Introduction
Non-alcoholic fatty liver disease (NAFLD) is a major
cause of chronic liver disease in numerous countries and has become
an international public health threat. NAFLD represents a wide
spectrum of liver diseases ranging from simple steatosis to
nonalcoholic steatohepatitis (NASH). While simple steatosis is
usually non-progressive, patients with NASH may develop cirrhosis
and associated complications, including liver failure and
hepatocellular carcinoma (1). In
addition to its liver-related complications, NAFLD is increasingly
considered to be the hepatic manifestation of metabolic syndrome
and is strongly associated with obesity, diabetes mellitus and
cardiovascular disease (2).
The pathogenesis of NAFLD remains to be elucidated
but a ‘two-hit’ mechanism has been widely accepted to describe its
development. The first ‘hit’ is hepatic steatosis, which is
characterized by excess hepatic lipid accumulation caused by
increased de novo lipogenesis and adipose tissue lipolysis.
Hepatic steatosis is important in the initiation and development of
NAFLD. The second ‘hit’ is oxidative stress and inflammatory
response, which induce hepatocellular injury and fibrosis.
Intrahepatic triglyceride level can be reduced following
diet-induced weight loss and regular physical activity. Similarly,
NAFLD-associated metabolic derangements can be restored by
lifestyle modification as well. However, there are no FDA-approved
treatments for this problem (3).
Previous studies have focused on identifying the active ingredients
of natural products or herbal extracts that can inhibit
adipogenesis and induce adipocyte apoptosis (4,5).
Radix Hedysari (RH) is the dried root of
Hedysarum polybotrys Hand-Mazz and has been widely used in
Chinese traditional medicine for its tonifying Qi, diuretic and
circulatory effects. Radix Hedysari polysaccharide (RHP) is
the main bioactive ingredient of RH, and possesses several
pharmacological activities, including antitumor, anti-inflammatory,
antioxidant and immunomodulatory effects (6,7).
Previous studies have also suggested that RHP may have hypoglycemic
and hypolipidemic properties and may improve insulin resistance
(8), however, the effect of RHP on
NAFLD remains to be elucidated.
The present study aimed to examine the
anti-hyperlipidemic and hepatoprotective effects of RHP in a
high-fat diet (HFD)-induced rat model of NAFLD, and the possible
mechanisms underlying these effects were evaluated.
Materials and methods
RHP preparation
RH roots were gathered from the Wudu County mountain
region (Gansu, China). The RHP was purified by the Institute of
Combined Traditional Chinese and Western Medicine, School of Basic
Medical Science at Lanzhou University (Lanzhou, China) (6,9). The
polysaccharide content was 97.3%.
Animals
Male Sprague-Dawley rats (Gansu University of
Traditional Chinese Medicine, Lanzhou, China) weighing 160±20 g
were fed in a particular pathogen-free laboratory environment with
free access to standard pellet chow and sterile water and were
maintained under a 12 h light/dark circadian cycle. Food intake and
rat body weight were monitored weekly. The study was approved by
the Ethics Committee of Gansu College of Traditional Chinese
Medicine (Lanzhou, China) and appropriate measures were taken to
reduce any pain or discomfort of the rats.
Experimental design
Following the acclimatization period, 42 rats were
randomly divided into two groups. Of these, 10 animals were fed a
regular diet (RD) and 32 animals were fed a HFD for 8 weeks. The
diets were analyzed for nutritional content as indicated in
Table I. The established rat model
was confirmed by histopathological examination in two rats and the
30 remaining rats were separated into three random groups (10
animals/group).
 | Table INutritional values of rat diets. |
Table I
Nutritional values of rat diets.
| Component | Regular diet | High-fat diet |
|---|
| g/100 g wet
matter |
| Moisture | 9.20 | 5.12 |
| Protein | 22.10 | 12.31 |
| Fat | 5.28 | 2.94 |
| Ash | 5.20 | 2.90 |
| Fibre | 4.12 | 2.29 |
| Carbohydrate | 54.10 | 30.13 |
| Lard | - | 17.80 |
| Saccharose | - | 11.30 |
| Casein | - | 11.20 |
| Gunk | - | 2.00 |
| Maltodextrin | - | 2.00 |
| kcal/100 g wet
matter | 352.00 | 444.70 |
| % of total
energy |
| Protein | 25.7 | 20.00 |
| Fat | 13.8 | 42.00 |
| Carbohydrate | 60.5 | 38.00 |
The first group (RD) received a regular diet and an
oral gavage of physiological saline solution. The second group
(HFD) received a HFD and an oral gavage of physiological saline
solution. The third group (HFD + RHP50) and the fourth
group (HFD + RHP150) received a HFD and were
administered with oral gavages of 50 and 150 mg/kg RHP liquid,
respectively. All rats were treated once daily for 8 weeks. Every
effort was made to minimize any animal suffering and to reduce the
number of animals used based on the Chinese Guidelines for the Care
and Use of Laboratory Animals.
Blood and tissue collection
At the end of the treatment period, food was removed
for 12 h and drinking water was allowed ad libitum. Rats were then
anesthetized with sodium pentobarbital (60 mg/kg, i.p.; Harbin
Pharmaceutical Group Co., Ltd., Harbin, China). Blood samples were
collected from the femoral artery and were immediately placed into
ice-chilled silicon disposable glass tubes (Shanghai Showbio
Biotech, Inc., Shanghai, China) and allowed to stand for 30 min.
Blood samples were centrifuged at 2,500 × g for 15 min at 4°C to
obtain serum, which was stored in aliquots at −80°C prior to
analysis. Liver tissues were dissected, weighed, frozen in liquid
nitrogen and stored at −80°C prior to use.
Liver morphological analysis and lipid
staining
Hematoxylin and eosin (H&E) staining was
performed on liver sections according to standard instructions
(10). The livers were fixed,
dehydrated, dipped in paraffin (Shanghai Showbio Biotech, Inc.),
sectioned (8 μm) and stained with H&E. Slides were examined
using a DP70 light microscope (Olympus, Tokyo, Japan).
To observe hepatic lipid accumulation, frozen liver
tissue sections were stained with Oil Red O (ORO; Shanghai Showbio
Biotech, Inc.) in accordance with a previously described method
(11). Briefly, liver tissues were
cryosectioned (6 μm thick), fixed in 10% formalin solution
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at room
temperature for 10 min and dipped in 60% isopropanol (Sinopharm
Chemical Reagent Co., Ltd.) for 3 min. The slides were then
immersed in 1% ORO solution for 10 min and washed in 60%
isopropanol followed by distilled water. The slides were
counterstained with Mayer’s hematoxylin and were mounted onto
glycerin gelatin (Shanghai Showbio Biotech, Inc.).
Biochemical parameters of liver
function
An automatic biochemical analyzer (Sysmex Chemix
180; Sysmex Corp., Kobe, Japan) was used to determine the serum
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
high-density lipoprotein cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C), total cholesterol (TC) and
triglyceride (TG) concentrations, according to the manufacturer’s
instructions.
Hepatic TG content measurement
To determine hepatic TG content, frozen liver tissue
was homogenized in phosphate-buffered saline and methanol
(Sinopharm Chemical Reagent Co., Ltd.) was added to the lysate.
Lipids were extracted according to the method of Bligh and Dyer
(12), and TG content was detected
using a TG kit (Abcam, Cambridge, UK).
RNA extraction and reverse
transcription
Total RNA was extracted from the liver tissues using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The
concentration and quantity of isolated total RNA were measured
using a Nanodrop Spectrophotometer (ND-1000; NanoDrop Technologies,
Inc., Wilmington, DE, USA). Reverse transcription was performed in
a 25 μl reaction volume using 2 μg RNA with moloney murine leukemia
virus reverse transcriptase (Promega Corporation, Madison, WI,
USA).
Semi-quantitative and quantitative
polymerase chain reaction (qPCR)
Semi-quantitative RT-PCR and qPCR were performed on
a Thermal Cycler Dice™ Detection System with SYBR green dye (Takara
Bio, Inc., Shiga, Japan) according to the method described
previously (13). The β-actin gene
was used as a reference for the normalization of data. The primers
used in the present study are presented in Table II.
 | Table IIPrimers used for semi-qPCR and
qPCR. |
Table II
Primers used for semi-qPCR and
qPCR.
| Gene | Primer
sequence | Product (bp) |
|---|
| IL-1β | Forward:
5′-CCTCTGTGACTCGTGGGATG-3′
Reverse: 5′-GGGTGTGCCGTCTTTCATCA-3′ | 277 |
| TNF-α | Forward:
5′-TGAACTTCGGGGTGATCGGT-3′
Reverse: 5′-CTCCTCCGCTTGGTGGTTTG-3′ | 158 |
| PPARα | Forward:
5′-AGACACCCTCTCTCCAGCTT-3′
Reverse: 5′-ACGCCAGCTTTAGCCGAATA-3′ | 200 |
| SREBP-1c | Forward:
5′-GCCGAGGTGTGCGAAATG-3′
Reverse: 5′-GCACGGACGGGTACATCTT-3′ | 292 |
| CPT-1 | Forward:
5′-CCCTAAGCCCACAAGGCTAC-3′
Reverse: 5′-TCTCTGTCCTCCCTTCTCGG-3′ | 275 |
| ATGL | Forward:
5′-TCCTCGGGGTCTACCACATT-3′
Reverse: 5′-AATCAGCAGGCAGGGTCTTC-3′ | 267 |
| HSL | Forward:
5′-CTAGCCATACAGCAGCCCTC-3′
Reverse: 5′-ATGCTGTGTGAGAATGCCGA-3′ | 269 |
| FASN | Forward:
5′-AGACGATGACAGGAGGTGGA-3′
Reverse: 5′-GAGTGAGGCCGGGTTGATAC-3′ | 199 |
| SCD-1 | Forward:
5′-ACCTTGCTCTGGGGGATATT-3′
Reverse: 5′-TTCCGCCCTTCTCTTTGACA-3′ | 298 |
| MTTP | Forward:
5′-TCTGTGGTACCGCGAGTCTA-3′
Reverse: 5′-GGGTACTGGGAGAACTGCAC-3′ | 165 |
| β-actin | Forward:
5′-CCCGCGAGTACAACCTTCTTG-3′
Reverse: 5′-ACCCATACCCACCATCACAC-3′ | 206 |
Western blot analysis
Western blot analysis was performed according to the
method described previously (14).
Total protein (50 μg) was separated onto 8% sodium dodecyl
sulfate-polyacrylamide minigels and transferred onto Hybond-C
nitrocellulose membranes (Amersham Life Science, Buckinghamshire,
UK). Following blocking with PBS containing 5% nonfat milk, the
membrane was incubated with rabbit monoclonal antibodies specific
for rat adenosine monophosphate-activated protein kinase α (AMPKα),
phosphorylated-AMPKα (p-AMPKAα; Thr172), acetyl-CoA carboxylase
(ACC), rabbit polyclonal antibody specific for rat phosphor-ACC
(Ser79; 1:1,000; Cell Signaling Technology, Inc., Beverly, MA,
USA), and mouse monoclonal antibody specific for rat β-actin
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
room temperature for 2 h or at 4°C overnight, followed by
incubation with IRDye 800CW or 680Rd goat anti-rabbit or anti-mouse
secondary antibodies (1:10,000; Li-COR Biosciences, Lincoln, NE,
USA) at room temperature for 30 min. β-actin served as a control
for sample loading and integrity. The sample bands were revealed
using the Odyssey Infrared Imaging System (Li-COR Biosciences).
Statistical analysis
All data were obtained from at least three separate
experiments and are expressed as the mean ± standard deviation from
10 rats. The statistical significance was evaluated using one-way
analysis of variance followed by a Newman-Keuls post-hoc test for
multiple comparisons (GraphPad Software, Inc., San Diego, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
RHP ameliorates lipid metabolism
disorders
A HFD was fed to rats to establish an NAFLD model
and the phenotypes were observed following simultaneous RHP
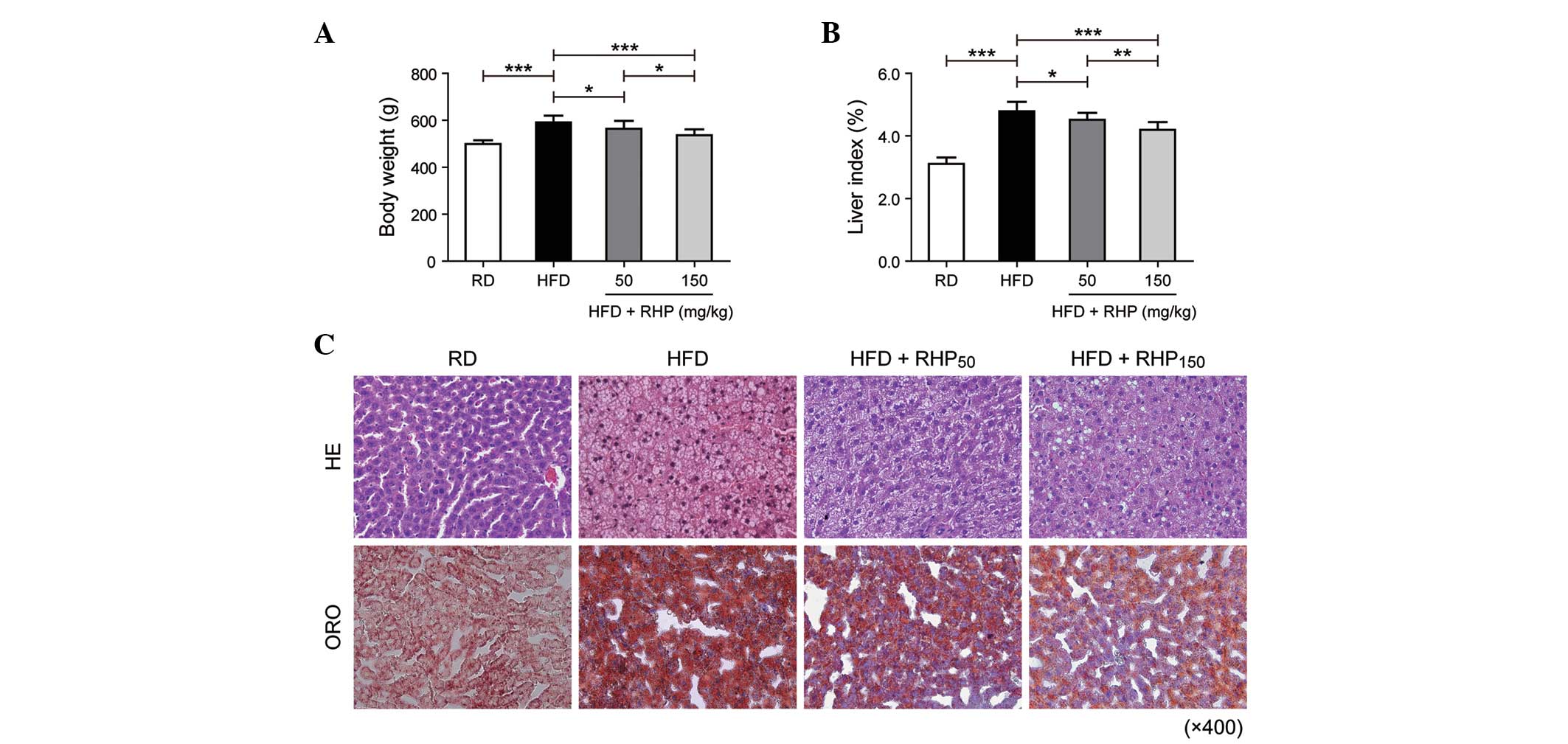
administration. At the beginning of the investigation, the body
weights of the rats in each group were not significantly different
(P>0.05). At week 16, the body weights and the liver index
(liver weight (g) / 100 g body weight) of the HFD group had
increased (P<0.001; Fig. 1A and
B) compared with the RD group. Compared with the HFD group, the
intervention group body weights and liver index (HFD +
RHP50 and HFD + RHP150) had markedly
decreased (P<0.05, P<0.001; Fig.
1A and B).
When liver histology was evaluated by H&E
staining, RD rat tissue exhibited well-arranged hepatic cords,
cells with round and central nuclei, a lobular structure and an
array of wheel-shaped cells along the centrilobular vein. However,
in the HFD group, lipid droplets were observed in the liver
sections (Fig. 1C). The
accumulated lipids in rat liver tissues obtained from each
experimental group were detected as red spots on histological
analysis using ORO staining. ORO staining of liver sections
confirmed that rats in the HFD group had an increased accumulation
of hepatic lipids compared with the RD group. Lipid droplet volumes
and quantities were reduced by simultaneous RHP administration.
These findings demonstrated that feeding a HFD to
rats was able to successfully establish a model of disordered lipid
metabolism. Furthermore, RHP intervention improved this lipid
metabolism disturbance.
RHP regulates the concentration of serum
TC, TG, LDL-C, and HDL-C as well as the hepatic TG content
Selected lipid metabolism parameters were detected
in the four experimental groups. At the end of the 16th week,
significant increases in serum TC, TG and LDL-C levels (P<0.001;
Fig. 2A) and hepatic TG content
were identified in the HFD group compared with the RD group
(P<0.001; Fig. 2B), whereas
HDL-C levels were unaltered (P>0.05; Fig. 2A). Significant decreases in serum
TC, TG and LDL-C levels and hepatic TG content were identified in
the RHP intervention groups (P<0.05; Fig. 2A and B). No significant difference
in serum HDL-C levels was identified following RHP administration
(P>0.05; Fig. 2A). Notably, the
majority of lipid metabolism parameters exhibited a dose-effect
association between these two intervention groups (P<0.05;
Fig. 2A and B).
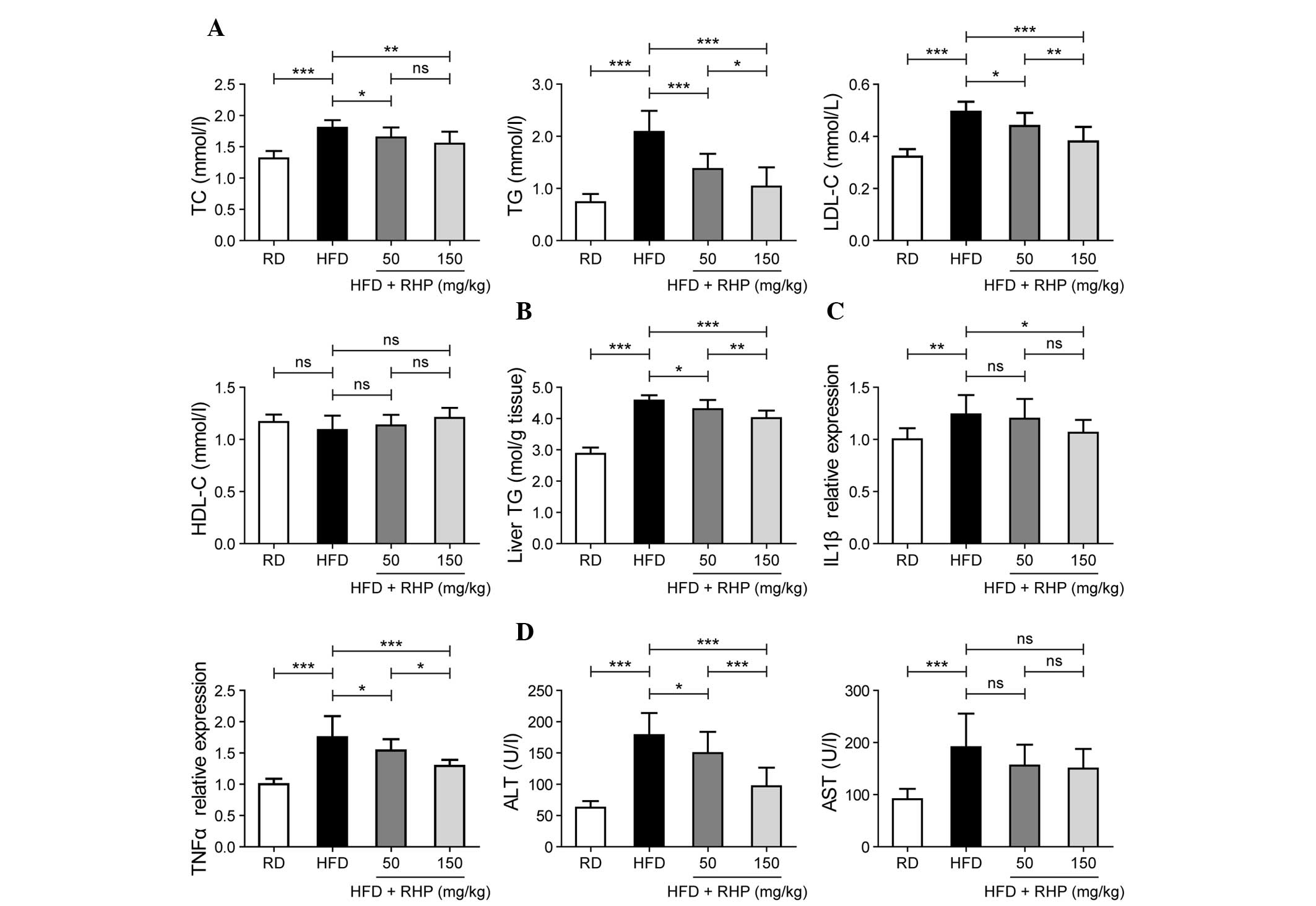 | Figure 2Effects of RHP administration on lipid
metabolism parameters, inflammation and damage. (A) Evaluation of
serum TC, TG, LDL-C and HDL-C contents in the four rat groups. (B)
Evaluation of liver TG content in the four rat groups. (C)
Evaluation of liver IL-1β and TNF-α mRNA expression levels in the
four rat groups. (D) Evaluation of serum ALT and AST
concentrations. Values are expressed as the mean ± standard
deviation (n=10 rats/group). All experiments were repeated at least
three times to confirm results. *P<0.05,
**P<0.01, ***P<0.001 between the two
indicated groups; ns indicates no statistical significance. RHP,
Radix Hedysari polysaccharide; HFD, high-fat diet; TC, total
cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein
cholesterol; HDL-C, high-density lipoprotein cholesterol. IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; ALT, alanine
aminotransferase; AST, aspartate aminotransferase. |
These findings also suggested that lipid metabolism
disorders were present in the rat model and that RHP interventions
were able to correct them.
RHP treatment improves liver inflammation
and damage
In addition to hepatic lipid accumulation,
inflammation and liver cell damage also occurred frequently. As a
result of the fatty liver and inflammatory condition, the mRNA
expression levels of interleukin-1β (IL-1β) and tumor necrosis
factor-α (TNF-α) were evaluated as markers of liver inflammation.
In HFD-fed rat livers, the mRNA expression of IL-1β and TNF-α
significantly increased (P<0.01; Fig. 2C). RHP administration to HFD-fed
rats prevented the upregulation of IL-1β and TNF-α expression
(P<0.05). Compared with the RD group, HFD-fed rats were
characterized by significantly higher serum ALT and AST activities
(P<0.001; Fig. 2D). However,
following RHP administration, ALT activity declined (P<0.05).
Although AST activity was also reduced in the two RHP groups, no
significant changes were observed compared with the HFD group.
Dose-effect associations in TNF-α expression and ALT activity
levels were also identified between these two intervention groups
(P<0.05; Fig. 2C and D).
These data revealed that a HFD caused hepatic
inflammation and hepatocellular damage, which was alleviated by
continuous RHP administration.
RHP activates AMPK and regulates
transcription factor expression
The above-mentioned results obtained from the
phenotypic experiment revealed that RHP treatment regulated lipid
metabolism and alleviated hepatocellular inflammation and damage.
The present study subsequently aimed to determine the mechanisms by
which RHP treatment achieved this effect. AMPK signaling is an
important pathway regulating glycolipid metabolism in hepatocytes.
A decrease in the levels of p-AMPK in HFD-fed rat livers compared
with RD-treated rats was observed (P<0.001; Fig. 3A and B). In rats treated with
different doses of RHP, increased levels of p-AMPK were observed
compared with HFD-fed rats (P<0.05). ACC phosphorylation, the
activity of an important rate-limiting enzyme in lipogenesis,
exhibited the same trend as p-AMPK (P<0.05; Fig. 3A and B). Relative mRNA expression
levels of peroxisome proliferator-activated receptor α (PPARα) and
sterol regulatory element binding protein-1c (SREBP-1c), which are
transcription factors downstream of AMPK, were assessed in rat
livers via semi-qPCR (Fig. 3C) and
qPCR (Fig. 3D), respectively. The
HFD-fed rat livers exhibited a significant decrease in PPARα mRNA
expression compared with the RD rats (P<0.001) and RHP
administration in HFD rats increased the mRNA expression of PPARα
(P<0.05). By contrast, the expression of SREBP-1c significantly
increased in the HFD group (P<0.001) and reduced following
administration of RHP (P<0.01). Therefore, these data supported
the hypothesis that RHP activated AMPK and regulated the expression
of the downstream transcription factors PPARα and SREBP-1c.
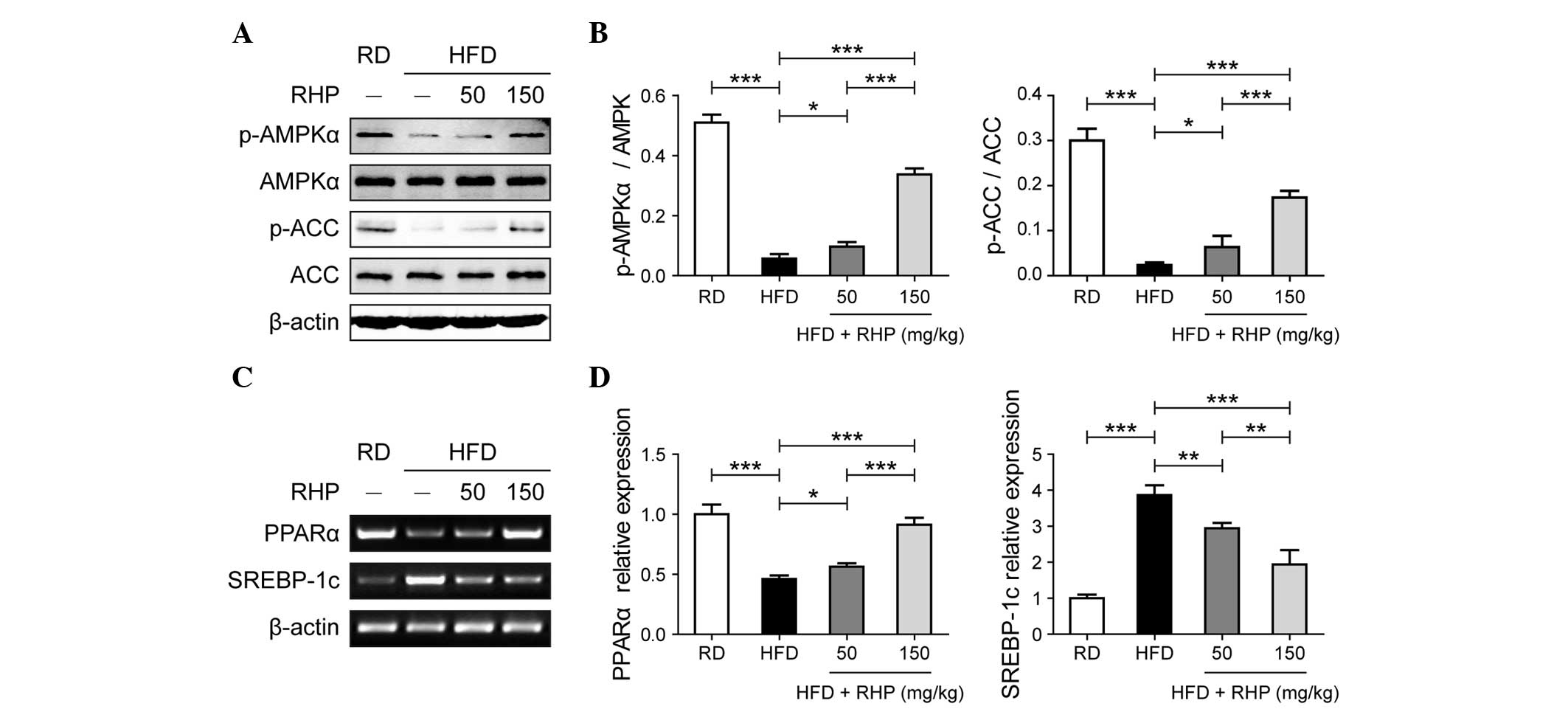 | Figure 3Effects of RHP treatment on AMPK
signaling and transcription factor mRNA expression in rat livers.
(A) Western blot analysis of RHP-induced AMPK and ACC
phosphorylation in rat livers. (B) Relative protein band
quantification was performed by optical density scanning of (A). (C
and D) mRNA expression of transcription factors PPARα and SREBP-1c
was assessed (panels C and D were obtained from
semi-quantitative-PCR and quantitative PCR, respectively). All
experiments were repeated at least three times to confirm results.
*P<0.05, **P<0.01,
***P<0.001 between the two indicated groups. RD,
regular diet; HFD, high-fat diet; RHP, Radix Hedysari
polysaccharide; p-AMPKα, phosphorylated-adenosine
monophosphate-activated protein kinase α; p-ACC, phospho acetyl-CoA
carboxylase; PPARα, peroxisome proliferator-activated receptor α;
SSREBP-1c, sterol regulatory element binding protein-1c; PCR,
polymerase chain reaction. |
RHP affects lipid metabolism gene
expression
Lipid metabolism involves lipolysis, lipogenesis and
lipid transport (15). Certain key
genes involved in lipid metabolism were detected in the present
study. The mRNA expression levels of the lipolytic genes carnitine
palmitoyltransferase 1 (CPT1) and adipose triglyceride lipase
(ATGL) were significantly decreased in the livers of the HFD group
and increased in the livers of the HFD + RHP150 group
(P<0.01; Fig. 4A), whereas no
significant alteration was observed in hormone-sensitive lipase
(HSL) in HFD rats compared with RD rats (P>0.05). No significant
changes in HSL transcription were observed following RHP
administration to HFD rats (P>0.05). Conversely, the expression
levels of fatty acid synthase (FASN) and stearoyl-coenzyme A
desaturase 1 (SCD-1), two important lipogenic enzymes required for
lipid synthesis, were significantly increased in the HFD group
(P<0.01; Fig. 4B). In addition,
administration of RHP reversed these increased levels of expression
(P<0.01). Expression of the lipid transporter microsomal
triglyceride transfer protein (MTTP) was also assessed. MTTP
expression was downregulated in the HFD group (P<0.05; Fig. 4C) but no significant change in
expression following RHP administration was identified
(P>0.05).
 | Figure 4Effects of RHP administration on lipid
metabolism-related gene mRNA expression in rat livers. (A)
Expression of hepatic lipogenesis, (B) lipolysis and (C) lipid
transport genes from the four rat groups. Data are expressed as the
mean ± standard deviation (n=10 rats/group). All experiments were
repeated at least three times to confirm results.
*P<0.05, **P<0.01,
***P<0.001 between the two indicated groups; ns
indicates no statistical significance. HFD, high-fat diet; RD,
regular diet; RHP, Radix Hedysari polysaccharide; CPT1,
carnitine palmitoyltransferase I; ATGL, adipose triglyceride
lipase; HSL, hormone-sensitive lipase; FASN, fatty acid synthase;
SCD-1, stearoyl-CoA desaturase-1; MTTP, microsomal triglyceride
transfer protein. |
These results demonstrated that RHP affected the
expression of genes involved in lipid metabolism, with a
dose-response association in the majority of the lipid metabolism
gene expression in these two intervention groups.
Discussion
Currently, NAFLD is the focus of significant
scientific and clinical studies. In previous decades, several
advances have been made in medical treatments of NAFLD. However,
significant therapeutic success in clinical studies of NAFLD
remains to be accomplished. To date, although lifestyle
interventions remain the most effective method to control and
remedy NAFLD in affected patients, pharmaceutical treatment is
required to strengthen the therapeutic effects of lifestyle
modifications (16–18).
Several pharmaceutical interventions have been used
in present clinical NAFLD treatments, including antioxidants
(4), insulin sensitizers
(metformin and thiazolidinediones) (19), lipid-lowering drugs (20), angiotensin II receptor antagonists
(irbesartan and losartan) (21,22)
and other drugs, including ursodeoxycholic acid (23) and L-carnitine (24). However, there remains no definitive
treatment for NAFLD as its pathology remains to be elucidated
(5).
RHP is the major component of RH, a traditional
Chinese medicine, and reportedly possesses several pharmacological
activities. The present study demonstrated that RHP may be
important in improving NAFLD by regulating adipose lipogenesis and
lipolysis.
AMPK is a serine/threonine protein kinase that acts
as a hepatocyte energy sensor, thereby regulating a wide variety of
pathways involved in glucose and lipid metabolism (25). The present study demonstrated that
RHP treatment decreased AMPK-α phosphorylation. The downstream
target ACC, which is phosphorylated and inactivated by AMPK, can
directly regulate lipid metabolism (26). In the present study, p-ACC
demonstrated a similar change to p-AMPK, suggesting that
RHP-induced alterations in lipid oxidation were mediated via AMPK
activation.
Furthermore, AMPK regulates hepatic lipid metabolism
by mediating the mRNA expression and transcriptional activity of
PPARa and SREBP-1c, which regulate lipogenic and lipolytic gene
expression, respectively (27,28).
PPARα is a nuclear transcription factor that regulates lipid
metabolism and fatty acid oxidation target genes (29). SREBP-1c is a major transcriptional
regulator that controls the expression of key lipogenic enzymes to
facilitate hepatic lipogenesis (30). In the present study, RHP treatment
increased the expression of PPARα and decreased the expression of
SREBP-1c compared with the HFD group. RHP treatment also promoted
the expression of lipolytic genes and inhibited the expression of
lipogenic genes in the liver, suggesting that RHP treatment may
enhance hepatic fatty acid oxidation and suppress hepatic TG
biosynthesis.
HSL is another catabolic rate-limiting steatolysis
enzyme and is associated with TG metabolism (31). The present study demonstrated no
significant change in the expression of HSL and it was hypothesized
that administration of RHP may not affect all lipid metabolism
genes.
In conclusion, the present study demonstrated that
RHP treatment is effective at alleviating NAFLD in rats and its
mechanism of action may be associated with AMPK activation and the
regulation of genes involved in lipid metabolism.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities (grant no. lzujbky-2013-159) and
the Scientific Research Project of Traditional Chinese Medicine
Administration in Gansu Province (grant no. GZK-2012-65). The
authors would like to thank Xiao-Liang Jin for assistance in the
animal experiments.
References
|
1
|
Farrell GC and Larter CZ: Nonalcoholic
fatty liver disease: from steatosis to cirrhosis. Hepatology.
43:S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Targher G: Non-alcoholic fatty liver
disease, the metabolic syndrome and the risk of cardiovascular
disease: the plot thickens. Diabetic Med. 24:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koot BG, van der Baan-Slootweg OH,
Tamminga-Smeulders CL, et al: Lifestyle intervention for
non-alcoholic fatty liver disease: prospective cohort study of its
efficacy and factors related to improvement. Arch dis Child.
96:669–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park HJ, DiNatale DA, Chung MY, et al:
Green tea extract attenuates hepatic steatosis by decreasing
adipose lipogenesis and enhancing hepatic antioxidant defenses in
ob/ob mice. J Nutr Biochem. 22:393–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quan HY, Kim do Y, Kim SJ, Jo HK, Kim GW
and Chung SH: Betulinic acid alleviates non-alcoholic fatty liver
by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling
pathway. Biochem Pharmacol. 85:1330–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei D, Cheng W, Wei Y and Zhang L:
Phosphorylated modification and in vitro antioxidant activity of
Radix Hedysari polysaccharide. Glycoconj J. 29:167–172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hui HP, Feng SL, Hu FD, Cui F and Wu YQ:
Study on antioxidative activity of polysaccharide from radix
hedysari in vitro. J Anhui Agri Sci. 38:4056–4057. 2010.
|
|
8
|
Liu J, Deng W, Fan L, et al: The role of
radix hedysari polysaccharide on the human umbilical vein
endothelial cells (HUVECs) induced by high glucose. Eur J Intern
Med. 23:287–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei D, Wei Y, Cheng W and Zhang L:
Sulfated modification, characterization and antitumor activities of
Radix hedysari polysaccharide. Int J Biol Macromol. 51:471–476.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grasselli E, Voci A, Canesi L, et al:
Direct effects of iodothyronines on excess fat storage in rat
hepatocytes. J Hepatol. 54:1230–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koopman R, Schaart G and Hesselink MK:
Optimisation of oil red O staining permits combination with
immunofluorescence and automated quantification of lipids.
Histochem Cell Biol. 116:63–68. 2001.PubMed/NCBI
|
|
12
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YP, Huang LY, Sun WM, et al: Insulin
receptor tyrosine kinase substrate activates EGFR/ERK signalling
pathway and promotes cell proliferation of hepatocellular
carcinoma. Cancer Lett. 337:96–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang LY, Wang YP, Wei BF, et al:
Deficiency of IRTKS as an adaptor of insulin receptor leads to
insulin resistance. Cell Res. 23:1310–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duvnjak M, Lerotic I, Barsic N, Tomasic V,
Virovic Jukic L and Velagic V: Pathogenesis and management issues
for non-alcoholic fatty liver disease. World J Gastroenterol.
13:4539–4550. 2007.PubMed/NCBI
|
|
17
|
Xiao J, Guo R, Fung ML, Liong EC and Tipoe
GL: Therapeutic approaches to non-alcoholic fatty liver disease:
past achievements and future challenges. Hepatobiliary Pancreat Dis
Int. 12:125–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakata R, Nakamura T, Torimura T, Ueno T
and Sata M: Green tea with high-density catechins improves liver
function and fat infiltration in non-alcoholic fatty liver disease
(NAFLD) patients: a double-blind placebo-controlled study. Int J
Mol Med. 32:989–994. 2013.
|
|
19
|
Vuppalanchi R and Chalasani N:
Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:
Selected practical issues in their evaluation and management.
Hepatology. 49:306–317. 2009. View Article : Google Scholar
|
|
20
|
Musso G, Cassader M and Gambino R:
Cholesterol-lowering therapy for the treatment of nonalcoholic
fatty liver disease: an update. Curr Opin Lipidol. 22:489–496.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matthew Morris E, Fletcher JA, Thyfault JP
and Rector RS: The role of angiotensin II in nonalcoholic
steatohepatitis. Mol Cell Endocrinol. 378:29–40. 2013.PubMed/NCBI
|
|
22
|
Kato J, Koda M, Kishina M, et al:
Therapeutic effects of angiotensin II type 1 receptor blocker,
irbesartan, on non-alcoholic steatohepatitis using FLS-ob/ob male
mice. Int J Mol Med. 30:107–113. 2012.PubMed/NCBI
|
|
23
|
Leuschner UF, Lindenthal B, Herrmann G, et
al: High-dose ursodeoxycholic acid therapy for nonalcoholic
steatohepatitis: a double-blind, randomized, placebo-controlled
trial. Hepatology. 2:472–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malaguarnera M, Gargante MP, Russo C, et
al: L-carnitine supplementation to diet: a new tool in treatment of
nonalcoholic steatohepatitis - a randomized and controlled clinical
trial. Am J Gastroenterol. 105:1338–1345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long YC and Zierath JR: AMP-activated
protein kinase signaling in metabolic regulation. J Clin Inv.
116:1776–1783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gray S and Kim JK: New insights into
insulin resistance in the diabetic heart. Trends Endocrinol Metab.
22:394–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawaguchi T, Osatomi K, Yamashita H,
Kabashima T and Uyeda K: Mechanism for fatty acid ‘sparing’ effect
on glucose-induced transcription: regulation of
carbohydrate-responsive element-binding protein by AMP-activated
protein kinase. J Biol Chem. 277:3829–3835. 2002.
|
|
28
|
Zhou G, Myers R, Li Y, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshimura Y, Nishii S, Zaima N, Moriyama T
and Kawamura Y: Ellagic acid improves hepatic steatosis and serum
lipid composition through reduction of serum resistin levels and
transcriptional activation of hepatic ppara in obese, diabetic
KK-A(y) mice. Biochem Biophys Res Commun. 434:486–491. 2013.
View Article : Google Scholar
|
|
30
|
Xu X, So JS, Park JG and Lee AH:
Transcriptional control of hepatic lipid metabolism by SREBP and
ChREBP. Semin Liver Dis. 33:301–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Moon MH, Jeong JK, et al:
Sulforaphane induced adipolysis via hormone sensitive lipase
activation, regulated by AMPK signaling pathway. Biochem Biophys
Res Commun. 426:492–497. 2012. View Article : Google Scholar : PubMed/NCBI
|