Introduction
According to the website of the World Health
Organization (http://www.who.int/diabetes/en/), 346 million
individuals worldwide have diabetes. The chronic cardiovascular and
metabolic complications of diabetes carry significant risks of
morbidity and mortality, requiring that the disease is carefully
managed. Type-1 diabetes, an autoimmune disorder, is characterized
by the loss of insulin-producing β cells. Pancreatic islet
transplantation is one of the most successful therapeutic
strategies for diabetes (1–4).
However, there are several obstacles for islet transplantation that
must be overcome, including the adverse effects resulting from
treatment with immunosuppressive drugs, preservation of islet
function and the shortage of islet donors (5). Therefore, generating functional β
cells would be a promising strategy in providing adequate numbers
of β cells for transplantation.
Stem cells, including embryonic and adult stem
cells, possess the potential for self-renewal, proliferation and
differentiation into various types of cells. Stem cells may be
isolated from embryo, pancreas, liver and bone marrow tissue and
used as β cells for transplantation (6,7).
Hepatic stem cells have the highest potential for differentiation
into insulin-producing cells, as the pancreas and liver have common
precursor cells during embryogenesis. A study by Yang et al
demonstrated the production of insulin-secreting β cells from
hepatic oval stem cells, this may be a therapeutic strategy for
autologous stem cell transplantation (8). However, this application is limited
since stem cells are difficult to obtain.
Bone marrow stromal cells (BMSCs), which have high
plasticity and are therefore easily manipulated in vitro,
are abundant in bone marrow and are readily obtained (9,10).
BMSCs may be isolated from other cells and cultured in
vitro. Previous studies have shown that BMSCs differentiate
into chrondrocytes, osteocytes, adipocytes and fibroblasts
(11,12). Tang et al demonstrated that
mouse BMSCs differentiate into insulin-producing cells (13). Moreover, genetically manipulated
human BMSCs show stable transgene expression from over 17 passages
in vitro and over 3 months in vivo(14). Therefore, BMSCs are a promising
source of insulin-producing cells for autologous
transplantation.
Nestin, a type VI intermediate filament protein,
promotes the phosphorylation-dependent disassembly of vimentin
intermediate filaments during mitosis (15) and is also used as a marker of
neural precursor cells (16) and
pancreatic stem cells (17).
Studies suggest that cerebral microglia expressing nestin and NG2,
a chondroitin sulfate proteoglycan, have high plasticity similar to
neonatal brain neurons (18,19).
Moreover, Milanesi et al demonstrated that nestin-positive
cells differentiate into pancreatic endocrine cells in
vitro(20). Taguchi and Otsuki
reported that PDX-1 staining was detected in small evaginations of
the main pancreatic duct and in the nuclei of islet cells;
nestin-positive staining was also detected in small evaginations of
the main duct, islets and spindle-shaped cells in the connective
tissue around the main duct (17).
These authors proposed that the nestin and PDX-1 double-positive
cells were pancreatic stem cells. PDX-1 plays a significant role
during the formation of the pancreas by regulating insulin
secretion. PDX-1 has been reported to be capable of reprogramming
extrapancreatic tissue towards a β-cell phenotype (21). When implanted under the renal
capsule of NOD-SCID mice, PDX-1-transfected liver cells
transdifferentiated into insulin-producing cells, which reduced
hyperglycemia (22). Therefore,
the PDX-1 gene is significant in regulating the production of
insulin.
The objective of the current study was to examine
bone marrow-derived neural stem cells (NSCs) as a renewable source
of insulin-producing cells for autologous transplantation.
Materials and methods
Isolation and culture of BMSCs and
neuron-like cells
Sprague-Dawley rats 3–4 weeks old (60–70 g) were
obtained from the Laboratory Animal Center of South Medical
University (Guangzhou, China). The rats were sacrificed by cervical
dislocation and placed in 75% alcohol for 1 min. The two femurs
were removed and bone marrow cells were flushed from the marrow
with a 1-ml syringe filled with L-DMEM containing 100 U/ml heparin.
The cell suspension was centrifuged at 400 rpm for 8 min. The
supernatant was discarded and the cells were resuspended with
L-DMEM; cells were then layered over lymphocyte isolation reagent
(Institute of Hematology and Blood Diseases Hospital, Chinese
Academy of Medical Sciences) and centrifuged at 2,500 rpm for 20
min at 25°C. Mononuclear cells were removed from the gradient
interface and washed twice with PBS. For BMSCs, the pellet was
resuspended with L-DMEM (containing 10% FBS) and plated in
tissue-culture flasks at a density of 1×106 cells/ml.
For neuronal stem cells, the pellet was resuspended with neuronal
stem cell culture medium (patent no. 02134314.4, Neurosurgery Lab,
South Medical University) and plated in tissue-culture flasks at a
density of 1×107 cells/ml. Half of the culture medium
was removed following the first 48 h. The cells were then passaged
until cell growth reached 70–80% confluence. The study was approved
by the ethics committee of Shenzhen Longgang Central Hospital,
Shenzhen, China.
Immunocytochemical staining
The fourth passage of MSCs and first passage of NSCs
were fixed with 4% paraformaldehyde for 15 min, washed 3 times with
distilled water and incubated with 0.03% Triton X-100 for 10 min.
Cells were washed 3 times with PBS and subsequently incubated with
30% H2O2 in methanol (1:50) for 30 min. Cells
were then washed with distilled water 3 times and non-specific
binding was blocked with 5% BSA for 20 min at room temperature.
Cells were then incubated with primary antibodies against nestin
(Beijing Biosysnthesis Biotechnoloy Co., Ltd., Beijing, China) with
1:100 dilutions for 1 h at room temperature, washed 3 times with
PBS and incubated with SABC reagent (Beijing Biosysnthesis
Biotechnology) for 20 min at room temperature. The cells were
counter-stained with propidium iodide (PI) to detect nuclei,
observed using an Olympus phase contrast microscope (CK-2; Olympus
Optical Co. Ltd., Tokyo, Japan) and images were captured with an
Olympus PM-CBAD exposure control unit (Olympus, Centre Valley, PA,
USA). Fluorescence of cells was observed with an inverted
fluorescence microscope (Leica, Mannheim, German).
Reverse-transcription polymerase chain
reaction (RT-PCR)
Total RNA of BMSCs and NSCs was extracted with
TRIzol (Gibco, Carlsbad, CA, USA) and reverse transcribed to cDNA
with an RT-kit (Tiangen Biotech, Beijing, China). RT-PCR was
performed as follows: the amplification reaction was carried out
with preliminary denaturing at 95°C for 3 min followed by 35 cycles
of the following conditions: 94°C for 55 sec, 55°C for 45 sec, 72°C
for 1 min and 72°C for 8 min. The following primers for nestin were
used: forward, 5′-GCGGGGCGG TGCTGATAC-3′; reverse,
5′-AGGCAAGGGGGAAGA GAAGGATGT-3′ and the expected size of the PCR
product was 326 bp. PDX-1 primers were forward, 5′-CGGCCACAC
AGCTCTACAAGG-3′; reverse, 5′-GAGGTTACGGCA CAATCCTGA-3′ and the
expected size of the PCR product was 667 bp. The insulin primers
were forward, 5′-CGGGAG GATGGGCTTTTCTG-3′; reverse, 5′-AGCTGCTTTTGG
TTGAGCACAG-3′ and the expected size was 191 bp.
Transfection
The fourth passage of MSCs and 7-day cultures of
nestin-positive cells were dissociated from culture dishes with
0.25% trypsin-EDTA. Cells were washed and resuspended at a density
of 3 to 4×106 cells/ml in Nucleofector®
solution. Cell suspension (100 μl) and 5 μg
pBluescript-EGFP-C2-PDX-1 plasmid were mixed and transferred into
electroporation cuvettes. The A33 Nucleofector® Program
was selected to transfect cells. Following transfection, 4 ml
L-DMEM was added for culture and after 24 h, cellular morphology
was observed by fluorescence microscopy.
Fluorescent-activated cell sorting
(FACS)
Cells were detached from the dish with 0.25%
trypsin-EDTA 24 h following transfection. Cells were then washed
and resuspended in 500 ml PBS buffer. Transfection efficiency was
determined by the percentage of green fluorescent-positive cells
using a FACS system (Beckman Coulter, Miami, FL, USA).
Western blotting
Cells were collected and washed with PBS 3 times.
Cell extraction solution (100 μl) was then added and the mixture
was sonicated at 240 W for 6 sec and the cells were centrifuged at
13,000 × g for 10 min. Cell pellets were placed in 4X denatured
buffer and boiled for 3–5 min. Protein sample (15 μl) was then
added into each well of a 12% polyacryamide gel for electrophoresis
and the blot was transferred onto a PVDF membrane at 90 V for 90
min. Non-specific binding was blocked with 5% non-fat milk/PBS for
30 sec and subsequently 4 μl of the primary antibody in PBS was
added and incubated for 90 min at RT. The mixture was washed with
PBS 3 times and then incubated with 4 μl secondary antibody in PBS
for 90 min at room temperature. This mixture was then washed 3
times with PBS and the signal was developed with 50 μg/ml BCIP +
100 μg/ml NBT developing buffer and incubated for 5–10 min.
Enzyme-linked immunosorbent assay
(ELISA)
Insulin and C-peptide were determined by ELISA kit
(Diagnostic Systems Laboratories, Webster, TX, USA). Standards and
equal culture supernatant were added to the 96-well plates. The
absorbance was then measured with GENios spectrometer (Tecan Group
Ltd., Männedorf, Switzerland) at 450 nm. A relative optical density
value was plotted using these standards and the concentration of
insulin and C-peptide in the culture supernatant derived.
Results
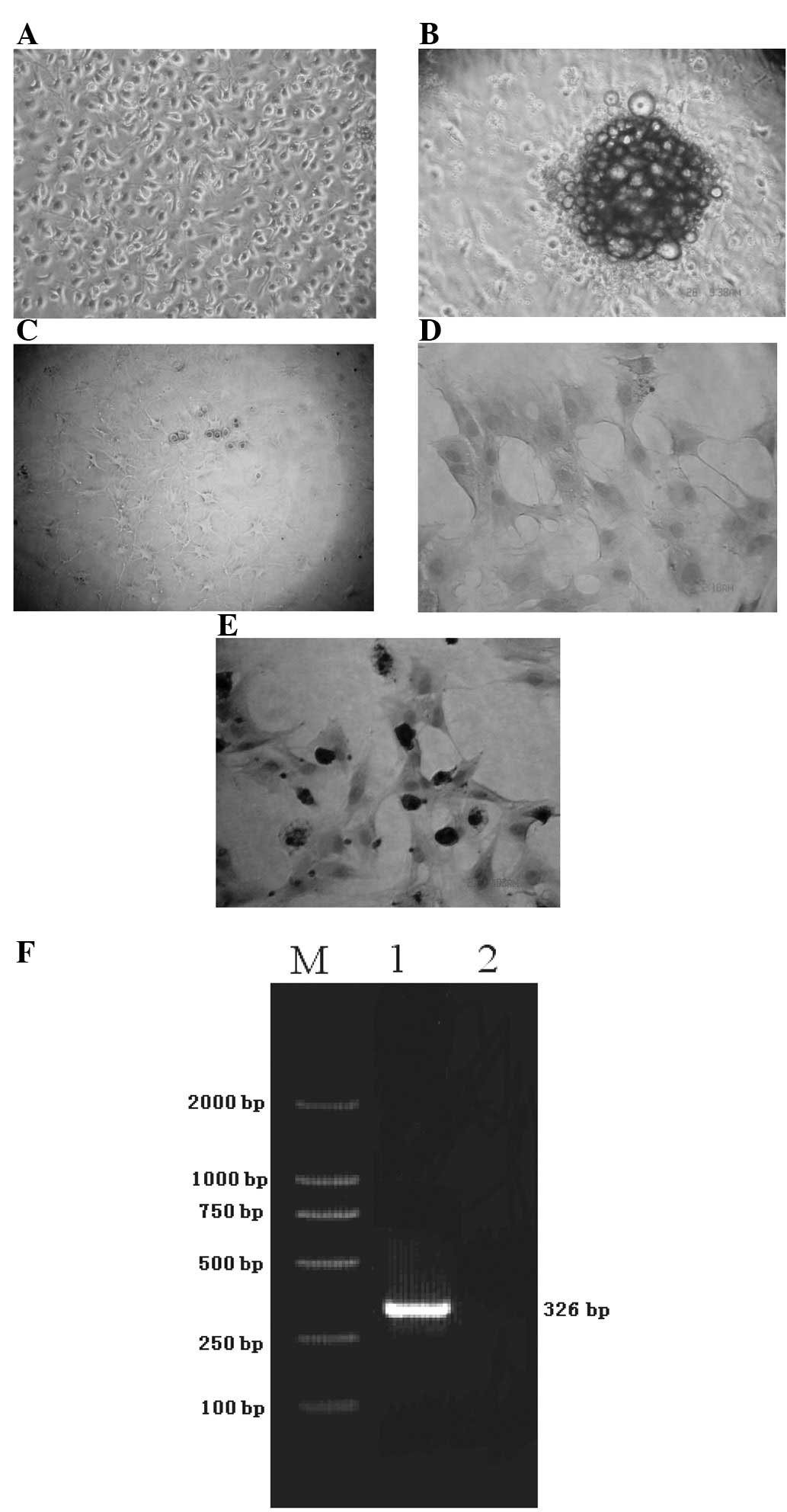
Nestin mRNA is expressed in NSCs
Bone marrow cells from the femurs of SD rats were
harvested and separated by Ficoll. Cells were cultured in L-DMEM
with 10% FBS for the generation of BMSCs and cultured in neurostem
cell medium for the generation of NSCs. BMSCs adhered to the
culture dishes and grew rapidly following 6 days of culture
(Fig. 1A). However, the NSCs
formed neurospheres after 3 days (Fig.
1B) and dendrites of the NSCs appeared after 8–10 days
(Fig. 1C). To characterize BMSCs
and NSCs, immunocytochemical staining and RT-PCR of nestin were
performed. Strong staining of nestin was observed in NSCs but not
BMSCs (Fig. 1D and E). The RT-PCR
analysis also confirmed that nestin mRNA was expressed in NSCs, but
not in BMSCs (Fig. 1F).
Cells transfected with
pBluescript-EGFP-C2-PDX-1 express mRNA (667 bp) and protein (46
kDa)
To induce cell differentiation, NSCs were
transfected with pBluescript-EGFP-C2-PDX-1. Following 7 days of
transfection, the green fluorescence of
pBluescript-EGFP-C2-PDX-1-transfected cells was observed by
fluorescence microscope (Fig. 2A)
and the transfection efficiency was 78.6% by FACS analysis
(Fig. 2B). The cells became round
with larger nuclei and, 3 days after PDX-1 transfection, the
nuclei-to-plasma ratio increased (Fig.
2C). The RNA of vector-only (pBluescript-EGFP-C2) or
pBluescript-EGFP-C2-PDX-1-transfected cells was extracted and the
expression of PDX-1 mRNA was examined by RT-PCR and PDX-1 protein
was determined by western blot analysis (Fig. 2D and E). Cells transfected with
pBluescript-EGFP-C2-PDX-1 expressed mRNA (667 bp) and protein (46
kDa). Neither PDX-1 mRNA nor protein expression was observed in
vector-transfected cells.
 | Figure 2PDX-1 expression in NSCs. NSCs were
transiently transfected with pEGFP-C2-PDX-1 plasmids (30 μg) for 24
h. (A) Fluorescent images of transfected cells were captured with a
fluorescence microscope. (B) The transfection efficiency was
quantified by flow cytometry. The M2 region indicates the
percentage of GFP expression in total cells. (C) The cell
morphology of pEGFP-C2-PDX-1-transfected NSCs was observed on the
third day. (D, left) Total RNA was extracted 2 days following
transfection and underwent electrophoresis. (D, right) The
expression of PDX-1 mRNA on pEGFP-C2 and pEGFP-C2-PDX-1-transfected
NSCs was analyzed by RT-PCR. Lane M, DNA ladder; lane 1,
pEGFP-C2-PDX-1-transfected cells; lane 2, pEGFP-C2-transfected
cells. (E) Protein expression of PDX-1 was analyzed by western
blotting. Lane 1, pEGFP-C2-PDX-1-transfected cells; lane 2,
pEGFP-C2-transfected cells. PDX-1, pancreatic duodenal homeobox-1;
NSC, neural stem cell; GFP, green fluorescent protein. |
Insulin mRNA was induced in the presence
of glucose, GLP1 and glucose + GLP1
To determine whether the PDX-1-transfected NSCs were
responsive to glucose or glucagon-like peptide 1 (GLP1) challenges,
insulin mRNA and insulin protein were measured using RT-PCR and
ELISA. Following 7 days of culture, NSCs were transfected with
vector or pBluescript-EGFP-C2-PDX-1. Cells were then cultured in
20% FBS/L-DMEM only (control group) or with glucose (group A), GLP1
(group B) or glucose + GLP1 (group C). Vector
(pBluescript-EGFP-C2)-transfected cells were cultured with glucose
+ GLP1 (group D) as a control. The PDX-1 mRNA was detected by
RT-PCR in all EGFP-C2-PDX-1-transfected cells (Fig. 3A). Subsequently, whether insulin
was induced by glucose or GLP1 was examined. As shown in Fig. 3B, insulin mRNA was induced in the
presence of glucose (lane 2), GLP1 (lane 3) and glucose + GLP1
(lane 4). Neither pEGFP-C2-PDX-1-transfected cells cultured with
medium (lane 1) nor vector-transfected cultured with glucose + GLP1
(lane 5) produced insulin. To determine whether the
PDX-1-transfected NSCs synthesized insulin protein, the
supernatants were collected and determined by ELISA. Fig. 3C shows that insulin was detected in
the control group (145 pg/ml), group A (144±57.28 pg/ml), group B
(208±117 pg/ml), group C (261.25±69.88 pg/ml) and group D
(271.25±89.68 pg/ml) prior to glucose/GLP-1 stimulation. However,
the insulin was not significantly released following a
glucose/GLP-1 challenge. Similar results were observed in the
production of C-peptide (Fig.
3D).
 | Figure 3Effects of glucose and GLP-1 on
PDX-1-transfected NSCs. (A) The PDX-1-transfected nestin-positive
NSCs were treated with glucose and/or GLP-1. After 2 h, the cells
were harvested and the mRNA expression levels of (A) PDX-1 and (B)
insulin in NSCs after various treatments were determined by RT-PCR.
Lane M, DNA ladder; lanes 1–4, EGFP-C2-PDX-1-transfected NSCs with
treatment with medium (lane 1), glucose (lane 2), GLP-1 (lane 3),
GLP-1 and glucose (lane 4); lane 5, EGFP-C2-transfected NSCs +
GLP-1 and glucose. Before (black bar) and after (grey bar)
treatment of glucose, GLP-1, or glucose + GLP-1, (C) production of
insulin and (D) C-peptide was determined by ELISA. Control and
groups A-C were EGFP-C2-PDX-1-transfected NSCs with treatment of
medium (control), glucose (group A), GLP-1 (group B) and GLP-1 +
glucose (group C). Group D, EGFP-C2-transfected NSCs treated with
GLP-1 and glucose. GLP-1, glucagon-like peptide-1; PDX-1,
pancreatic duodenal homeobox-1; NSC, neural stem cell. |
Discussion
Previous studies have shown that insulin-producing
cells are generated from progenitor cells from various sources,
including liver (8), pancreas
(23,24), intestinal epithelium (25) and embryonic stem cells (26). However, overcoming the rejection of
transplanted β cells remains a challenge. Moreover, it is difficult
to obtain sufficient stem cells from these organs. Bone marrow is
an abundant source of adult stem cells. This study examined the
possibility of using bone marrow cells that differentiate into NSCs
by transfecting the PDX-1 gene into BMSCs to generate
insulin-producing cells. The production of insulin from
PDX-1-transfected NSCs was confirmed by RT-PCR and ELISA. The
current study suggests that bone marrow contains pluripotent cells
that are capable of being reprogrammed to differentiate into
insulin-producing cells.
In pancreatic β cells, GLP-1 regulates numerous
genes that control insulin, glucokinase, glucose transporter-1
(GLUT-1), GLUT2 and hexokinase I (27–29).
GLP-1 also enhances insulin secretion in a glucose-dependent
manner. However, significantly increased insulin release was not
observed in the presence or absence of glucose, GLP-1, or with
glucose + GLP-1 in the PDX-1-transfected NSCs of the current study.
The PDX-1-transfected NSCs spontaneously released insulin without
glucose/GLP-1 challenge. These data suggest that PDX-1-transfected
NSCs are β-like cells and further induction, including with
nicotinamide and exendin 4, is required to reach a higher degree of
differentiation, as observed in native pancreatic β cells (13,30).
Campbell and Macfarlane reported that glucose, GLP-1
and insulin positively regulate the PDX-1 gene promotor in
pancreatic β cells (31). In the
current study, glucose or GLP-1 induced the expression of mRNA and
insulin in PDX-1-transfected nestin-positive cells, but no
synergistic effect was identified. There are three possible reasons
for this. Firstly, the expression level of plasmid DNA may have
changed as the transfection system is different. In the current
study, the plasmid was transfected by Nucleofector into the nucleus
directly, not into the cytoplasm. Secondly, the concentration of
glucose or GLP-1 was too high to induce a synergistic effect and
thirdly, PDX-1 may be regulated through a pathway independent of
glucose and GLP-1.
Multiple growth factors, including insulin, were
added to the cell culture prior to transfection. However, neither
PDX-1-transfected nestin-positive cells nor nestin-positive cells
in the presence of glucose and GLP-1 produced insulin, suggesting
that insulin production was induced by glucose and GLP-1
stimulation in PDX-1-transfected nestin-positive cells. Conversely,
insulin production was not induced in nestin-positive cells in the
presence of GLP-1 and glucose. That PDX-1 is necessary for glucose
and GLP-1 to induce insulin expression was consistent with the
results of previous studies (32,33).
The length of time required for BM-derived
nestin-positive cells to differentiate into insulin-producing cells
was shorter in the present study compared with that in others
(13,34). There are two reasons for this
difference. Firstly, the plasmid DNA was transfected into the
nucleus directly and the expression reached a peak after 24 h.
Additionally, the amount of time for PDX-1 expression was shorter
in the current study compared with that of others. Secondly, in the
study by Gao et al, high concentrations of serum inhibited
the differentiation of β cells (35). However, in the current study, high
concentrations of serum enhanced cell proliferation and cell-cell
contact, resulting in a more rapid differentiation.
This study provides evidence that PDX-1-transfected
BM-derived nestin-positive cells differentiated into
insulin-producing cells following stimulation with glucose and
GLP-1. However, more research is required to clarify the mechanisms
of a single or synergistic effect of growth factors. Based on these
findings, the source of insulin-producing cells appears to be
unlimited for autologous transplantation for type-1 diabetes.
Acknowledgements
This study was supported by the Doctoral Initiating
Project of Guangdong Province Foundation for Natural Sciences
(project number, 8445583547-7001223).
References
|
1
|
Robertson RP, Davis C, Larsen J, Stratta R
and Sutherland DE: Pancreas and islet transplantation for patients
with diabetes. Diabetes Care. 23:112–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar
|
|
3
|
Weir GC and Bonner-Weir S: Scientific and
political impediments to successful islet transplantation.
Diabetes. 46:1247–1256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weir GC and Bonner-Weir S: Islet
transplantation as a treatment for diabetes. J Am Optom Assoc.
69:727–732. 1998.PubMed/NCBI
|
|
5
|
Ryan EA, Paty BW, Senior PA, Bigam D,
Alfadhli E, Kneteman NM, Lakey JR and Shapiro AM: Five-year
follow-up after clinical islet Transplantation. Diabetes.
54:2060–2069. 2005.PubMed/NCBI
|
|
6
|
Roche E, Reig JA, Campos A, Paredes B,
Isaac JR, Lim S, Calne RY and Soria B: Insulin-secreting cells
derived from stem cells: clinical perspectives, hypes and hopes.
Transpl Immunol. 15:113–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roche E, Santana A, Vicente-Salar N and
Reig JA: From stem cells to insulin-producing cells: towards a
bioartificial endocrine pancreas. Panminerva Med. 47:39–51.
2005.PubMed/NCBI
|
|
8
|
Yang L, Li S, Hatch H, Ahrens K, Cornelius
JG, Petersen BE and Peck AB: In vitro trans-differentiation
of adult hepatic stem cells into pancreatic endocrine
hormone-producing cells. Proc Natl Acad Sci USA. 99:8078–8083.
2002. View Article : Google Scholar
|
|
9
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tuan RS, Boland G and Tuli R: Adult
mesenchymal stem cells and cell-based tissue engineering. Arthritis
Res Ther. 5:32–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang DQ, Cao LZ, Burkhardt BR, Xia CQ,
Litherland SA, Atkinson MA and Yang LJ: In vivo and in vitro
characterization of insulin-producing cells obtained from murine
bone marrow. Diabetes. 53:1721–1732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee K, Majumdar MK, Buyaner D, Hendricks
JK, Pittenger MF and Mosca JD: Human mesenchymal stem cells
maintain transgene expression during expansion and differentiation.
Mol Ther. 3:857–866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou YH, Khuon S, Herrmann H and Goldman
RD: Nestin promotes the phosphorylation-dependent disassembly of
vimentin intermediate filaments during mitosis. Mol Biol Cell.
14:1468–1478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taguchi M and Otsuki M: Co-localization of
nestin and PDX-1 in small evaginations of the main pancreatic duct
in adult rats. J Mol Histol. 35:785–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yokoyama A, Sakamoto A, Kameda H, Imai Y
and Tanaka J: NG2 proteoglycan-expressing microglia as multipotent
neural progenitors in normal and pathologic brains. Glia.
53:754–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yokoyama A, Yang L, Itoh S, Mori K and
Tanaka J: Microglia, a potential source of neurons, astrocytes and
oligodendrocytes. Glia. 45:96–104. 2004.PubMed/NCBI
|
|
20
|
Milanesi A, Lee JW, Xu Q, Perin L and Yu
JS: Differentiation of nestin-positive cells derived from bone
marrow into pancreatic endocrine and ductal cells in vitro. J
Endocrinol. 209:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferber S, Halkin A, Cohen H, Ber I, Einav
Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N and
Karasik A: Pancreatic and duodenal homeobox gene 1 induces
expression of insulin genes in liver and ameliorates
streptozotocin-induced hyperglycemia. Nat Med. 6:568–572. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sapir T, Shternhall K, Meivar-Levy I,
Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I,
Goldberg I, Pri-Chen S, et al: Cell-replacement therapy for
diabetes: Generating functional insulin-producing tissue from adult
human liver cells. Proc Natl Acad Sci USA. 102:7964–7969. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonner-Weir S, Taneja M, Weir GC,
Tatarkiewicz K, Song KH, Sharma A and O’Neil JJ: In vitro
cultivation of human islets from expanded ductal tissue. Proc Natl
Acad Sci USA. 97:7999–8004. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramiya VK, Maraist M, Arfors KE, Schatz
DA, Peck AB and Cornelius JG: Reversal of insulin-dependent
diabetes using islets generated in vitro from pancreatic stem
cells. Nat Med. 6:278–282. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki A, Nakauchi H and Taniguchi H:
Glucagon-like peptide 1 (1–37) converts intestinal epithelial cells
into insulin-producing cells. Proc Natl Acad Sci USA.
100:5034–5039. 2003.
|
|
26
|
Assady S, Maor G, Amit M, Itskovitz-Eldor
J, Skorecki KL and Tzukerman M: Insulin production by human
embryonic stem cells. Diabetes. 50:1691–1697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Egan JM, Raygada M, Nadiv O, Roth
J and Montrose-Rafizadeh C: Glucagon-like peptide-1 affects gene
transcription and messenger ribonucleic acid stability of
components of the insulin secretory system in RIN 1046-38 cells.
Endocrinology. 136:4910–4917. 1995.PubMed/NCBI
|
|
28
|
Wang Y, Perfetti R, Greig NH, Holloway HW,
DeOre KA, Montrose-Rafizadeh C, Elahi D and Egan JM: Glucagon-like
peptide-1 can reverse the age-related decline in glucose tolerance
in rats. J Clin Invest. 99:2883–2889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MacDonald PE, El-kholy W, Riedel MJ,
Salapatek AF, Light PE and Wheeler MB: The multiple actions of
GLP-1 on the process of glucose-stimulated insulin secretion.
Diabetes. 51(Suppl 3): S434–S442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohgawara H, Kawamura M, Honda M, et al:
Reversal of glucose insensitivity of pancreatic B-cells due to
prolonged exposure to high glucose in culture: effect of
nicotinamide on pancreatic B-cells. Tohoku J Exp Med. 169:159–166.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Campbell SC and Macfarlane WM: Regulation
of the pdx1 gene promoter in pancreatic beta-cells. Biochem Biophys
Res Commun. 299:277–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao LZ, Tang DQ, Horb ME, Li SW and Yang
LJ: High glucose is necessary for complete maturation of
Pdx1-VP16-expressing hepatic cells into functional
insulin-producing cells. Diabetes. 53:3168–3178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hui H, Wright C and Perfetti R:
Glucagon-like peptide 1 induces differentiation of islet duodenal
homeobox-1-positive pancreatic ductal cells into insulin-secreting
cells. Diabetes. 50:785–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hori Y, Gu X, Xie X and Kim SK:
Differentiation of insulin-producing cells from human neural
progenitor cells. PLoS Med. 2:e1032005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao R, Ustinov J, Pulkkinen MA, Lundin K,
Korsgren O and Otonkoski T: Characterization of endocrine
progenitor cells and critical factors for their differentiation in
human adult pancreatic cell culture. Diabetes. 52:2007–2015. 2003.
View Article : Google Scholar : PubMed/NCBI
|