Introduction
Proliferation and migration of vascular smooth
muscle cells (VSMCs) from the tunica media to the tunica intima is
an early event in response to vascular injury, including
atherosclerosis and angioplasty-induced restenosis (1). Interactions between the cells and
surrounding extracellular matrix (ECM) have important roles in
numerous cellular processes, including differentiation,
proliferation, migration, cytoskeletal organization and survival
(2). Fibronectin is an
extracellular glycoprotein that has important roles in cell
adhesion, migration, growth and differentiation (3). Integrin (ITG) α5β1 is a dominant
fibronectin receptor comprised of the α5 and β1 subunits, which is
known to be abundantly expressed on the surface of VSMCs (4). ITGβ is able to activate signaling
pathways, including the focal adhesion kinase (FAK), Src and
Ras/extracellular signal-regulated kinase (ERK) pathways, which are
dependent on the interactions between various extracellular matrix
(ECM) proteins and ITG (5). FAK
and its autonomously expressed C-terminal inhibitor FAK-related
non-kinase (FRNK), are important regulators of VSMC spreading and
migration (6). Integrin-linked
kinase (ILK) is a downstream mediator of ITGβ1 activity. ILK
activity has previously been shown to be associated with numerous
cell functions (7). It has
previously been suggested that ILK has a role in the cell adhesion,
proliferation, migration, matrix remodeling and survival of various
types of tissue and cells (7).
The role of ITG α5β1 in the proliferation and
migration of VSMCs has not yet been reported. The present study
aimed to explore the regulatory effects of ITG α5β1 on the
proliferation and migration of VSMCs, investigate alterations to
the FAK and ILK signaling pathways and understand the underlying
mechanisms. The present study constructed a lentiviral expression
vector of ITGα5β1 as well as a small interfering RNA (siRNA)
lentiviral vector of ITGα5β1 in order to obtain VSMC with ITGα5β1
overexpression and knockdown, respectively. The proliferation,
migration, cell cycle distribution and mRNA expression levels of
transfected VSMCs were then analyzed.
Materials and methods
Reagents
Fetal bovine serum (FBS) and RPMI 1640 were
purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
Trypsin was obtained from HyClone (GE Healthcare, Logan, UT, USA).
RNase and propidium iodide (PI) were obtained from Sigma-Aldrich
(St. Louis, MO, USA). pGEM-T vector was purchased from Tiangen
Biotech Co., Ltd. (Beijing, China). The lentiviral expression
vector pLenti, and lentivirus packaging plasmids pLP1 and pLP/VSVG
were a generous gift from by Dr Huijun Zhi (Department of
Microbiology and Immunology, Uniformed Services University of the
Health Sciences, National Institutes of Health, Bethesda, MA, USA).
pCMV-SPORT6-ITGα5 and pCMV-SPORT6-ITGβ1 were purchased from OriGene
Technologies, Inc. (Rockville, MD, USA). The plasmid
pRNAT-U6.2/Lenti was obtained from GenScript (Piscataway, NJ, USA).
Restriction enzymes BamHI, XhoI, KpnI and
MluI were purchased from Promega Corp. (Madison, WI, USA).
Lipofectamine®2000 was purchased from Invitrogen (Thermo
Fisher Scientific). ITGα5 mouse monoclonal antibody (cat. no.
SC-166681), ITGβ1 monoclonal antibody (cat. no. SC-71386), GAPDH
monoclonal antibody (cat. no. SC-137179) and horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (cat no.
SC-2005) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). All antibodies were diluted at a 1:300 ratio and
all incubations were performed for 1 h at room temperature. ITGα5
and ITGβ1 reverse-transcription polymerase chain reaction (RT-PCR)
kits and primers were purchased from Qiagen China (Beijing, China).
The other reagents used in the present study were of analytical
grade.
Cell culture
The 293FT cells were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in RPMI 1640 supplemented with
10% FBS. Human aortic VSMCs (no. PCS-100-021) were purchased from
the American Type Culture Collection (Manassas, VA, USA) and were
cultured in RPMI 1640 supplemented with 10% FBS and antibiotics
(100 IU/ml penicillin and 100 µg/ml streptomycin; all from
Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere.
Construction of the lentiviral expression
vector and siRNA lentiviral vector of ITGα5 and ITGβ1
The entire cDNA sequences of ITGα5 and ITGβ1 were
amplified by PCR of pCMV-SPORT6-ITGα5 and pCMV-SPORT6-ITGβ1. The
upstream and downstream primers both contained KpnI and
MluI endonuclease sites. The cDNA was then ligated with the
pGEM-T vector. The ligation products were transfected into
Escherichia coli DH5α cells (Beijing Hua Yueyang
Biotechnology Co. Ltd., Beijing, China), which were maintained at
37°C. The positive recombinant clones pGEM-T-ITGα5 and pGEM-T-ITGβ1
were then selected and maintained in LB medium for 4 h at 37°C.
Subsequently, 1 µl bacterial medium was used as a template
and PCR was performed using the EconoTaq PLUS 2X Master Mix
(Lucigen, Madison, WI, USA) with T7 (5′-TAATACGACTCACTATAGGGAGA-3′)
and SP6 (5′-CATACGATTTAGGTGACACTATAG-3′) primers according to
manufacturer's instructions. The positive clones were subjected to
DNA sequencing by Shanghai Sengong Biotech (Shanghai, China). The
cloning vector and the lentivirus were cut using KpnI and
MluI restriction endonucleases, following which they were
ligated and transfected. Enzyme analysis and gene sequencing
analysis were used to verify the accuracy of the recombinant
vectors pLenti-ITGα5 and pLenti-ITGβ1.
According to the nucleotide sequence of the ITGα5
and ITGβ1 genes in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and the
principles of siRNA design, two segment sequences were selected
from each: 735–753 nt and 970–988 nt, and 600–618 nt and
1,283–1,301 nt, respectively. The effective siRNA sequences
targeting ITGα5 and ITGβ1 were then designed using the siRNA
Designer web tool from Promega Corp. and synthesized by Sengong
Biotech. The full list of siRNAs and their sequences used in the
present study is shown in Table I.
Both ends of the hairpin target sequences contained BamHI
and XhoI endonuclease sites. The cDNA containing both the
sense and antisense strands of the targeting sequence was designed,
synthesized and cloned into the pRNAT-U6.2/Lenti vector, which
contained the H1 promoter and green fluorescent protein (GFP). The
resulting lentiviral vectors containing ITGα5 or ITGβ1 siRNA were
named pRNAT-U6.2/Lenti-si ITGα5-1, pRNAT-U6.2/Lenti-siITGα5-2,
pRNAT-U6.2/Lenti-siITGβ1-1 and pRNAT-U6.2/Lenti-siITGβ1-2.
Restriction endonuclease digestion and DNA sequencing were
conducted to confirm the generation of the recombinant vectors. PCR
and gene sequencing analysis were used to verify the accuracy of
the recombinant vectors.
 | Table IsiRNAs used in the present study. |
Table I
siRNAs used in the present study.
| siRNA | Sequence |
|---|
| IA11 |
5′-GATCCGGACCAGGAAGCTATTTCTTTCAAGAGAAGAAATAGCTTCCTGGTCCTTTTTTC-3′ |
| IA12 |
5′-TCGAGAAAAAAGACCAGGAAGCTATTTCTTCTCTTGAAAGAAATAGCTTCCTGGTCCG-3′ |
| IA21 |
5′-GATCCGCTATGTCACCATCCTTAATTCAAGAGATTAAGGATGGTGACATAGCTTTTTTC-3′ |
| IA22 |
5′-TCGAGAAAAAAGCTATGTCACCATCCTTAATCTCTTGAATTAAGGATGGTGACATAGCG-3′ |
| IB11 |
5′-GATCCGAGCCACAGACATTTACATTTCAAGAGAATGTAAATGTCTGTGGCTCTTTTTTC-3′ |
| IB12 |
5′-TCGAGAAAAAAGAGCCACAGACATTTACATTCTCTTGAAATGTAAATGTCTGTGGCTCG-3′ |
| IB21 |
5′-GATCCGTCAGCAGTAGGAACATTATTCAAGAGATAATGTTCCTACTGCTGACTTTTTTC-3′ |
| IB22 |
5′-TCGAGAAAAAAGTCAGCAGTAGGAACATTATCTCTTGAATAATGTTCCTACTGCTGACG-3′ |
Lentivirus packaging plasmid mixtures containing
Lentivirus-ITGα5 or Lentivirus-ITGβ1, or
pRNAT-U6.2/Lenti-siITGα5-1, pRNAT-U6.2/Lenti-siITGα5-2,
pRNAT-U6.2/Lenti-siITGβ1-1 or pRNAT-U6.2/Lenti-siITGβ1-2 were
co-transfected into the 293FT cells. All transfections were
performed using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions. The titer of the viral stock solutions
was assessed via quantification of the expression levels of GFP as
previously described (8).
VSMC transfection
Recombinant lentiviruses were trans-fected into the
VSMCs in order to establish the following cell lines with
upregulated and downregulated ITGα5 and ITGβ1 gene expression:
Lentivirus-ITGα5, Lentivirus-ITGβ1, pRNAT-U6.2/Lenti-siITGα5-1,
pRNAT-U6.2/Lenti-siITGα5-2, pRNAT-U6.2/Lenti-siITGβ1-1,
pRNAT-U6.2/Lenti-siITGβ1-2, pLentiGFP empty vector and
pRNAT-U6.2/Lenti empty. Lipofectamine 2000 (Invitrogen) was used
for all transfections according to the manufacturer's instructions.
Screening with G418 (Sigma-Aldrich) was used to obtain stably
transfected VSMCs. The transfected VMSC cell lines were named:
ITGα5-overexpressing cell line (EX-ITGα5), ITGβ1-overexpressing
cell line (EX-ITGβ1), ITGα5-knockdown cell line (si-ITGα5),
ITGβ1-knockdown cell line (si-ITGβ1), pLentiGFP empty
vector-transfected cell line (Con-Ex) and pRNAT-U6.2/Lenti empty
vector-transfected cell line (Con-si), respectively.
Post-transfection, lentivirus-ITGα5 was trans-fected into the
EX-ITGβ1 cell line in order to generate a cell line overexpressing
both ITGα5 and ITGβ1 - this cell line was named D-EX. In addition,
pRNAT-U6.2/Lenti-siITGα5-1 was transfected into the si-ITGβ1-2
cells in order to generate a cell line exhibiting both ITGα5 and
ITGβ1 knockdown - this cell line was named D-si. Quantitative
(q-PCR) and western blotting were used to detect the changes to
ITGα5 and ITGβ1 gene and protein expression levels in all of the
stably transfected cell lines (4,9).
Cell growth was observed using microscopy (Inverted microscope
IX83; Olympus, Japan).
Proliferation assay
All experiments were conducted using the nine cell
lines: EX-ITGα5, EX-ITGβ1, D-EX, si-ITGα5, si-ITGβ1, D-si, Con-Ex,
Con-si and normal VSMCs. The nine cell lines were divided into
three groups: Overexpressing groups, including EX-ITGα5, EX-ITGβ1
and D-EX; knockdown groups, including si-ITGα5, si-ITGβ1 and D-si;
and control groups, including Con-Ex, Con-si and normal VSMCs.
All of the cell lines were cultured in 96-well
microtiter plates (Corning, Inc., Corning, NY, USA) at
1×104 cells/well and incubated for five days at 37°C.
Subsequently, 20 µl MTT (5 mg/ml; Jiemei Gene
Pharmaceutical, Shanghai, China) was added to each well and
incubated for 4 h at 37°C. Following removal of the supernatant,
150 µl dimethyl sulfoxide (Jiemei Gene Pharmaceutical) was
added to each well, and the absorbance values were measured using a
microplate reader (iMark™ Microplate Absorbance reader; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 490 nm (9).
Migration assay
The migration assay was performed using a Transwell
system, which allows cells to migrate through an 8-µm pore
polycarbonate membrane in a millicell (10,11).
Briefly, the cells were serum-starved for 24 h and then plated
(1×104 cells/well) in serum-free medium (Gibco) in the
upper chamber of a 24-well Transwell plate (Corning). The lower
chamber was filled with 1.5 ml medium supplemented with 500
µl chemotactic factor (Jiemei Gene Pharmaceutical). After 48
h, the cells that remained on the upper surface of the membrane
were removed using a cotton swab, and the cells on the lower
surface of the membrane were fixed with cold methanol for 15 min
and stained with 0.2% crystal violet (Jiemei Gene Pharmaceutical).
The cells that had migrated to the bottom of the membrane were
visualized and counted using an inverted microscope. For each
repetition, cells in four randomly selected fields were counted and
averaged. Results were expressed as a ratio of the untreated
group.
Cell cycle analysis
The cells were cultured in six-well culture plates
for 24 h and incubated for a further 24 h in serum-free medium. The
cells were then incubated in medium containing serum for another 48
h. The cell cycle was assessed according to the intensity of PI
staining, as described previously (12). Briefly, ethanol-fixed cells were
centrifuged and washed twice with phosphate-buffered saline.
Subsequently, cells were treated with 100 µl RNase (Promega)
for 30 min at 37°C, followed by the addition of 100 µl
propidium iodide dye (1 µg/m;l Sigma-Aldrich) and incubation
for 30 min in the dark. Cell cycle analysis was performed using a
flow Cytometer (FACS Aria II; BD Biosciences, Franklin Lakes, NJ,
USA). Data were analyzed using Modfit LT software, version 3.2 (BD
Biosciences).
RT-qPCR
Total RNA was isolated using a Total RNA Extraction
kit (Qiagen, Hilden, Germany). Total RNA was then
reverse-transcribed in a 20-µl reaction solution containing
Revert AID™ First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific). The subsequent RT-qPCR, used to determine the
expression levels of ILK and FAK, was conducted as described
previously (13,14). The sequences of the individual
pairs of ILK, FAK, ITGα5, ITGβ1 and GAPDH primers (Qiagen) are
presented in Table II. The PCR
reactions were performed using the ABI StepOne Plus PCR system
(Applied Biosystems; Thermo Fisher Scientific) in 96-well reaction
plates. The thermocycling conditions were as follows: 95°C for 10
min followed by 95°C for 10 sec and 60°C 60 sec for 40 cycles. Gene
expression was quantified by 2−ΔΔCT method (15). Relative mRNA levels for each gene
were normalized, and values are expressed as the ratio of target
gene mRNA to GAPDH expression.
 | Table IISequence details of individual pairs
of primers. |
Table II
Sequence details of individual pairs
of primers.
| Gene | Sense | Antisense |
|---|
| ILK1 |
5′-CTGGCAGCCAGTCATGGACAC-3′ |
5′-ATGCTGACAAGGGCCCCATTT-3′ |
| FAK |
5′-TTGCGGAGAATATGGCTGACCTAA-3′ |
5′-TGGTATTGATGGCAAAGCCCGTTC-3′ |
| ITGα5 |
5′-AGTGCACCCCCATTGAATTTG-3′ |
5′-GAACTGTTGCCCCGAACCACT-3′ |
| ITGβ1 |
5′-ACAGCAGTTGGTTTTGCGATT-3′ |
5′-TCCAATTCTGAAGTCCGAAGT-3′ |
| GAPDH |
5′-GGGAAGGTGAAGGTCGGAGTC-3′ |
5′-CCCACTTGATTTTGGAGGGAT-3′ |
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses of differences between the groups
were performed using Student's t-test and analysis of variance.
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction of overexpression and RNAi
vectors
The results of PCR, enzyme analysis and DNA
sequencing analysis confirmed that the ITGα5 and ITGβ1 genes had
been successfully cloned (full length, 3,150+176 bp and 2,397+176
bp, respectively), and the pGEM-T-ITGα5 and pGEM-T-ITGβ1
recombinant clones were confirmed by gene sequencing analysis.
Enzyme analysis and gene sequencing indicated that the lentiviral
expression vectors Lentivirus-ITGα5 and -ITGβ1 had been
successfully constructed. PCR and DNA sequencing demonstrated that
the lentivirus RNAi vectors psiRNA-ITGα5 and -ITGβ1 were also
successfully constructed (Fig. 1).
The resulting lentiviral vectors were named
pRNAT-U6.2/Lenti-siITGα5 and pRNAT-U6.2/Lenti-siITGβ1. The viral
titers were all >7.7×105 IU/ml, and were determined
by detecting the expression levels of GFP.
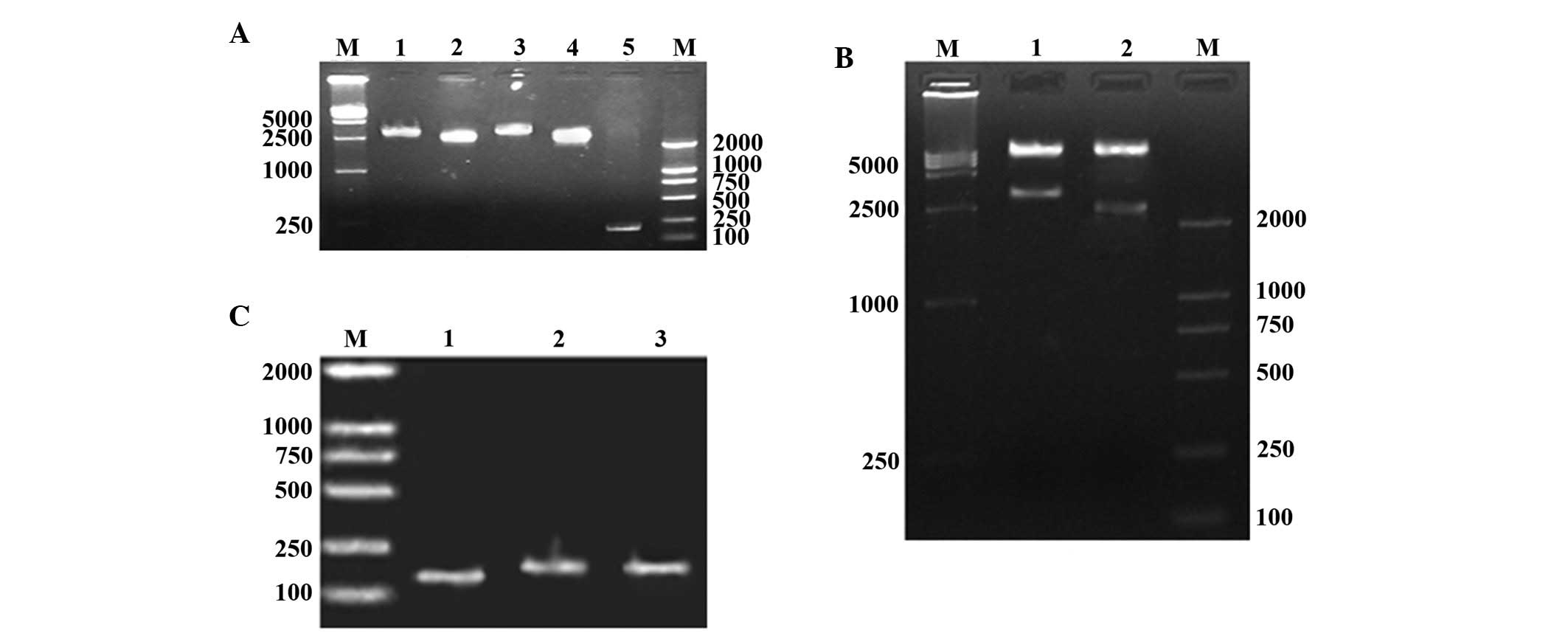 | Figure 1Target genes ITGα5 and ITGβ1, and
recombinant clones. (A) PCR cloning of ITGα5 and ITGβ1 genes. M,
marker; lane 1, ITGα5; lane 2, ITGβ1; lane 3, pGEM-ITGα5; lane 4,
pGEM-ITGβ1; lane 5, control vector pGEM-T. (B) Double digestion of
recombinant clones. M, marker; lane 1, pLent-ITGα5; lane 2,
pLent-ITGβ1. (C) siRNA gene recombinant clones. M, marker; lane 1,
pRNAT-U6.2/Lenti; lane 2, pRNAT-U6.2/Lenti-siITGα5; lane 3,
pRNAT-U6.2/Lenti-siITGβ1. ITG, integrin; siRNA, small interfering
RNA. |
Confirmation of transfected cell
lines
As shown in Fig.
2A, the mRNA expression levels of ITGα5 and ITGβ1 were measured
by RT-qPCR. The expression levels in the control groups were as
follows: Con-si, ITGα5 mRNA (0.252±0.026) and ITGβ1 mRNA
(0.516±0.056); Con-Ex, ITGα5 mRNA (0.251±0.021) and ITGβ1 mRNA
(0.505±0.062); and Con, ITGα5 (0.238±0.021) and ITGβ1
(0.471±0.051). There were no significant differences between the
expression levels in the control groups (P>0.05). The mRNA
expression levels of ITGα5 in the EX-ITGα5 and D-EX cells were
significantly increased (P<0.05), and were significantly
decreased in the si-ITGα5-1 and D-si cells (P<0.05), as compared
with those in the control groups. In addition, the mRNA expression
levels of ITGβ1 in the EX-ITGβ1 and D-EX cells were significantly
increased (P<0.05), while being significantly decreased in the
si-ITGβ1-1 and D-si cells (P<0.05). The same trend was observed
with regards to the protein expression levels of ITGα5 and ITGβ1,
as detected by western blot analysis (Fig. 2B). As observed under an inverted
microscope, the EX-ITGα5, EX-ITGβ1 and D-EX cells grew faster, as
compared with the control cells, which exhibited better cell
morphology and were spindle-shaped. The cells of the si-ITGα5,
si-ITGβ1 and D-si groups had a similar morphology to that of the
control cells; however, their growth rate was slightly slower as
compared with that of the control cells (Fig. 3).
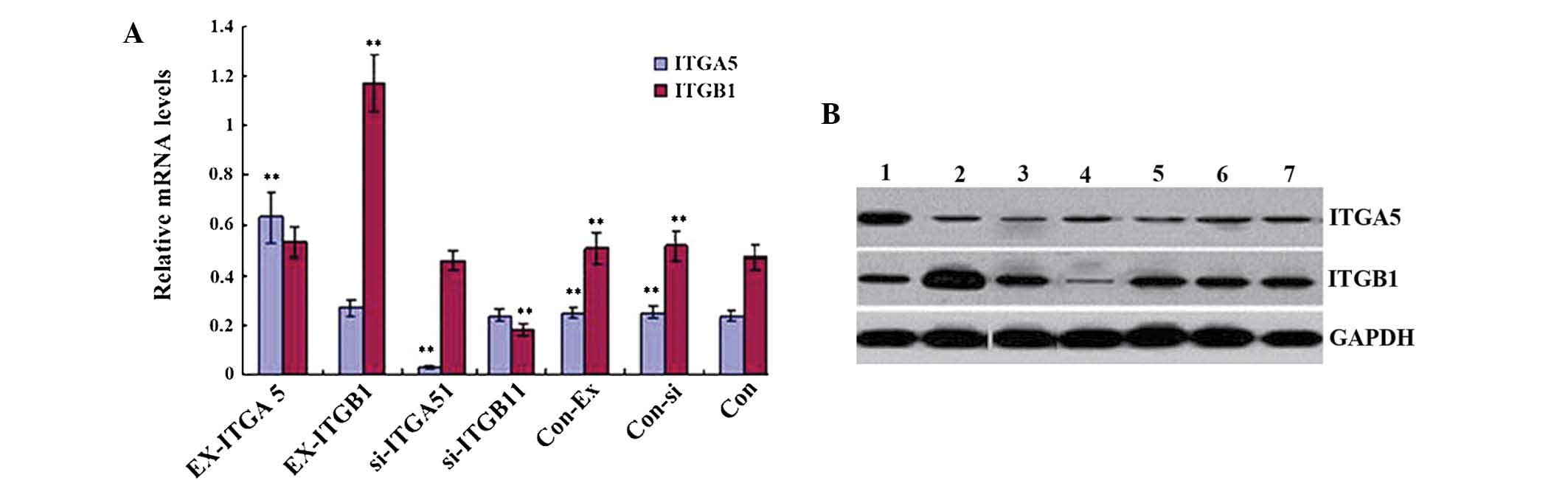 | Figure 2mRNA and protein expression levels of
ITGα5 and ITGβ1 in the seven types of vascular smooth muscle cells.
(A) mRNA expression levels of ITGα5 and ITGβ1 in the seven cell
groups. (B) Protein expression levels of ITGα5 and ITGβ1 in the
seven cell groups. Values are expressed as the mean ± standard
error of the mean (n=3). **P<0.05 vs. Con. Lane 1,
EX-ITGα5; lane 2, EX-ITGβ1; lane 3, si-ITGα5; lane 4, si-ITGβ1;
lane 5, Con-Ex; lane 6, Con-si; lane 7, Con. ITG, integrin; si,
small interfering; Con, control; EX, overexpressing. |
 | Figure 3VSMCs were transfected with various
lentiviruses (magnification, ×400). (A) EX-ITGα5, VSMCs transfected
with pLent-ITGα. (B) EX-ITGβ1, VSMCs transfected with pLent-ITGβ1.
(C) si-ITGα5, VSMCs transfected with pRNAT-U6.2/Lenti-siITGα5. (D)
si-ITGβ1, VSMCs transfected with pRNAT-U6.2/Lenti-siITGβ1. (E)
Con-Ex, VSMCs transfected with pLentiGFP. (F) Con-si, VSMCs
transfected with pRNAT-U6.2/Lenti. VSMC, vascular smooth muscle
cell; ITG, integrin; si, small interfering; Con, control; GFP,
green fluorescent protein; EX, overexpressing. |
Effects of ITGα5 and ITGβ1 on cell
growth
Fig. 4 shows the
proliferative activity of the cells. There were no significant
differences between the control groups, and on the second day there
were no significant differences in cell proliferation between any
of the groups. From the third day, the absorbance values of MTT
were significantly increased in the EX-ITGβ1 and D-EX cells
(P<0.05), and were significantly decreased in the si-ITGβ1 and
D-Si cells (P<0.05), as compared with those in the control
group; however, there was no significant difference between the
EX-ITGα5 and si-ITGα5 cells (P>0.05). These results suggested
that upregulation of ITGβ1 expression is able to induce cell
proliferation, and downregulation of ITGβ1 expression may hinder
cell proliferation. By contrast, ITGα5 expression had no effect on
proliferation of the cells.
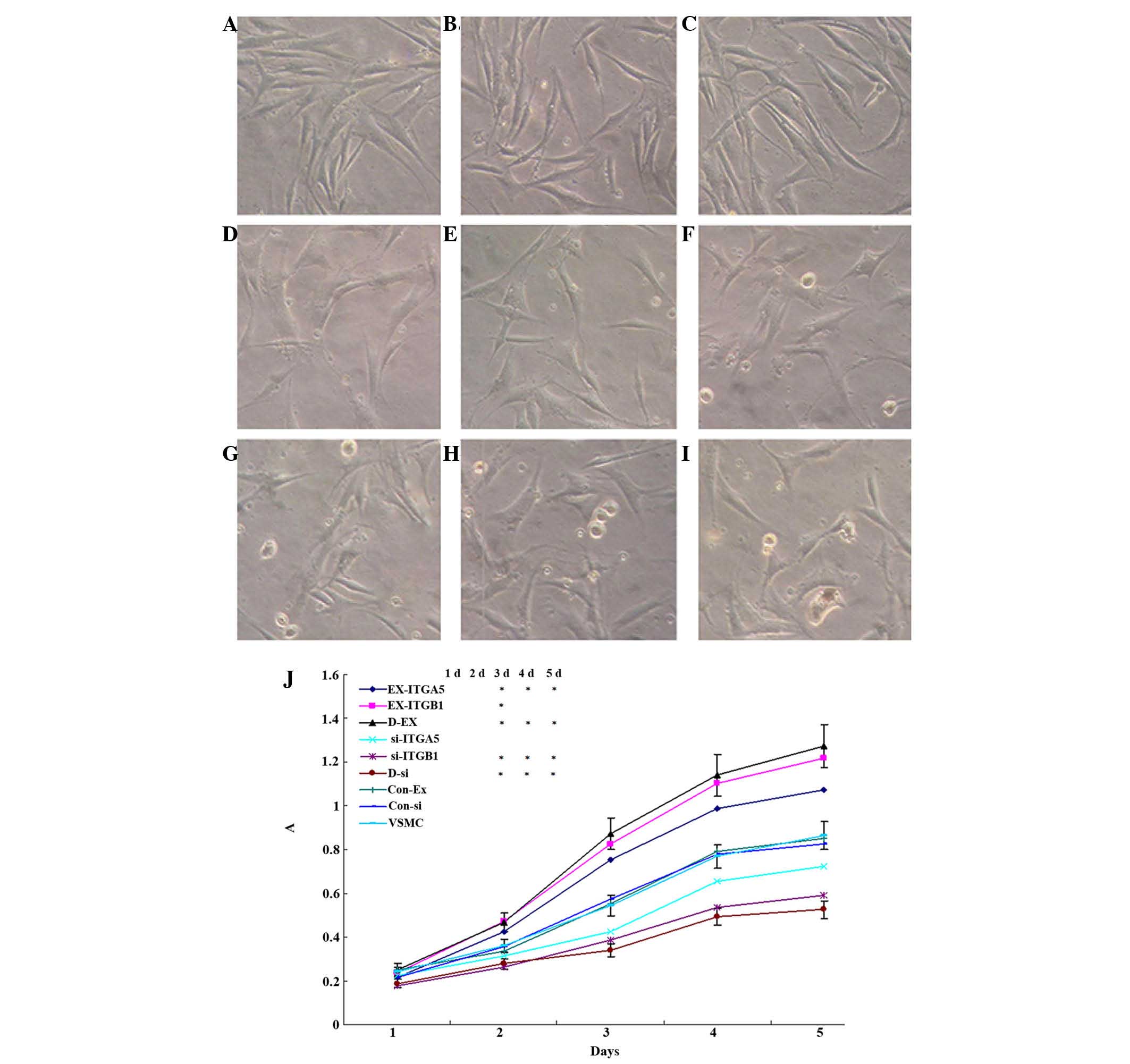 | Figure 4Effects of ITGα5 and ITGβ1 regulation
on the proliferation of VSMCs. (A-I) VSMC morphology visualized
under an inverted microscope (magnification, ×400). The following
cell groups were visualized: (A) EX-ITGα5, (B) EX-ITGβ1, (C) D-EX,
(D) VSMC, (E) Con-Ex, (F) Con-si, (G) si-ITGα5, (H) si-ITGβ1, (I)
D-si. (J) Effects of ITGα5 and ITGβ1 regulation on the
proliferation of VSMCs, as determined by MTT assay. Values are
expressed as the mean ± standard error of the mean (n=3).
*P<0.05 vs. VSMC. ITG, integrin; VSMC, vascular
smooth muscle cell; si, small interfering; Con, control; EX,
overexpressing; A, absorbance. |
Effects of ITGα5 and ITGβ1 on cell
migration
A Transwell chamber migration assay was used to
measure the number of cells that passed through an artificial
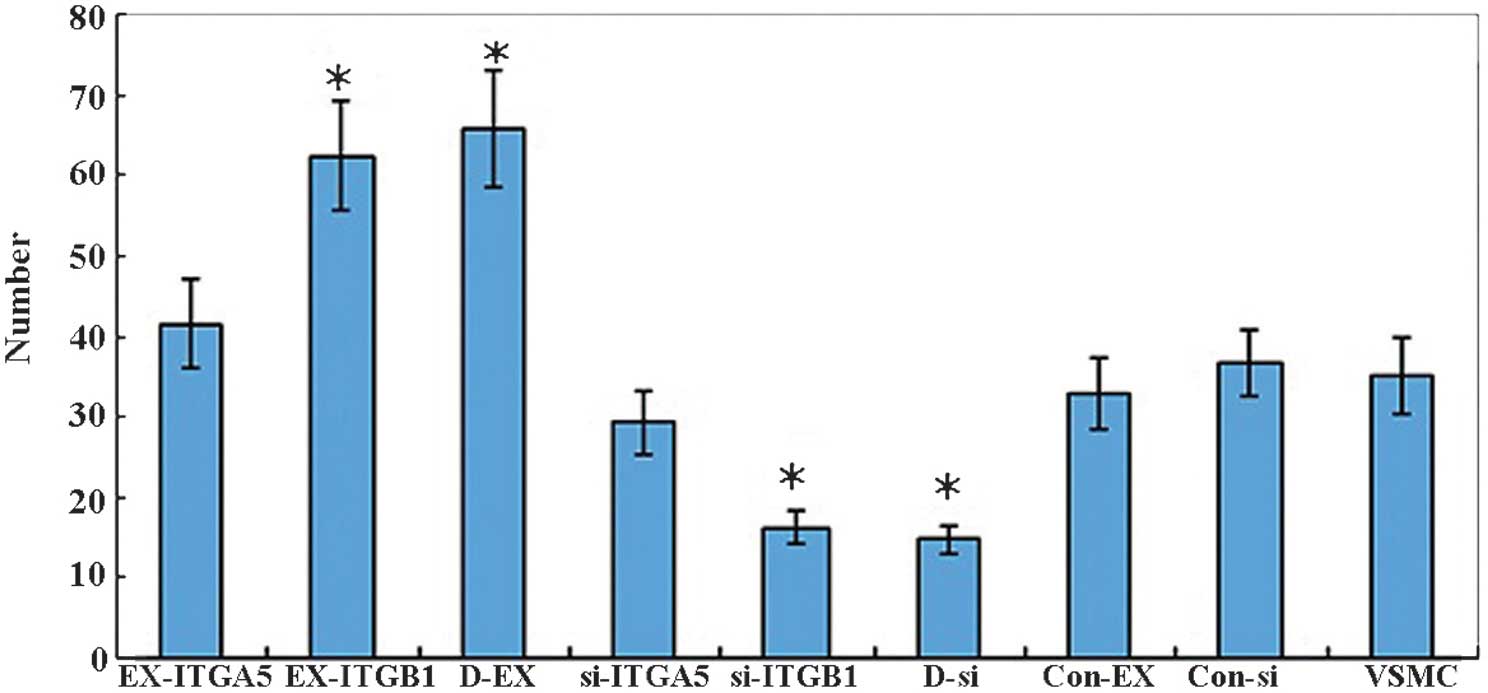
basement membrane (Fig. 5). The
numbers of migrated cells in the control groups were as follows:
Con-Ex, 32.9±4.4; Con-si, 36.6±4.1; and Con, 35.2±4.7, and there
was no statistical difference between them. There was no difference
between the number of migrated cells in the EX-ITGα5 (41.5±5.6) and
si-ITGα5 (29.3±3.9) groups, as compared with that in the control
groups (P>0.05); however, the number of migrated cells in the
EX-ITGβ1 (62.3±6.8) and D-EX (65.7±7.2) groups was markedly
increased (P<0.05). In addition, the number of migrated cells in
the si-ITGβ1 (16.2±2.1) and D-si (14.8±1.7) groups was
significantly decreased (P<0.05). These results indicated that
ITGβ1 may affect the migratory ability of VSMCs. However, ITGα5 had
no effect on VSMC cell migration.
Effects of ITGα5 and ITGβ1 on the cell
cycle distribution
The results of the flow cytometric cell cycle
analysis showed that, among the three control groups, the
proportion of cells in G0/G1, G2/M
and S phase were similar (P>0.05; Fig. 6). There was a higher proportion of
cells in G0/G1 phase, as compared with that
in S phase and G2/M phase. In the EX-ITGα5, EX-ITGβ1 and
D-EX groups, the number of cells in G0/G1
phase was lower, whereas the number of cells in S phase was
increased compared with that in the control groups. In addition,
there was a higher proportion of cells in
G0/G1 phase in the si-ITGα5, si-ITGβ1 and
D-si groups, and a lower proportion of cells in S and
G2/M phase as compared with that in the control groups.
These results suggested that upregulation of ITGβ1 expression may
be able to induce cell division.
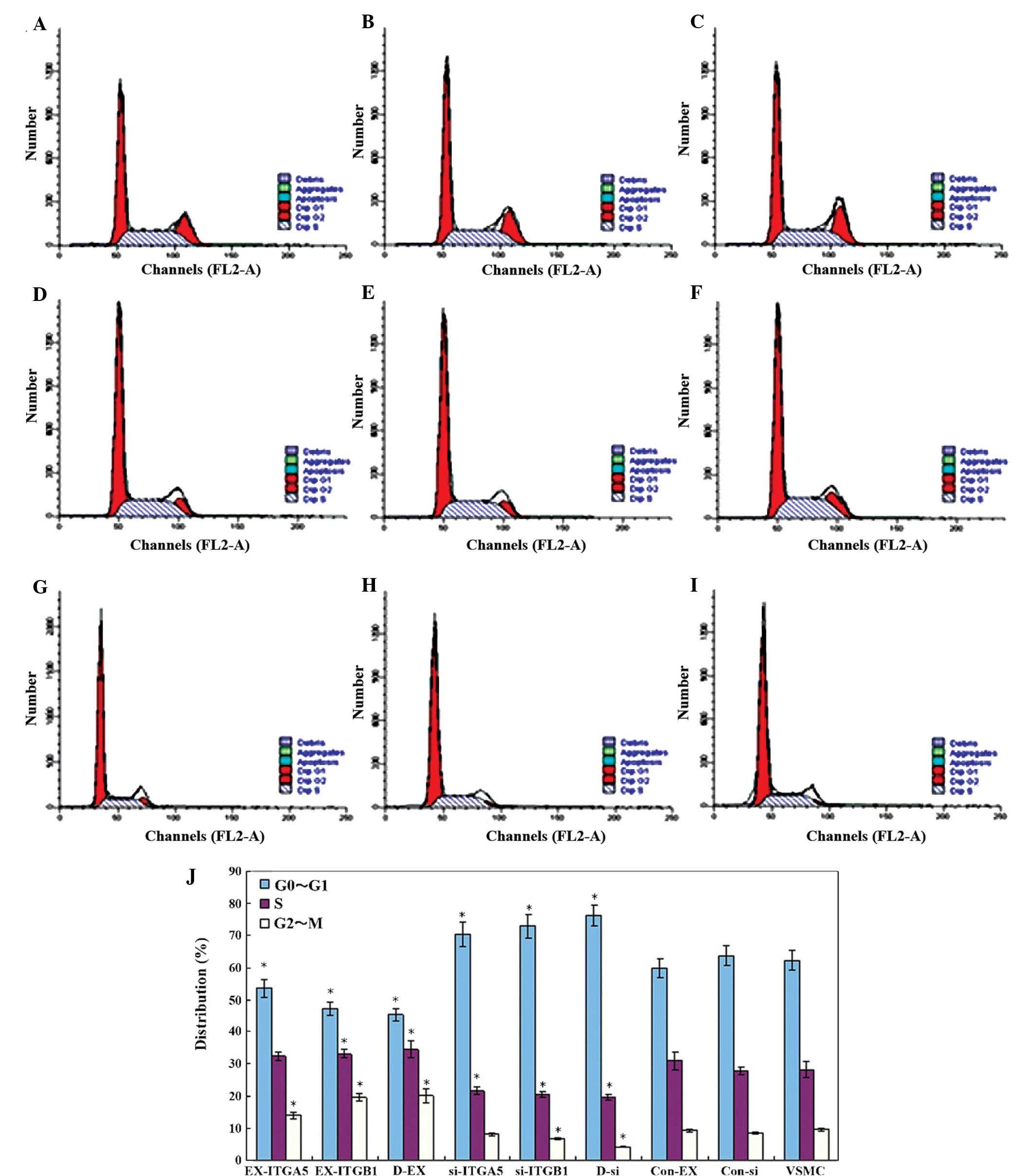 | Figure 6Effects of ITGα5 and ITGβ1 regulation
on the cell cycle of VSMCs. Cell cycle distribution was analyzed in
the following groups: (A) EX-ITGα5, (B) EX-ITGβ1, (C) D-EX, (D)
VSMC, (E) Con-Ex, (F) Con-si, (G) si-ITGα5, (H) si-ITGβ1 and (I)
D-si. (J) Quantification of cell cycle distribution. Values are
expressed as the mean ± standard error of the mean (n=3).
*P<0.05 vs. VSMC group. ITG, integrin; si, small
interfering; Con, control; EX, overexpressing; VSMC, vascular
smooth muscle cell. |
Effects of ITGα5 and ITGβ1 on the mRNA
expression levels of ILK and FAK
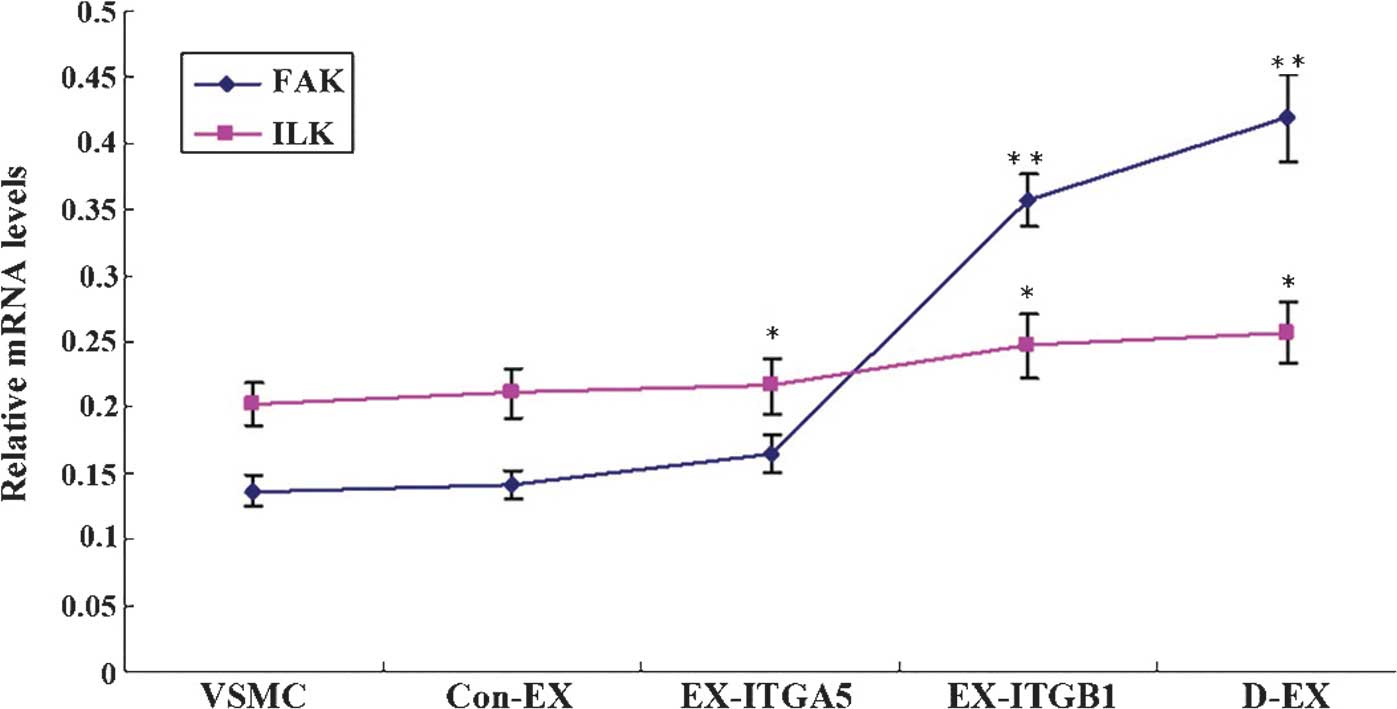
The expression levels of ILK in the control groups
were as follows: Con-Ex (0.142±0.011), Con-si (0.129±0.012) and Con
(0.137±0.012), and there were no differences detected between them
(P>0.05). The mRNA expression levels of FAK in the EX-ITGβ1
(0.357±0.0194) and D-EX cells (0.419±0.033) were significantly
increased as compared with those in the control groups (P<0.01;
Fig. 7). The mRNA expression
levels of FAK in the si-ITGβ1 (0.054±0.008) and D-si (0.034±0.004)
cells were decreased compared with those in the control
(P<0.01). There were no marked changes in ILK mRNA expression
levels between the groups. These results indicated that ITGβ-1 may
be a major regulator of FAK mRNA expression.
Discussion
In the present study, six types of transfected VSMCs
were successfully constructed: and ITGα5-overexpressing cell line
(EX-ITGα5), an ITGβ1-overexpressing cell line (EX-ITGβ1), an ITGα5-
and ITGβ1-overexpressing cell line (D-EX), an ITGα5 knockdown cell
line (si-ITGα5), an ITGβ1 knockdown cell line (si-ITGβ1) and an
ITGα5- and ITGβ1-knockdown cell line (D-si). A pLentiGFP empty
vector cell line (Con-Ex), pRNAT-U6.2/Lenti empty vector cell line
(Con-si) and normal VSMC cell line were also used, alongside the
six experimental cell lines, to analyze cell cycle distribution,
proliferation and migration.
The results of the present study demonstrated that
ITGβ1 upregulation was able to induce VSMCs to re-enter the cell
division cycle, and increase proliferation and migration.
Conversely, downregulation of ITGβ1 resulted in the opposite effect
in VSMCs. Furthermore, ITGα5 regulation had no effect on the
proliferation and migration of VSMCs. Abnormal proliferation and
migration of VSMCs is the main cause of early vascular restenosis
(16). Integrins are a family of
glycoprotein cell surface receptors that participate in cell
adhesion with the ECM, and have an important role in regulating
cell growth, differentiation and proliferation. ITG α5β1 is
associated with the migration, proliferation and vascular injury
repair process of VSMCs (17). The
present study compared three cell groups: Overexpressing cells,
including EX-ITGα5, EX-ITGβ1 and D-EX; knockdown groups, including
si-ITGα5, si-ITGβ1 and D-si; and the control cells, including
Con-Ex, Con-si and normal VSMCs. ITGβ1 was shown to be able to
positively regulate the proliferation and migration of VSMCs and
induce cell division. By contrast, ITGα5 did not have any effect on
the functions of VSMCs. These results indicated that ITGβ1 may be
involved in the proliferation and migration of VSMCs.
During ITG-mediated proliferation and migration of
VSMCs, FAK and ILK were shown to be involved in the signaling
pathway. Biochemical evidence has previously suggested that ILK may
interact with ITGβ1 as well as ITGβ3 (18). ITGβ1 and the associated ILK have a
crucial role in cell survival, tissue homeostasis and
carcinogenesis. ILK associates with ITG tails and thereby links
ITGs with the actin cytoskeleton and various signaling pathways
(19,20). FAK may be the main structural basis
for the signal conduction of VSMC proliferation and migration
regulation (2). The results of the
present study showed that the mRNA expression levels of FAK in the
EX-ITGβ1 and D-EX cells were increased, whereas they were decreased
in the si-ITGβ1 and D-si cells. However, there were no marked
changes in ILK mRNA expression levels. These results suggested that
ITGβ1 may be a major regulator of FAK mRNA expression.
In conclusion, the present study investigated the
role of ITG α5β1 in the proliferation and migration of VSMCs. ITGβ1
was shown to be involved in the proliferation and migration of
VSMCs, and FAK was involved in the signaling pathways of ITGβ1.
These results may provide a possible therapeutic target for the
prevention and treatment of VSMC-associated early vascular
disease.
Acknowledgments
The present study was supported by a grant from the
Basic and Advanced Technology Research Projects of Henan Province,
China (no. 1223004110184).
References
|
1
|
Han M, Wen JK, Zheng B, Liu Z and Chen Y:
Blockade of integrin beta3-FAK signaling pathway activated by
osteopontin inhibits neointimal formation after balloon injury.
Cardiovasc Pathol. 16:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang S, Sun Z, Li Z, Martinez-Lemus LA
and Meininger GA: Modulation of microvascular smooth muscle
adhesion and mechanotransduction by integrin-linked kinase.
Microcirculation. 17:113–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruoslahti E: Fibronectin and its
receptors. Annu Rev Biochem. 57:375–413. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pickering JG, Chow LH, Li S, Rogers KA,
Rocnik EF, Zhong R and Chan BM: alpha5beta1 integrin expression and
luminal edge fibronectin matrix assembly by smooth muscle cells
after arterial injury. Am J Pathol. 156:453–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li JJ, Han M, Wen JK and Li AY:
Osteopontin stimulates vascular smooth muscle cell migration by
inducing FAK phosphorylation and ILK dephosphorylation. Biochem
Biophys Res Commun. 356:13–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koshman YE, Engman SJ, Kim T, Iyengar R,
Henderson KK and Samarel AM: Role of FRNK tyrosine phosphorylation
in vascular smooth muscle spreading and migration. Cardiovasc Res.
85:571–581. 2010. View Article : Google Scholar :
|
|
7
|
Brakebusch C and Fässler R: The
integrinactin connection, an eternal love affair. EMBO J.
22:2324–2333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YG, Guo X, Zhou JJ, Yu B and Liu B:
Sorting of packaging cells for retroviral vector carrying green
fluorescent gene and viral titer determination. Di Yi Jun Yi Da Xue
Xue Bao. 25:30–32. 362005.In Chinese.
|
|
9
|
Zhou Y, Luo W, Zheng L, Li M and Zhang Y:
Construction of recombinant FGFR1 containing full-length gene and
its potential application. Plasmid. 64:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams LS, Phung S, Yee N, Seeram NP, Li L
and Chen S: Blueberry phytochemicals inhibit growth and metastatic
potential of MDA-MB-231 breast cancer cells through modulation of
the phosphatidylinositol 3-kinase pathway. Cancer Res.
70:3594–3605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zhang J, Dai B, Wang N and He L:
Anti-proliferative and apoptotic effects of the novel taspine
derivative tas41 in the Caco-2 cell line. Environ Toxicol
Pharmacol. 31:406–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YM, Dai BL, Zheng L, et al: A novel
angiogenesis inhibitor impairs lovo cell survival via targeting
against human VEGFR and its signaling pathway of phosphorylation.
Cell Death Dis. 3:e4062012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Zheng L, Zhang J, Dai B, Wang N,
Chen Y and He L: Antitumor activity of taspine by modulating the
EGFR signaling pathway of Erk1/2 and Akt in vitro and in vivo.
Planta Med. 77:1774–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Shi L, Ji Y, Jiang X, et al: Liraglutide
attenuates high glucose-induced abnormal cell migration,
proliferation, and apoptosis of vascular smooth muscle cells by
activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt
signaling pathways. Cardiovasc Diabetol. 14(18)2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedrich EB, Clever YP, Wassmann S,
Werner N, Böhm M and Nickenig G: Role of integrin-linked kinase in
vascular smooth muscle cells: Regulation by statins and angiotensin
II. Biochem Biophys Res Commun. 349:883–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, et al: Regulation of cell adhesion and
anchorage-dependent growth by a new beta 1-integrin-linked protein
kinase. Nature. 379:91–96. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Böttcher RT, Lange A and Fässler R: How
ILK and kindlins cooperate to orchestrate integrin signaling. Curr
Opin Cell Biol. 21:670–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanasaki K, Kanda Y, Palmsten K, et al:
Integrin beta1-mediated matrix assembly and signaling are critical
for the normal development and function of the kidney glomerulus.
Dev Biol. 313:584–593. 2008. View Article : Google Scholar
|