fibroblasts induce matrix
metalloproteinase-mediated cetuximab resistance in head and neck
squamous cell carcinoma cells. Mol Cancer Res. 10:1158–1168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sinha N, Mukhopadhyay S, Das DN, Panda PK
and Bhutia SK: Relevance of cancer initiating/stem cells in
carcinogenesis and therapy resistance in oral cancer. Oral Oncol.
49:854–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsushita Y, Yanamoto S, Takahashi H,
Yamada S, Naruse T, Sakamoto Y, Ikeda H, Shiraishi T, Fujita S,
Ikeda T, et al: A clinicopathological study of perineural invasion
and vascular invasion in oral tongue squamous cell carcinoma. Int J
Oral Maxillofac Surg. 44:543–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Velez-delValle C, Marsch-Moreno M,
Castro-Muñozledo F, Galván-Mendoza IJ and Kuri-Harcuch W:
Epithelial cell migration requires the interaction between the
vimentin and keratin intermediate filaments. Sci Rep. 6:243892016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramaekers FC, Haag D, Kant A, Moesker O,
Jap PH and Vooijs GP: Coexpression of keratin- and vimentin-type
intermediate filaments in human metastatic carcinoma cells. Proc
Natl Acad Sci USA. 80:2618–2622. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pagan R, Martín I, Alonso A, Llobera M and
Vilaró S: Vimentin filaments follow the preexisting cytokeratin
network during epithelial-mesenchymal transition of cultured
neonatal rat hepatocytes. Exp Cell Res. 222:333–344. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grigore AD, Jolly MK, Jia D, Farach-Carson
MC and Levine H: Tumor Budding: The Name is EMT. Partial EMT J Clin
Med. 5:E512016. View Article : Google Scholar
|
|
42
|
Pradella D, Naro C, Sette C and Ghigna C:
EMT and stemness: Flexible processes tuned by alternative splicing
in development and cancer progression. Mol Cancer. 16:82017.
View Article : Google Scholar : PubMed/NCBI
|