Introduction
The liver is predominantly comprised of polarized
epithelial cells, which regulate the intermediary metabolism,
detoxification, the manufacture of critical circulating proteins
and the production of bile for digestion (1). However, the liver is susceptible to
different types of injuries. Hepatocyte apoptosis is considered to
be a pivotal pathological process in a variety of liver injuries.
The damaged hepatocytes release apoptotic bodies that are engulfed
by resident kupffer and hepatic stellate cells (HSCs), which
release chemokines and cytokines that subsequently promote HSC
activation and transdifferentiation into myofibroblasts, resulting
in dysregulated hepatic fibrosis and cirrhosis (2). Cirrhosis is the worst consequence of
continuous liver injury, as it may lead to portal hypertension,
liver failure and mortality (3).
The optimal therapeutic strategy for several liver diseases is to
directly inhibit the mechanism that triggers the liver injury.
Clinically, antiviral therapies have been demonstrated to be
effective for viral hepatitis. However, there are no effective
treatments for a variety of liver diseases, including liver
fibrosis, and patients with hepatitis C (HCV) or hepatitis B
viruses (HBV) who are unresponsive to antiviral therapies. For
these patients, taking effective measures to protect the
hepatocytes from injury and inhibit apoptosis are important
therapeutic strategies (4).
Nerve growth factor (NGF) and its receptors are
produced in nervous and non-nervous system cells, which regulate
the differentiation, survival, development and apoptosis processes
of cells by means of autocrine or paracrine signaling and are also
involved in wound healing and tissue remodeling processes. The
biological effects of NGF are mediated through two membrane
receptors, the tropomyosin tyrosine kinase-A nerve growth factor
receptor (TrkANGFR) and the p75 pan-neurotrophin
receptor (p75NTR) (5).
Recent evidence has suggested that NGF has broader physiological
effects, which may be involved in the liver injury-repair process,
since its mRNA and protein expression levels become elevated whilst
regenerating hepatocytes. Furthermore, activated HSCs induced by
lead nitrate in rats (6), partial
hepatectomy (7) or CCl4
treatment in mice (8) and
exogenous recombinant NGF may promote activated HSC apoptosis
(8,9). Certain authors have demonstrated that
manipulation of the NGF/p75NTR axis represents a novel
means for regulating the progression and resolution of liver
fibrosis (10). However, little is
known with regards to the protective effect of NGF on damaged
hepatocytes.
In this study, we provide evidence that human
hepatocyte L-02 cells express NGF and its receptors,
TrkANGFR and p75NTR. Furthermore, we report
that exogenous human recombinant β-NGF attenuates injury and
inhibits the apoptosis of L-02 cells induced by D-galactosamine
(D-GalN), and that the protective mechanism of β-NGF is likely to
be mediated through the TrkANGFR signaling pathway.
Materials and methods
Chemicals
DMEM, fetal bovine serum (FBS), penicillin,
streptomycin, 0.25% EDTA-Trypsin, D-GalN and silymarin (Sily) were
purchased from Life Technologies (Gibco, Carlsbad, CA, USA).
Recombinant human β-NGF was obtained from R&D Systems
(Minneapolis, MN, USA) and rabbit polyclonal anti-NGF,
anti-TrkANGFR and anti-p75NTR antibodies were
derived from Boster Bioengineering Limited Company (Wuhan, China).
Human albumin (ALB), lactate dehydrogenase (LDH) activity,
malondialdehyde (MDA) and glutathione (GSH) assay kits were
supplied by Jiancheng Bioengineering Institute (Nanjing, China).
The BD™ MitoScreen (JC-1) kit was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). The cell proliferation reagent XTT was
purchased from Bio Basic Inc (BBI, Markham, ON, Canada). The
MaxVision™ kit and 3,3′-diaminobenzidine (DAB) were purchased from
the Maixin Bioengineering Institute (Fuzhou, China).
Cell cultures
The human liver L-02 cell line (Institute of
Biochemistry and Cell Biology, SIBS, Shanghai, China) was cultured
in DMEM medium supplemented with 10% FBS, 100 U/ml penicillin and
100 μg/ml streptomycin, and maintained in a humidified 5%
CO2 incubator at 37°C. The culture medium was replaced
every 2–3 days. The cells were sub-cultured and allowed to adhere
for 24 h prior to performing subsequent experiments.
Immunocytochemistry
L-02 cells (1×105 cells/well) were seeded
into 6-well plates with coverslips and fixed with 4% formaldehyde
in PBS at room temperature (RT) for 15 min, then washed three times
with PBS. L-02 cells were incubated overnight at 4°C with primary
rabbit polyclonal antibodies against NGF or TrkANGFR, or
p75NTR diluted (1:100) in PBS containing 0.1% Triton
X-100 detergent. Cells were washed three times with PBS and
subsequently incubated for 30 min at RT with the MaxVision kit
(HRP-Polymer anti-rabbit lgG) according to the manufacturer’s
instructions. The signal was detected using DAB as the chromogen.
Counterstaining was performed with Mayer’s hematoxylin (Invitrogen,
Carlsbad, CA, USA). Negative controls were obtained by omitting the
primary antibody. Stained cells were visualized using an inverted
optical microscope (Olympus CK40, Tokyo, Japan).
Light microscopy
L-02 cells were pretreated with β-NGF for 30 min and
subsequently incubated with D-GalN for 24 h. The alterations to
cell morphology were viewed using an inverted Olympus CK40
microscope. Microphotographs were captured with a digital Canon EOS
600D camera (Canon, Tokyo, Japan).
Cell proliferation assay
The primary culture of L-02 cells were seeded into
96-well plates at a density of 5×103 cells/well and
pretreated with β-NGF at various concentrations (0, 25, 50, 100,
200 and 400 μg/l) for 30 min prior to incubation with D-GalN (40
mmol/l). Sily (100 mg/l) was used as a positive control. In order
to evaluate whether the neurotrophin receptors, TrkANGFR
and/or p75NTR, were involved in the response of the L-02
cells to β-NGF, the cells were cultured in the presence of β-NGF
(100 μg/l) and anti-TrkANGFR (200 μg/l) or
anti-p75NTR (200 μg/l) for 30 min, then D-GalN was added
and incubated for a further 24 h. Following this period, the XTT
reagent was added to the cultured medium and incubated in a
humidified atmosphere for 4 h. The absorbance of the samples was
measured using a microplate reader at the dual wavelength mode of
450 and 630 nm, respectively. The experiment was performed in
tetramerous and repeated three times with consistent results.
Biochemical assay
Hepatocyte toxicity was determined by LDH activity
and the concentration of MDA in the culture medium. The production
of ALB, an important functional marker of hepatocytes, was
evaluated using a colorimetric method. The antioxidative condition
of hepatocytes was determined by measuring the levels of GSH in the
cell lysate.
Mitochondrial membrane potential
(MMP)
The change in MMP was evaluated from uptake of JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine
iodide), a cationic lipophilic fluorescent probe. In intact cells,
the healthy mitochondrial membrane was polarized and JC-1 was
rapidly taken up by mitochondria. This uptake increased the
concentration gradient of JC-1, which led to the formation of JC-1
aggregates in the mitochondrial matrix. JC-1 aggregates exhibited a
red spectral shift resulting in higher levels of red fluorescence
(Emmax 590 nm). In cells with an altered MMP, JC-1 did
not aggregate in the mitochondria and thus remained in the
cytoplasm as monomers, producing green fluorescence
(Emmax 527 nm). L-02 cells were incubated with JC-1 at
37°C for 15 min and centrifuged at 400 × g for 5 min at RT.
Subsequently, the cells were carefully removed, the supernatant was
discarded and the cells were resuspended in the JC-1 working
solution. L-02 cells were analyzed by flow cytometry at the
excitation and emission wavelengths of 527 and 590 nm,
respectively.
Statistical analysis
All values are expressed as the mean ± SD.
Differences between the groups were compared using a one-way
analysis of variance (ANOVA) test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of NGF, TrkANGFR
and p75NTR in L-02 cells
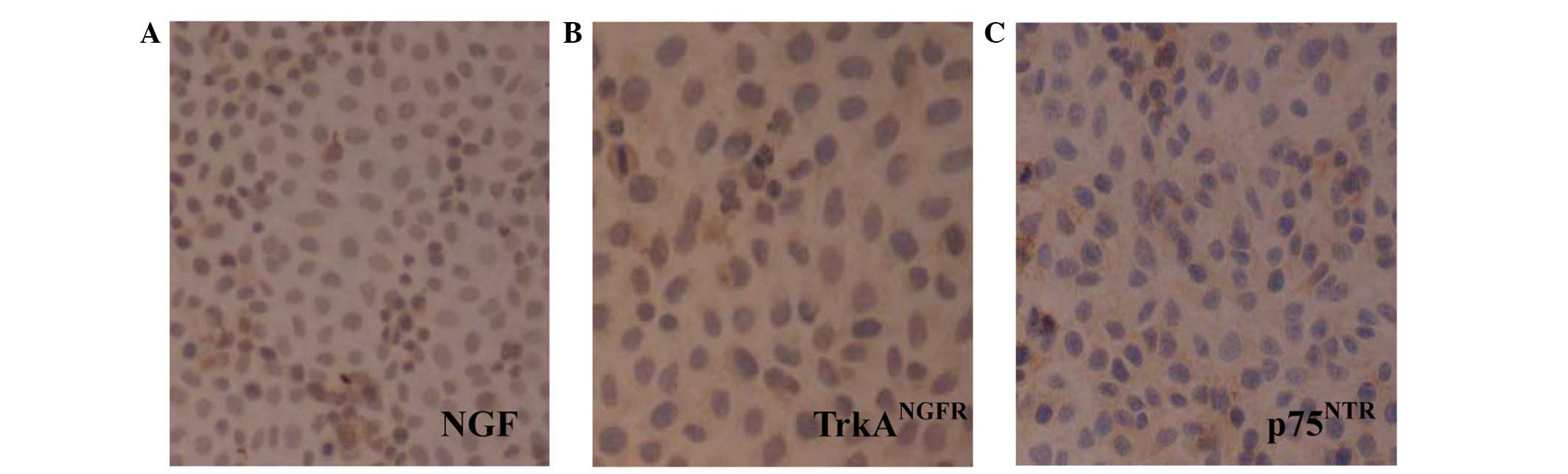
The expression of NGF, TrkANGFR and
p75NTR was examined in L-02 cells using
immunocytochemistry. Immunostained NGF was detected prominently in
the cytoplasm and on the nuclei, and TrkANGFR and
p75NTR immunoreactivities were observed on the cell
membrane and in the cytoplasm of L-02 cells (Fig. 1).
Morphological changes of L-02 cells in
the culture medium
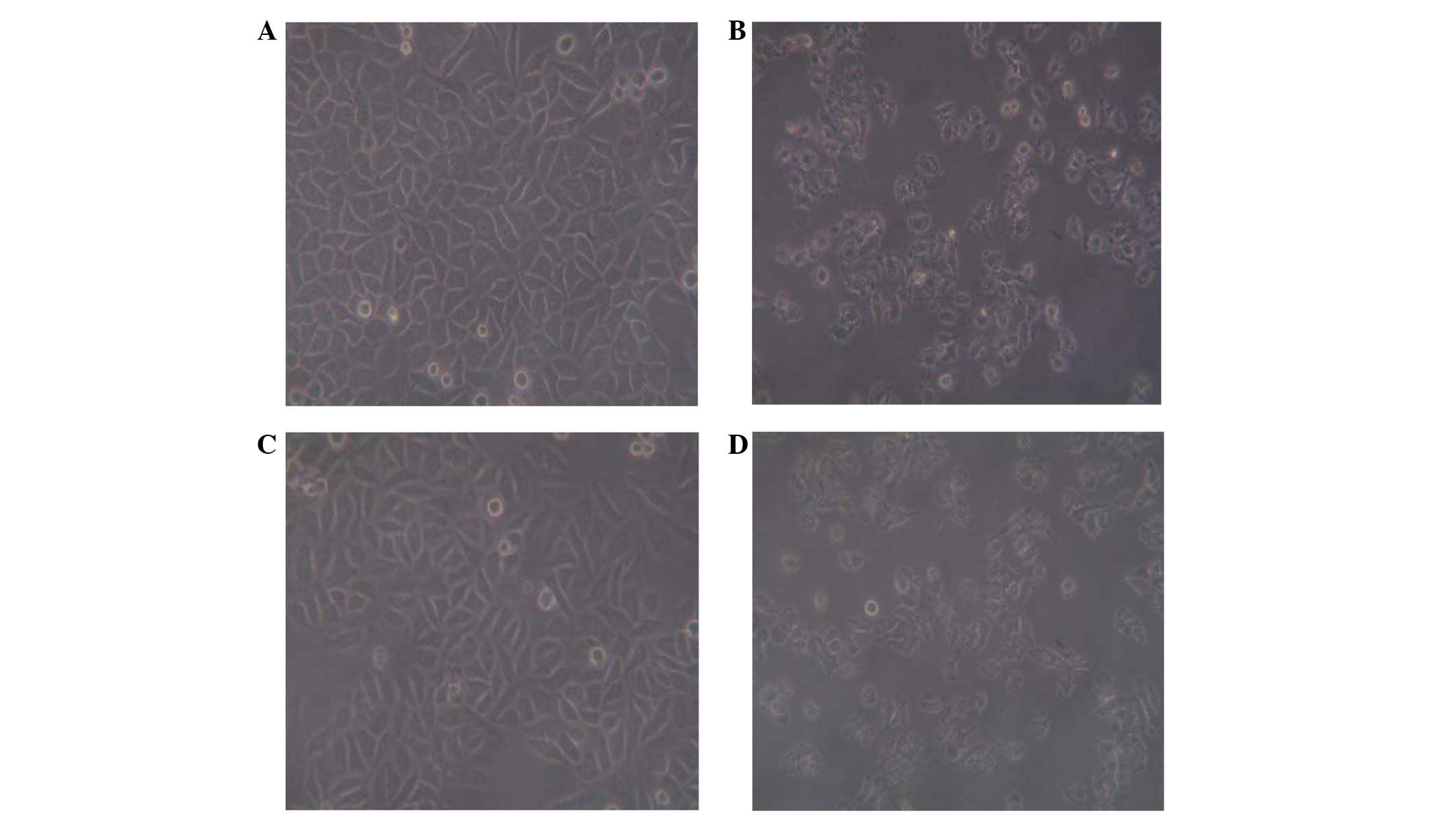
The morphology of L-02 cells incubated with D-GalN
demonstrated discontinuities of the cytoplasm membrane, its
spherical shape and highly granular cytoplasm formed a notable
contrast to the intact cells. β-NGF and Sily were capable of
markedly improving the D-GalN-induced morphological changes to L-02
cells (Fig. 2).
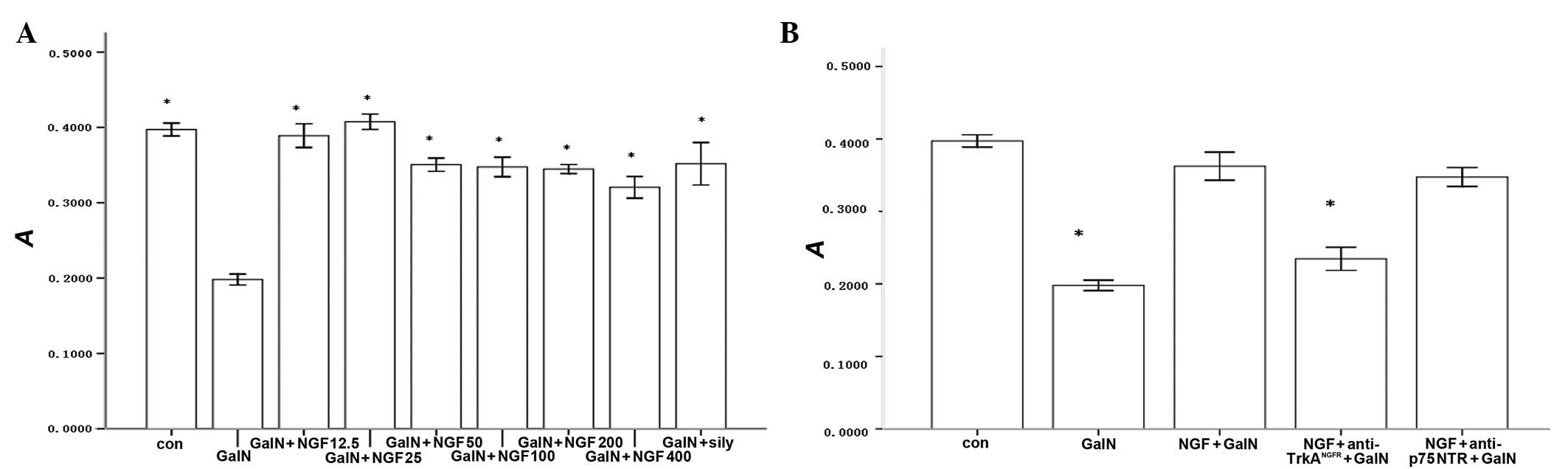
β-NGF promotes the proliferation of L-02
cells
Exogenous human recombinant β-NGF was capable of
promoting the proliferation of D-GalN-injured L-02 cells in a
dose-dependent manner. We observed a statistically significant
increase at concentrations between 25–400 μg/l. β-NGF and Sily
significantly promoted the proliferation of L-02 cells compared
with D-GalN alone. However, there were no differences between the
β-NGF and Sily groups (Fig. 3A).
To demonstrate which signaling pathway contributed to β-NGF
irritating the proliferation events of L-02 cells, we inhibited the
biological activity of TrkANGFR with
anti-TrkANGFR and observed that the promoted
proliferation effect of β-NGF on L-02 cells was blocked. However,
pretreatment of L-02 cells with the anti-p75NTR
antibody, which neutralized the binding of β-NGF to
p75NTR, did not affect the proliferation of L-02 cells
responding to β-NGF (Fig. 3B).
These data suggested that TrkANGFR participates in the
response of the L-02 cells to β-NGF.
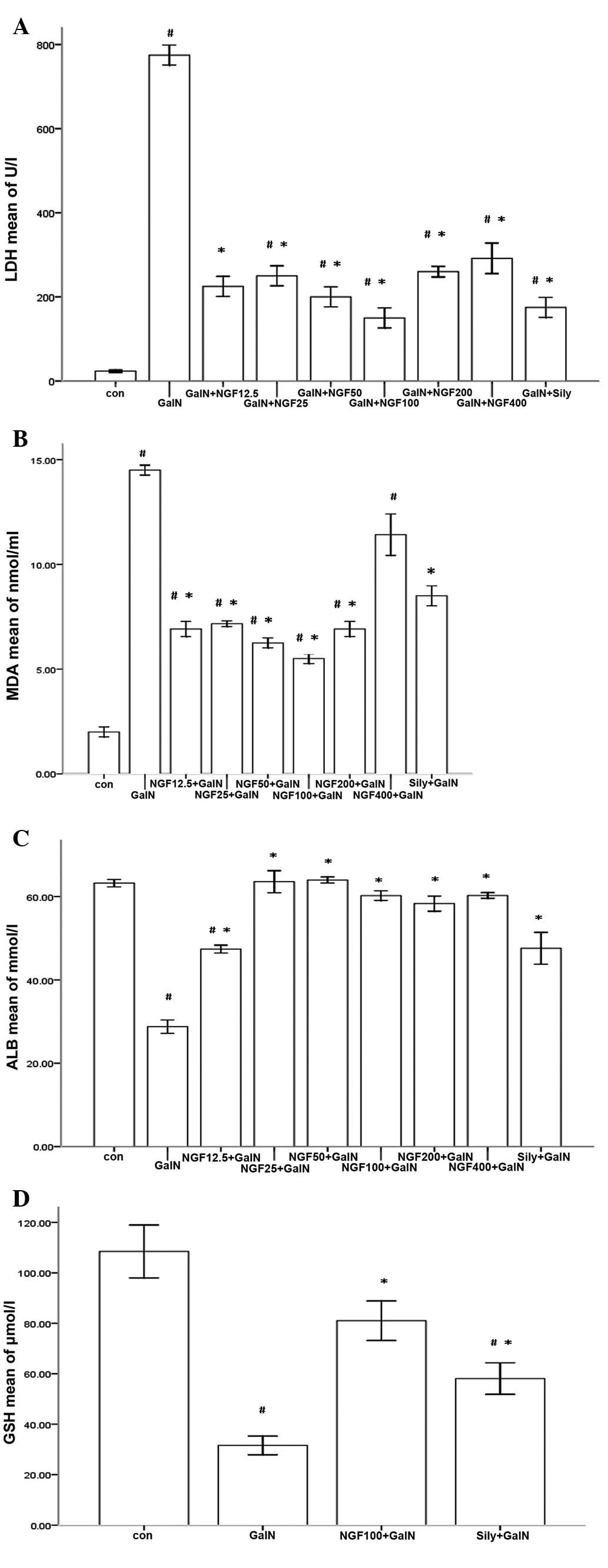
Biochemical changes of β-NGF on
D-GalN-induced L-02 cells
D-GalN severely injured L-02 cells in the primary
culture following 24 h incubation. D-GalN induced a marked increase
in LDH and MDA activity in the supernatant (Fig. 4A and B). Furthermore, ALB
production and the GSH content of the cells decreased (Fig. 4C and D). β-NGF reduced the levels
of LDH and MDA released from D-GalN-induced L-02 cells, while ALB
synthesis was markedly enhanced and the cell content of GSH was
increased.
Effect of β-NGF on the MMP of
D-GalN-induced L-02 cells
The MMP was measured using the fluorescent probe
JC-1. There was low accumulation of JC-1 in the mitochondrial
matrix of L-02 cells incubated with D-GalN. However, β-NGF
significantly decreased the permeability of the inner mitochondrial
membrane and JC-1 accumulated in the mitochondrial matrix,
recovering the MMP damaged by D-GalN. There was no statistical
difference in JC-1 accumulation in the mitochondrial matrix between
the β-NGF and Sily treatment groups (Fig. 5).
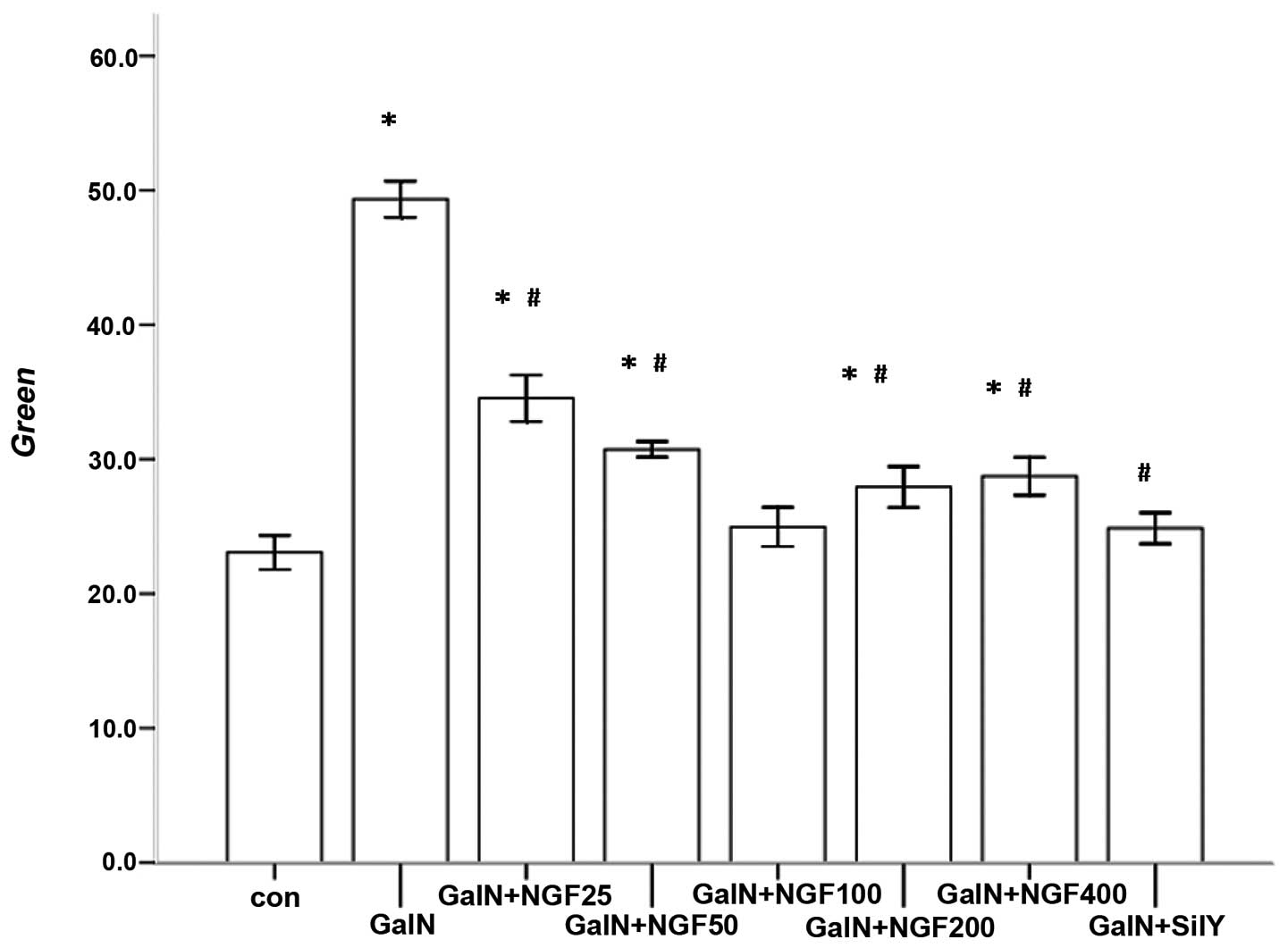 | Figure 5Accumulation of JC-1 in the cytoplasm
decreased following NGF treatment of L-02 cells injured by D-GalN.
*P<0.05 vs. control; #P<0.05 vs. GalN.
Con, control; D-GalN, D-galactosamine; NGF, nerve growth factor;
sily, silymarin; JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide. |
Discussion
D-GalN is frequently used in liver damage models to
replicate the effects of human viral hepatitis, which induces
diffuse hepatic injury by depleting uridine nucleotides, resulting
in the inhibition of the synthesis of messenger RNA and subsequent
proteins (11). A hepatotoxic dose
of D-GalN induces hepatic tissue lesions associated with a decrease
in GSH synthesis or an increase in the breakdown of GSH (12,13).
Mitochondrial permeability transition (MPT) leads to the
dissipation of MMP and is considered to be a fundamental mechanism
of cell apoptosis or necrosis (14). Liver regeneration driven by
hepatocyte proliferation is required for hepatic tissue
injury-repair and survival following acute/chronic liver injury
(15,16).
This study demonstrated that pretreatment with
exogenous human recombinant β-NGF promoted the proliferation and
inhibited apoptosis of D-GalN-induced L-02 cells compared with
D-GalN alone. LDH is an important marker for hepatocyte injury. We
identified that D-GalN significantly increased the activity of LDH
in the L-02 cell culture medium. However, pretreatment with β-NGF
markedly decreased the LDH activity of D-GalN-induced L-02 cells.
MDA is a marker for lipid peroxidation. Pretreatment with β-NGF
reduced the concentration of MDA and increased intracellular GSH
content compared with D-GalN alone. The production of ALB by
D-GalN-incubated L-02 cells was markedly reduced, but β-NGF may
improve the synthesis of ALB by D-GalN-induced L-02 cells. However,
high MMP in L-02 cells was completely eradicated by D-GalN. The
opening of high-conductance MPT pores increases the permeability of
the inner-mitochondrial membrane, which results in the collapse of
the MMP, a disruption in ionic homeostasis, cytochrome C release
and subsequently, apoptosis (17).
However, pretreatment with β-NGF may prevent MPT pores from opening
by repairing the plasma membrane injured by GalN-induced L-02
cells, which may be achieved by enhancing GSH synthesis. The
integrity of the cells was damaged by D-GalN, whose representations
were associated with discontinuities in the plasma membrane, a
spherical shape and a highly granular cytoplasm when compared with
intact cells (18). Treatment with
β-NGF markedly reduced D-GalN-induced morphological changes to the
cells. These results indicated that β-NGF is a potential drug for
protecting L-02 cells from D-GalN-induced injury.
To investigate the mechanism by which β-NGF promotes
the proliferation of L-02 cells, we performed immunocytochemical
staining for NGF, TrkANGFR and p75NTR in L-02
cells. We discovered that L-02 cells expressed NGF,
TrkANGFR and the p75NTR protein. In order to
evaluate which, or both, neurotrophin receptors were involved in
the response of L-02 cells to β-NGF, L-02 cells were pretreated
with β-NGF and anti-TrkANGFR or anti-p75NTR
for 30 min and subsequently damaged by D-GalN for 24 h. The
promoted proliferation effect of β-NGF on the L-02 cells was
inhibited by anti-TrkANGFR. Notably, this selectively
inhibited β-NGF binding to p75NTR with
anti-p75NTR, yet it did not prevent β-NGF from promoting
the proliferation of L-02 cells. These data demonstrate that
TrkANGFR may be involved in the response of L-02 cells
to β-NGF.
In conclusion, in this study we demonstrated that
NGF and its receptors, TrkANGFR and p75NTR,
are expressed in L-02 cells. Exogenous human recombinant β-NGF
exerts a hepatoprotective effect on D-GalN-induced L-02 cell
injury. The beneficial effects of this agent may be partially due
to the promotion of L-02 cell proliferation and the inhibition of
apoptosis via the NGF/TrkANGFR signaling pathway, which
increases GSH synthesis, attenuates the disruption of oxidative
stress and lipid peroxidation and reduces damage to the plasma
membrane. Thus, β-NGF is a viable therapeutic option for various
liver diseases.
References
|
1
|
Lalor PF and Adams DH: The liver: a model
of organ-specific lymphocyte recruitment. Expert Rev Mol Med.
12:1–16. 2002.
|
|
2
|
Friedman SL: Hepatic stellate cells:
protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malhi H, Guicciardi ME and Gores GJ:
Hepatocyte death: a clear and present danger. Physiol Rev.
90:1165–1194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen-Naftaly M and Friedman SL: Current
status of novel antifibrotic therapies in patients with chronic
liver disease. Ther Adv Gastroenterol. 4:391–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Micera A, Lambiase A, Stampachiacchiere B,
et al: Nerve growth factor and tissue repair remodeling: trkA(NGFR)
and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev.
18:245–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nemoto K, Miyata S, Nemoto F, et al: Gene
expression of neurotrophins and their receptors in lead
nitrate-induced rat liver hyperplasia. Biochem Biophys Res Commun.
275:472–476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asai K, Tamakawa S, Yamamoto M, et al:
Activated hepatic stellate cells overexpress p75NTR after partial
hepatectomy and undergo apoptosis on nerve growth factor
stimulation. Liver Int. 26:595–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oakley F, Trim N, Constandinou CM, et al:
Hepatocytes express nerve growth factor during liver injury:
evidence for paracrine regulation of hepatic stellate cell
apoptosis. Am J Pathol. 163:1849–1858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trim N, Morgan S, Evans M, et al: Hepatic
stellate cells express the low affinity nerve growth factor
receptor p75 and undergo apoptosis in response to nerve growth
factor stimulation. Am J Pathol. 156:1235–1243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kendall TJ, Hennedige S, Aucott RL, et al:
p75 Neurotrophin receptor signaling regulates hepatic myofibroblast
proliferation and apoptosis in recovery from rodent liver fibrosis.
Hepatology. 49:901–910. 2009. View Article : Google Scholar
|
|
11
|
Keppler DO, Rudigier JF, Bischoff E and
Decker KF: The trapping of uridine phosphates by D-galactosamine.
D-galactosamine, and 2-deoxy-D-galactose A study on the mechanism
of galactosamine hepatitis. Eur J Biochem. 17:246–253. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun F, Hamagawa E, Tsutsui C, et al:
Evaluation of oxidative stress during apoptosis and necrosis caused
by D-galactosamine in rat liver. Biochem Pharmacol. 65:101–107.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McMillan JM and Jollow DJ: Galactosamine
hepatotoxicity: effect of galactosamine on glutathione resynthesis
on rat hepatocyte cultures. Toxicol Appl Pharmacl. 115:234–240.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kon K, Kim JS, Jaeschke H and Lemasters
JJ: Mitochondrial permeability transition in acetaminophen-induced
necrotic and apoptotis of cultured mouse hepatocytes. Hepatology.
40:1170–1179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Issa R, Zhou X, Trim N, et al: Mutation in
collagen-1 that confers resistence to the action of collagenase
results in failure of recovery from CCl4-induced liver
fibrosis, persistence of activated hepatic stellate cells, and
diminished hepatocyte regeneration. FASEB J. 17:47–49.
2003.PubMed/NCBI
|
|
16
|
Taub R: Liver regeneration: from myth to
mechanism. Nat Rev Mol Cell Biol. 5:836–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drahota Z, Kriváková P, Cervinková Z, et
al: Tert-butyl hydroperoxide selectively inhibits mitochondrial
respiratory-chain enzymes in isolated rat hepatocytes. Physiol Res.
54:67–72. 2005.
|
|
18
|
Kucera O, Lotková H, Kand’ár R, et al: The
model of D-galactosamine-induced injury of rat hepatocytes in
primary culture. Acta Medica (Hradec Kralove). 49:59–65.
2006.PubMed/NCBI
|