Introduction
Drowning is one of the principal causes of
accidental deaths, and thus presents a serious public health risk
(1). Acute lung injury (ALI)
caused by drowning is a critical complication that often results in
mortality. Instances of seawater aspiration often lead to more
severe ALI than those of freshwater aspiration, and thus present a
higher mortality rate (2).
Seawater aspiration-induced ALI is often characterized by acute
inflammation of lung parenchyma and interstitial tissue with severe
hypoxemia and pulmonary edema (3,4).
However, the apoptosis of alveolar epithelial cells can also result
in high endothelial cell permeability and pulmonary edema in
seawater aspiration-induced ALI (5,6).
Epigallocatechin-3-gallate (EGCG), the main
ingredient in green tea, has been reported to ameliorate seawater
aspiration-induced ALI by regulating the inflammatory response in
rats (7). EGCG may also trigger
apoptosis in tumor tissues, due to the inactivation of NF-κB and
the decreased expression of cyclin D1 (8,9).
Conversely, EGCG may protect against ischemia/reperfusion-induced
apoptosis and cisplatin-induced apoptosis in heart and kidney
(10,11). Therefore, the current study aimed
to determine the effect of EGCG on alveolar epithelial cells
exposed to seawater aspiration-induced ALI.
EGCG has been reported to reduce signal transducer
and activator of transcription 1 (STAT1) phosphorylation in various
human cells (12). EGCG may reduce
inflammation and pulmonary edema in seawater aspiration-induced ALI
via inhibiting the JAK/STAT1 pathway (7). Furthermore, inhibiting STAT1 may
reduce cerebral and osteocyte cell apoptosis (13,14).
Therefore, EGCG may also be capable of suppressing alveolar
epithelial cell apoptosis in seawater aspiration-induced ALI via
inhibiting the STAT1-caspase-3/p21 pathway.
Materials and methods
Ethical approval
All experimental procedures were authorized by the
Animal Care and Use Committees of the Fourth Military Medical
University (Xi'an, China) and followed the protocols outlined in
the Guide for Care and Use of Laboratory Animals published by the
National Institutes of Health (publication no. 85–23, revised
1985).
Chemicals and reagents
EGCG (purity >99%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and prepared as a stock solution
in normal saline. Seawater [osmolality 1,300 mmol/l (pH 8.2),
specific weight 1.05; NaCl, 26.518 g/l; MgSO4, 3.305
g/l; MgCl2, 2.447 g/l; CaCl2, 1.141 g/l; KCl,
0.725 g/l; NaHCO3, 0.202 g/l; and NaBr, 0.083 g/l; all
reagents from Sigma-Aldrich) was prepared in order to mimic the
chemical composition of the East China Sea, provided by the Chinese
Ocean Bureau (Beijing, China). STAT1 (cat. no. 9172) and
phospho-STAT1-Tyr-701 (cat. no. 9167) rabbit monoclonal antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). p21 (cat. no. sc-271532) and β-actin (cat. no. sc-8432) mouse
monoclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Cleaved caspase-3 (cat. no. a0214) rabbit
monoclonal antibody was purchased from ABclonal Biotech Co., Ltd.
(Cambridge, MA, USA). An annexin V/fluo-rescein isothiocyanate
(FITC) kit was purchased from BD Biosciences (San Jose, CA, USA). A
TUNEL In Situ Cell Death Detection Kit, Fluorescein was purchased
from Roche Diagnostics (Laval, Canada). Evans Blue was obtained
from Sigma-Aldrich. The Total Protein Extraction kit and the
bicinchoninic acid protein assay kit were supplied by Thermo Fisher
Scientific, Inc. (Waltham, MA, USA).
Animals and groups
The seawater aspiration-induced ALI rat model used
in the current study followed the procedure outlined in Liu et
al (7). Prior to exposure of
the trachea, the rats were anesthetized with 3% pentobarbital
sodium [1.5 ml/kg, administered by intraperitoneal (i.p.)
injection]. A total of 24 adult male Sprague-Dawley rats (180–220
g) were obtained from the Animal Center of the Fourth Military
Medical University. They were randomly divided into four groups
(all n=6) as follows: (i) The control group, no treatment was
applied, only the trachea was exposed; (ii) the seawater-only
group, seawater (4 ml/kg) was instilled into rat lungs following
trachea exposure within 2 min, maintaining a constant speed using a
1 ml syringe; (iii) the seawater + EGCG group, EGCG (10 mg/kg; i.p.
injection) 30 min prior to seawater exposure; and (iv) the
EGCG-only group, EGCG (10 mg/kg; i.p. injection) was injected prior
to trachea exposure. The rats were sacrificed by aortic transection
6 h subsequent to treatment. The dosage of EGCG used was determined
on the basis of a previous study (15).
Lung edema and microvascular
permeability
The wet-to-dry weight ratio of the lung was as an
indication of lung edema. The upper lobes of the right lungs were
obtained 6 h following application of experimental treatment and
weighed immediately subsequent to removal, then subjected to
desiccation at 55°C for 72 h and weighed again. The ratio of
wet-to-dry was calculated by dividing the wet weight by the dry
weight (16).
Microvascular permeability was examined by the
extravasation of Evans Blue into the tissue. Evans Blue (20 mg/kg)
was administered through a tail vein 0.5 h prior to euthanasia. The
pulmonary circulation was flushed with 10 ml phosphate-buffered
saline (PBS; Sigma-Aldrich). The lungs were subsequently excised
and frozen in liquid nitrogen. The frozen tissue was homogenized
and incubated with formamide at 60°C for 16 h, prior to
centrifugation at 7,000 × g for 20 min. The absorbance (A620 and
A740) of the supernatant was measured using a spectrophotometer
[model no. 722; Suoyu (Shanghai) Electronic Co., Ltd., Shanghai,
China], and the Evans Blue content (μg/g lung) was
calculated against the generated Evans Blue standard absorbance
curves (5).
Histopathology
At the end of the experiments, lung tissues of the
same lobe from each rat were fixed with 10% formalin, embedded in
paraffin and stained with hematoxylineosin. The different group
tissue sections were blindly assessed by two independent
pathologists. They were scored based on edema, neutrophil
infiltration, hemorrhage, bronchiole epithelial desquamation and
hyaline membrane formation. A scale of 0–4 indicated the severity
of the lung tissue injury as follows: 0, no injuries (normal in
appearance); 1, limited injuries; 2, intermediate injuries; 3,
widespread injuries; and 4, prominent injuries (17).
Quantification of apoptosis in lung
tissue sections
The apoptotic cells were identified using the TUNEL
kit, which was conducted in accordance with the manufacturer's
protocol. Apoptotic cells were counted under a fluorescence
microscope (Eclipse Ti-SR; Nikon Corp., Tokyo, Japan). A total of 6
tissue sections from each group were randomly selected, and 10
fields were evaluated per section at x200 magnification. Image Pro
Plus, version 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA) was used to obtain the average number of apoptotic cells per
visual field.
Cell culture and treatment
Human lung alveolar epithelial cells (A549) were
obtained from the American Type Culture Collection (Rockville, MD,
USA). Cells were cultured in HyClone™ RPMI 1640 medium (GE
Healthcare Life Sciences, Logan, Utah, USA) supplemented with 10%
fetal bovine serum (Gibco BRL, Gaithersburg, MD, USA) at 37°C in a
humidified atmosphere with 5% CO2. In order to induce
apoptosis, the following concentrations of seawater were
administered for 6 h: 10, 20, 30 and 40%, equivalent to 0.1, 0.2,
0.3 and 0.4 ml per 1 ml total volume. The effect of EGCG on A549
cells treated with seawater was also examined. Two treatment groups
were established as follows: i) The seawater-only group, cells were
exposed to 30% seawater for 6 h; and ii) the seawater + EGCG group,
cells were initially treated with 10 μM EGCG for 30 min and
then exposed to the 6-h 30% seawater treatment.
Flow cytometry
The percentage of apoptotic A549 cells in each group
was determined using annexin V-FITC and prop-idium iodide (PI)
staining and analyzed by flow cytometric analysis. Following
digestion, the cells were centrifuged at 1,000 × g for 5 min. They
were then washed three times with PBS and resuspended in 400
μl annexin binding buffer. Subsequently, PI and
FITC-conjugated annexin V were added, and the cell suspension was
incubated for 15 min in the dark prior to analysis using a
FACSCalibur flow cytometer (BD FACSAria™ III; BD Biosciences)
(6).
Western blot analysis
Part of the right lung tissue from the experimental
rats was sampled along with A549 cells for each treatment group.
Total protein was extracted from whole-cell and lung tissues and
prepared according to the manufacturer's protocol with a Total
Protein Extraction kit. Protein concentrations were identified by
bicinchoninic acid protein assay kit. The samples were then
separated in parallel with 8% gradient (spacer gel, 80 V;
separation gel, 120 V), loaded onto a SDS-PAGE gel (Beyotime
Institute of Biotechnology, Shanghai, China) and transferred to an
Invitrogen™ nitrocellulose membrane (Thermo Fisher Scientific).
Membranes were blocked for 2 h, prior to overnight incubation at
4°C with the relevant primary antibodies against STAT1 (1:500),
P-STAT1 (1:500), caspase-3 (1:1,000), p21 (1:1,000) and β-actin
(1:1,000). The membranes were then washed five times and incubated
for 2 h with the secondary antibody (anti-mouse or anti-rabbit,
1:5,000; cat nos. 5571 and 7076; Cell Signaling Technology, Inc.).
Detection was performed using a chemiluminescence system
(ImageQuant LAS 4000 Mini; GE Healthcare Life Sciences, Chalfont,
UK).
Statistical analysis
Data are expressed as the mean ± standard error and
statistical analysis was performed with one-way analysis of
variance, followed by a Dunnett's test for multiple comparisons. As
the histological injury score data is not continuous, the
non-parametric, Kruskal-Wallis one-way analysis of variance was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of EGCG on lung edema and
microvascular permeability in seawater aspiration-induced ALI
The extent of lung edema and microvascular
permeability was evaluated using the lung wet-to-dry weight ratios
and the leak index. As demonstrated by Fig. 1, the wet-to-dry lung ratio was
significantly greater following seawater aspiration compared with
the control group (5.87±0.28 vs. 4.08±0.15; P<0.05; n=6), whilst
the EGCG pretreatment reduced the lung wet-to-dry weight ratio
compared with the seawater-only group (5.02±0.33 vs. 5.87±0.28;
P<0.05; n=6). In the seawater-only group, the leak of Evans Blue
was significantly greater compared with the control group
(87.2±3.61 vs. 28.98±1.90; P<0.05; n=6). The EGCG pretreatment
had a lower leak index compared with the control group (73.8±3.63
vs. 87.2±3.61; P<0.05; n=6).
Effects of EGCG on histopathological
changes and the lung injury scores in seawater aspiration-induced
ALI
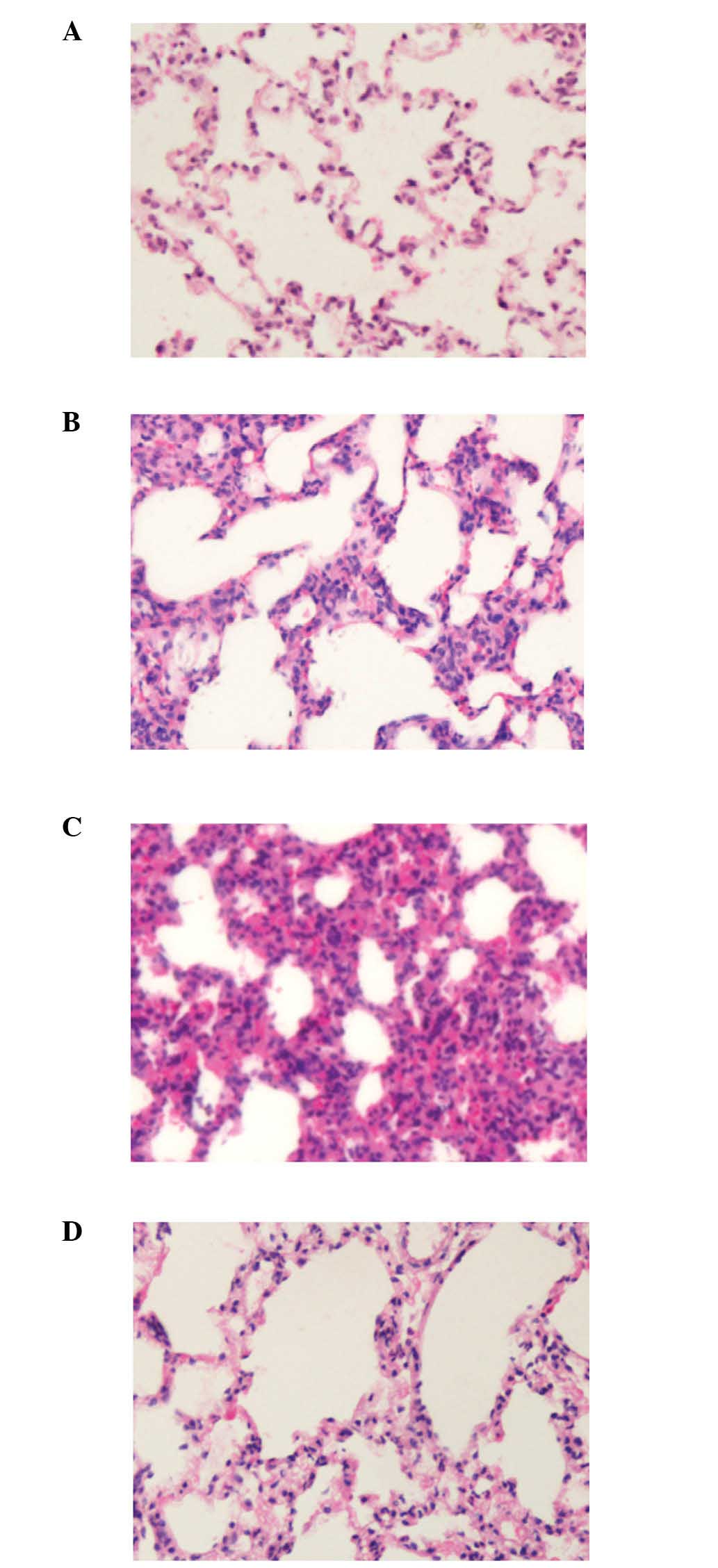
As demonstrated by Fig.
2, seawater aspiration induced lung edema, causing inflammatory
cells to infiltrate the lung tissues and alveoli, resulting in
hemorrhage, bronchiole epithelial desquamation and alveolar
collapse (Fig. 2B). EGCG
pretreatment ameliorated these histopathological changes (Fig. 2C). The lung injury scores of each
group are presented in Table I.
These results indicated that EGCG can reduce the lung injury scores
in seawater aspiration-induced ALI.
 | Table ILung injury scores in each group. |
Table I
Lung injury scores in each group.
| Group | Neutrophil
infiltration | Edema | Hemorrhage | Epithelial
desquamation |
|---|
| Control | 0.2±0.16 | 0.3±0.14 | 0.2±0.18 | 0.1±0.09 |
| Seawater | 3.5±0.28a | 3.4±0.31a | 3.1±0.25a | 3.4±0.12a |
| Seawater + EGCG | 1.9±0.24b | 2.0±0.23b | 1.8±0.31b | 1.4±0.25b |
| EGCG | 0.3±0.23 | 0.2±0.13 | 0.2±0.12 | 0.2±0.17 |
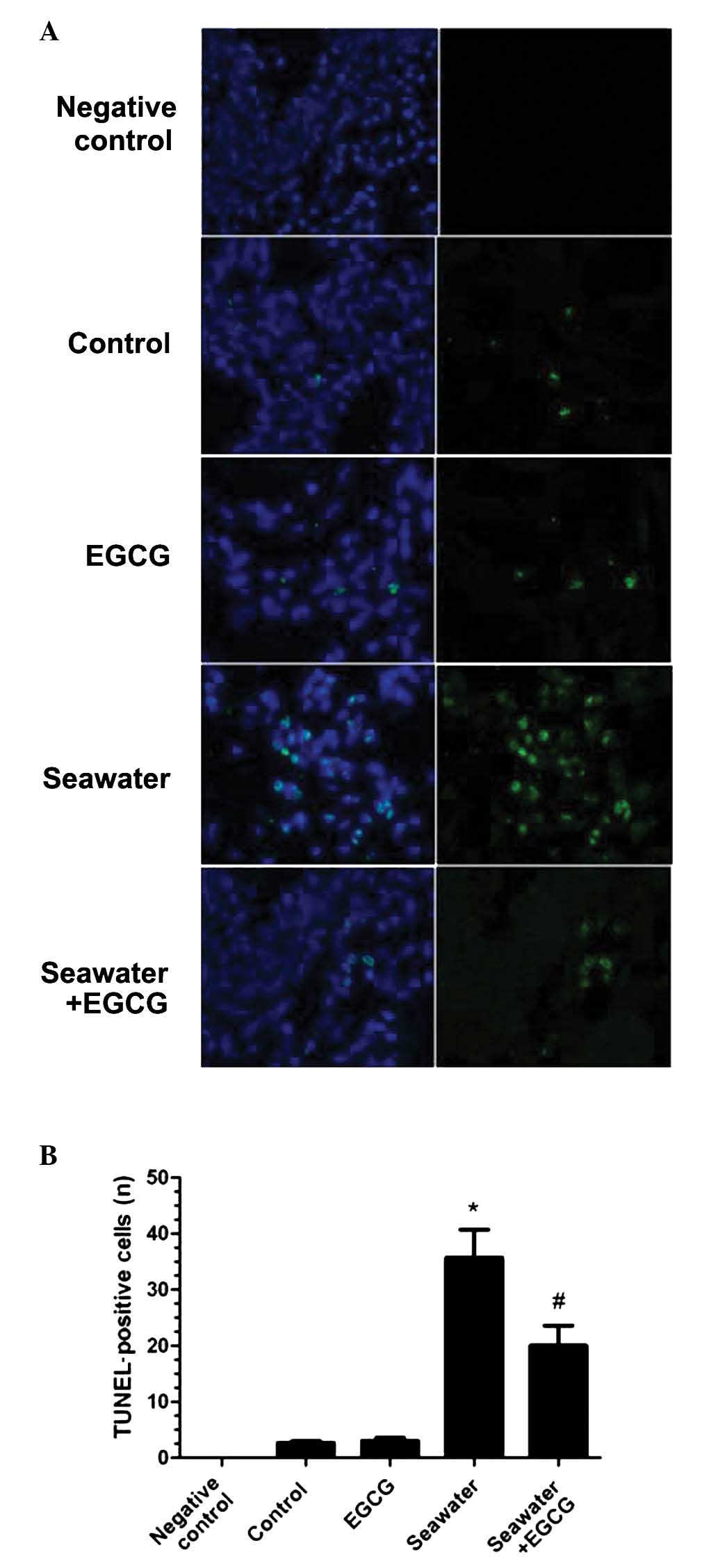
Effects of EGCG on apoptosis in seawater
aspiration-induced ALI
Fluorescence TUNEL staining on lung sections from
each group determined the effect of EGCG on the apoptosis of
seawater aspiration-induced ALI (Fig.
3A). The number of fluorescent TUNEL-positive cells
significantly increased in the seawater-only group compared with
the control group (P<0.05; Fig.
3B). EGCG pretreatment reduced the fluorescence of the
TUNEL-positive cells compared with the seawater group (P<0.05;
Fig. 3B). No significant
differences were identified between the control and EGCG
groups.
Effects of EGCG on the expression of
apoptosis-associated proteins in seawater aspiration-induced
ALI
Levels of the apoptosis-associated proteins
caspase-3 and p21 in the lungs of the rats that had been exposed to
seawater were assessed by western blot analysis (Fig. 4A). The results demonstrated that
caspase-3 and p21 expression levels were increased in the
seawater-only group (P<0.05 vs. the control group; Fig. 4A and B), whilst expression was
significantly decreased in the seawater + EGCG group (P<0.05 vs.
the seawater group; Fig. 4B and
C). No significant differences were identified between the
seawater + EGCG and control groups.
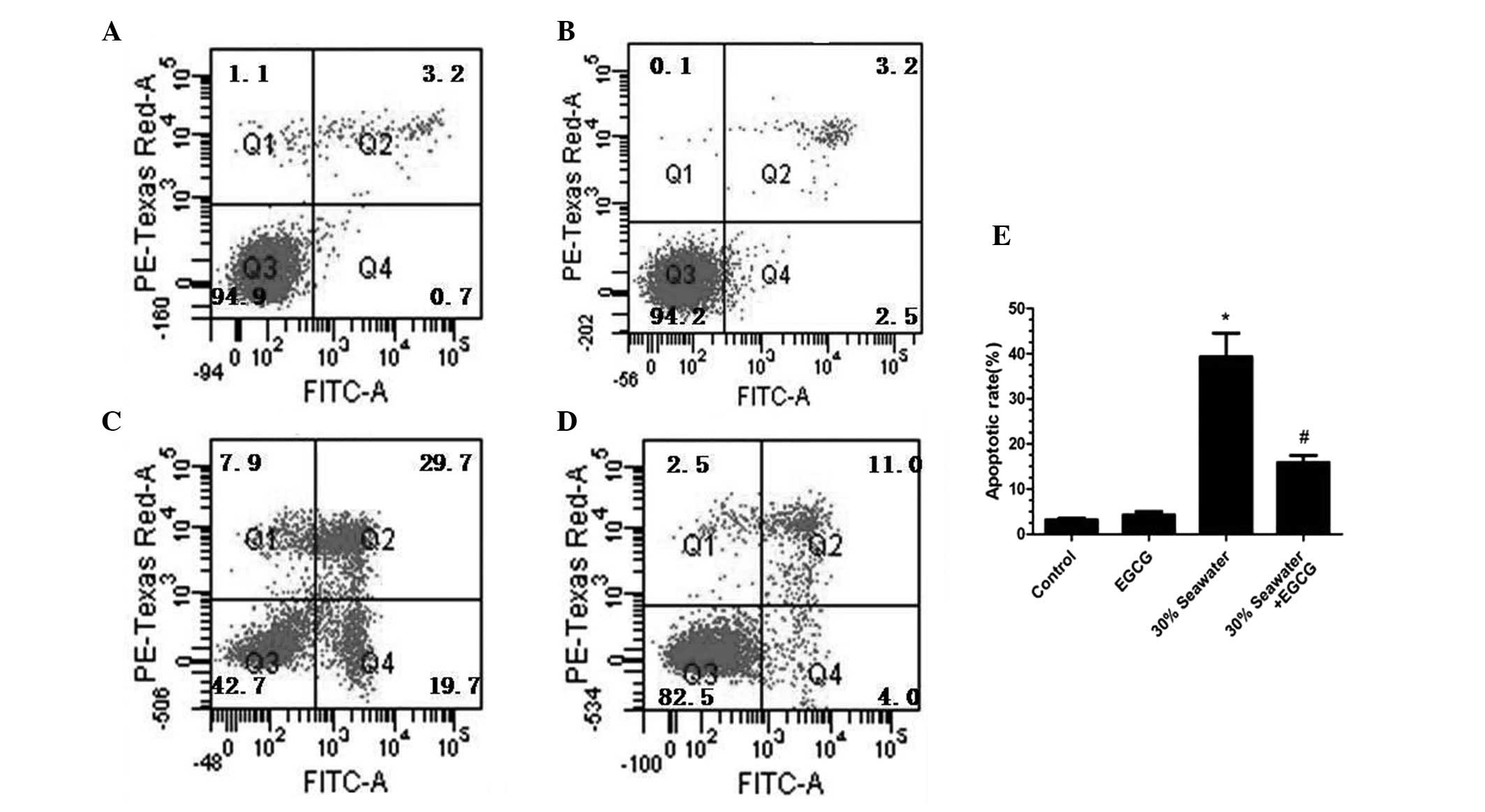
Effects of EGCG on the seawater-induced
apoptosis of the A549 cells
The effects of EGCG on the apoptosis of alveolar
epithelial cell line A549 when treated with seawater were explored.
As shown in Fig. 5, the percentage
of apoptotic A549 cells increased in a concentration-dependent
manner. The apoptotic rate reached 68.5% in the 40% seawater group
(P<0.05 vs. the control group; Fig.
5F). However, no significant difference between the control
group and 10% seawater group was identified.
The effect of EGCG on A549 cells treated with 30%
saltwater was further investigated. The EGCG pretreatment
significantly decreased the percentage of A549 cells undergoing
apoptosis (P<0.05 vs. the control group; Fig. 6). No significant differences were
identified between the percentage of cells undergoing apoptosis in
the control group and the EGCG-only group (P>0.05; Fig. 6).
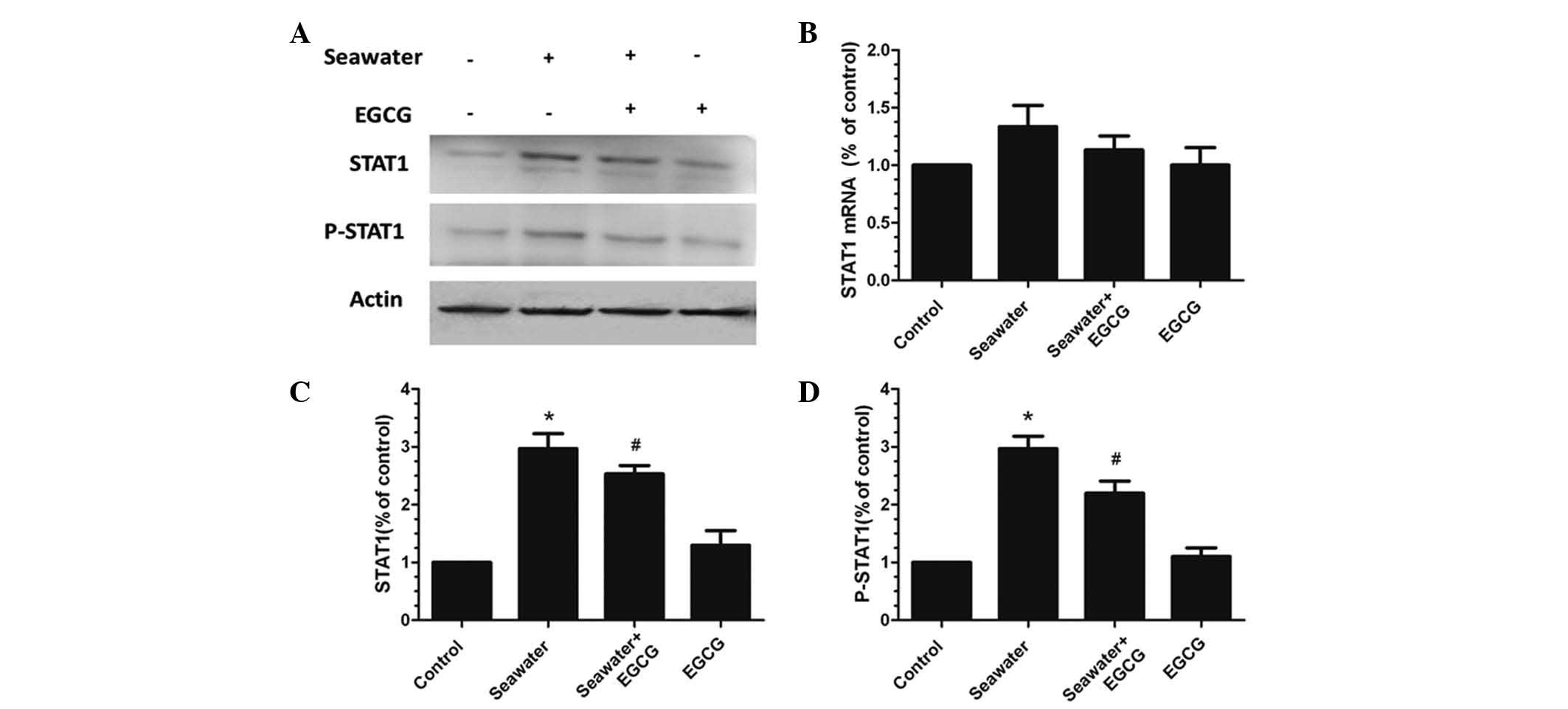
Effects of seawater and EGCG on the
expression of STAT1 and P-STAT1 in A549 cells
The effects of seawater and EGCG on the expression
of STAT1, a key upstream modulator of caspase-3 and p21, was
examined in A549 cells. No significant differences were identified
in the STAT1 mRNA expression levels between any of the treatment
groups (Fig. 7A and B). However,
the protein expression levels of STAT1 and P-STAT1 were
significantly elevated in the seawater-only group compared with the
control group (P<0.05; Fig. 7C and
D). The group pretreated with EGCG presented significantly
reduced protein expression levels of STAT1 and P-STAT1 compared
with the seawater-only group (P<0.05; Fig. 7C and D).
Discussion
In the present study, it was demonstrated that EGCG
pretreatment alleviated seawater aspiration-induced ALI. EGCG
pretreatment decreased the apoptosis of alveolar epithelial cells
in seawater aspiration-induced ALI. To further investigate the role
of EGCG in the apoptosis of alveolar epithelium in seawater
aspiration-induced ALI, the expression levels of caspase-3 and p21
were determined, as they are apoptosis-associated proteins.
Notably, protein levels were significantly higher in seawater
aspiration-induced ALI compared with the control group. By
contrast, the EGCG pretreatment group exhibited significantly lower
levels of the apoptotic proteins, suggesting that EGCG may limit
the damage that cells undergo during seawater aspiration-induced
ALI. The expression of the protein STAT1 and phosphorylated STAT1,
a key upstream protein of caspase-3 and p21, was identified to
increase in the seawater-only group. However, protein expression
levels of STAT1 and P-STAT1 decreased in the EGCG pretreatment
group compared with the seawater group.
The alveolar epithelium and the microvascular
endothelium constitute the alveolar-capillary barrier (18). The epithelial barrier is less
permeable than the endothelial barrier under physiological
conditions (19). The loss of
integrity of alveolar epithelium is an important pathophysiological
change during ALI, which leads to pulmonary edema and a decrease in
surfactant-associated proteins (18,20).
The apoptosis of alveolar epithelial cells is one of the main
factors damaging the alveolar-capillary barrier and thus,
represents an important prognostic marker of ALI (21). Seawater aspiration-induced ALI is
one of the most serious complications due to seawater drowning
(22). Previous studies have
indicated that the apoptosis of alveolar epithelium aggravated
seawater aspiration-induced ALI, which may also be mediated via the
Fas/FasL (6) and protein kinase
(ERK) pathways (5).
In previous studies, EGCG has been demonstrated to
exhibit anti-inflammatory (23),
anti-oxidative (24) and
anticancer (25) properties. In
addition, EGCG has been indicated to have a positive effect on cell
cycle arrest and apoptosis in prostate and breast cancer cells
(26). Furthermore, it has been
demonstrated to enhance cisplatin chemosensitivity in cervical
cancer cells via the induction of apoptosis (27). Other studies have indicated that
EGCG inhibits Cd2+-induced apoptosis through scavenging
reactive oxygen species activity (28), and prevents cardiac
ischemia/reperfusion-induced apoptosis (10). The findings of another previous
study suggest that EGCG ameliorates inflammation in seawater
aspiration-induced ALI by inhibiting the JAK/STAT1 pathways
(7). However, it remains largely
unknown whether EGCG protection during seawater aspiration-induced
ALI is also mediated by the suppression of apoptosis in alveolar
epithelial cells.
In the current study, it was determined that EGCG
pretreatment attenuated the degree of ALI and inhibited the
apoptosis of lung tissue and alveolar epithelial cells in
vivo and in vitro. Simultaneously, EGCG pretreatment
reduced the expression of the proteins STAT1, P-STAT1 and the
downstream proteins caspase-3 and p21. Overexpression of STAT1 may
induce osteocyte apoptosis in steroid-induced avascular necrosis of
the femoral head and promote the apoptosis of retinal pericytes
under high glucose conditions (29). The apoptosis-associated proteins
caspase-3 and p21, as the downstream proteins of STAT1, promoted
the apoptosis of alveolar epithelial cells in seawater
aspiration-induced ALI. Therefore, EGCG may inhibit the apoptosis
of alveolar epithelial cells in seawater aspiration-induced ALI via
decreasing the expression of STAT1, P-STAT1 and its downstream
proteins caspase-3 and p21.
In summary, the present study suggests that EGCG
attenuates seawater aspiration-induced ALI by suppressing the
apoptosis of alveolar epithelial cells, at least partly, via the
inhibition of the STAT1-caspase-3/p21 associated pathway.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81270124 and
81372129).
References
|
1
|
Salomez F and Vincent JL: Drowning: A
review of epidemiology, pathophysiology, treatment and prevention.
Resuscitation. 63:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rui M, Duan YY, Wang HL, Zhang XH and Wang
Y: Differences between seawater- and freshwater-induced lung
injuries. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 21:416–420.
2009.In Chinese. PubMed/NCBI
|
|
3
|
Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D,
Zhao P, Zhang B, Cao F, Wang Y, et al: Tanshinone IIA ameliorates
seawater exposure-induced lung injury by inhibiting aquaporins
(AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol.
176:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma L, Li Y, Zhao Y, Wang Q, Nan Y, Mu D,
Li W, Sun R, Jin F and Liu X: 3,5,4′-tri-O-acetylresveratrol
ameliorates seawater exposure-induced lung injury by upregulating
connexin 43 expression in lung. Mediators Inflamm. 2013:1821322013.
View Article : Google Scholar
|
|
5
|
Li JH, Xu M, Xie XY, Fan QX, Mu DG, Zhang
Y, Cao FL, Wang YX, Zhao PT, Zhang B, et al: Tanshinone IIA
suppresses lung injury and apoptosis, and modulates protein kinase
B and extracellular signal-regulated protein kinase pathways in
rats challenged with seawater exposure. Clin Exp Pharmacol Physiol.
38:269–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han F, Luo Y, Li Y, Liu Z, Xu D, Jin F and
Li Z: Seawater induces apoptosis in alveolar epithelial cells via
the Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 182:71–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Dong M, Bo L, Li C, Liu Q, Li Y, Ma
L, Xie Y, Fu E, Mu D, et al: Epigallocatechin-3-gallate ameliorates
seawater aspiration-induced acute lung injury via regulating
inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats.
Mediators Inflamm. 2014:6125932014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shankar S, Suthakar G and Srivastava RK:
Epigallocatechin-3-gallate inhibits cell cycle and induces
apoptosis in pancreatic cancer. Front Biosci. 12:5039–5051. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Townsend PA, Scarabelli TM, Pasini E,
Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS and
Stephanou A: Epigallocatechin-3-gallate inhibits STAT-1 activation
and protects cardiac myocytes from ischemia/reperfusion-induced
apoptosis. FASEB J. 18:1621–1623. 2004.PubMed/NCBI
|
|
11
|
Zou P, Song J, Jiang B, Pei F, Chen B,
Yang X, Liu G and Hu Z: Epigallocatechin-3-gallate protects against
cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int
J Clin Exp Pathol. 7:4607–4616. 2014.PubMed/NCBI
|
|
12
|
Menegazzi M, Tedeschi E, Dussin D, De
Prati AC, Cavalieri E, Mariotto S and Suzuki H: Anti-interferon
gamma action of epigallocatechin-3-gallate mediated by specific
inhibition of STAT1 activation. FASEB J. 15:1309–1311.
2001.PubMed/NCBI
|
|
13
|
Song CG, Yang X, Min LQ, Liu CX and Zhao
CS: The effect of procyanidin on expression of STAT1 in type 2
diabetes mellitus SD rats with focal cerebral ischemia. Neuro
Endocrinol Lett. 35:68–72. 2014.PubMed/NCBI
|
|
14
|
Xu X, Wen H, Hu Y, Yu H, Zhang Y, Chen C
and Pan X: STAT1-caspase 3 pathway in the apoptotic process
associated with steroid-induced necrosis of the femoral head. J Mol
Histol. 45:473–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae HB, Li M, Kim JP, Kim SJ, Jeong CW,
Lee HG, Kim WM, Kim HS and Kwak SH: The effect of epigallocatechin
gallate on lipopolysaccharide-induced acute lung injury in a murine
model. Inflammation. 33:82–91. 2010. View Article : Google Scholar
|
|
16
|
Shi Y, Zhang B, Chen XJ, Xu DQ, Wang YX,
Dong HY, Ma SR, Sun RH, Hui YP and Li ZC: Osthole protects
lipopoly-saccharide-induced acute lung injury in mice by preventing
down-regulation of angiotensin-converting enzyme 2. Eur J Pharm
Sci. 48:819–824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou ZH, Sun B, Lin K and Zhu LW:
Prevention of rabbit acute lung injury by surfactant, inhaled
nitric oxide, and pressure support ventilation. Am J Respir Crit
Care Med. 161:581–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiener-Kronish JP, Albertine KH and
Matthay MA: Differential responses of the endothelial and
epithelial barriers of the lung in sheep to Escherichia coli
endotoxin. J Clin Invest. 88:864–875. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greene KE, Wright JR, Steinberg KP,
Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T,
Kuroki Y, et al: Serial changes in surfactant-associated proteins
in lung and serum before and after onset of ARDS. Am J Respir Crit
Care Med. 160:1843–1850. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galani V, Tatsaki E, Bai M, Kitsoulis P,
Lekka M, Nakos G and Kanavaros P: The role of apoptosis in the
pathophysiology of Acute Respiratory Distress Syndrome (ARDS): An
up-to-date cell-specific review. Pathol Res Pract. 206:145–150.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li
JH, Xie XY, Fan QX, Liu W, Mu DG, et al: Tanshinone IIA attenuates
seawater aspiration-induced lung injury by inhibiting macrophage
migration inhibitory factor. Biol Pharm Bull. 34:1052–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YJ, Choi DY, Yun YP, Han SB, Oh KW and
Hong JT: Epigallocatechin-3-gallate prevents systemic
inflammation-induced memory deficiency and amyloidogenesis via its
anti-neuroinflammatory properties. J Nutr Biochem. 24:298–310.
2013. View Article : Google Scholar
|
|
24
|
Lee IT, Lin CC, Lee CY, Hsieh PW and Yang
CM: Protective effects of (−)-epigallocatechin-3-gallate against
TNF-α-induced lung inflammation via ROS-dependent ICAM-1
inhibition. J Nutr Biochem. 24:124–136. 2013. View Article : Google Scholar
|
|
25
|
Butt MS, Ahmad RS, Sultan MT, Nasir QM and
Naz A: Green tea and anticancer perspectives: Updates from last
decade. Crit Rev Food Sci Nutr. 55:792–805. 2015. View Article : Google Scholar
|
|
26
|
Wang P, Wang B, Chung S, Wu Y, Henning SM
and Vadgama JV: Increased chemopreventive effect by combining
arctigenin, green tea polyphenol and curcumin in prostate and
breast cancer cells. RSC Advances. 4:35242–35250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh M, Bhui K, Singh R and Shukla Y: Tea
polyphenols enhance cisplatin chemosensitivity in cervical cancer
cells via induction of apoptosis. Life Sci. 93:7–16. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An Z, Qi Y, Huang D, Gu X, Tian Y, Li P,
Li H and Zhang Y: EGCG inhibits Cd(2+)-induced apoptosis through
scavenging ROS rather than chelating Cd(2+) in HL-7702 cells.
Toxicol Mech Methods. 24:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin ES, Huang Q, Gurel Z, Palenski TL,
Zaitoun I, Sorenson CM and Sheibani N: STAT1-mediated Bim
expression promotes the apoptosis of retinal pericytes under high
glucose conditions. Cell Death Dis. 5:e9862014. View Article : Google Scholar : PubMed/NCBI
|