Introduction
The invasive capability and metastatic potential of
cancer cells, as well as their growth rate, are important factors
in determining the prognosis of cancer patients. It is well known
that the characteristic biological behavior of cancer cells, such
as invasion and metastasis, are intrinsically acquired through
multiple genetic and epigenetic changes (1). In addition, the importance of
extrinsic factors derived from the tumor microenvironment has
emerged in recent studies. Among numerous microenvironmental
factors, the focus has been on the role of inflammation in
carcinogenesis and cancer progression (2–4). It
has been suggested that inflammation in the tumor microenvironment
contributes to the invasion, metastasis, and resistance to
chemotherapeutic agents of various cancers (5–7). The
invasive and metastatic characteristics of oral cancer have been
major obstacles in the treatment of patients with this disease.
However, extrinsic, non-genetic factors that promote invasion and
metastasis of oral cancer remain largely unknown while few studies
have been conducted on the inflammatory factors involved in
acquisition of such pivotal characteristics (8,9).
Furthermore, the efficacy of chemotherapeutic agents to modulate
the invasiveness of chronic inflammation-induced progression as
well as apoptosis of oral cancer cells has been poorly described in
an experimental setting.
Periodontitis and oral squamous cell carcinoma
(OSCC), one of the most common chronic inflammatory diseases and
cancer in the oral cavity, respectively, mostly affect people
greater than 40 years of age (10,11).
OSCC patients are highly susceptible to chronic periodontitis,
suggesting OSCC cells exist in a chronic inflammatory state.
However, studies on the effect of periodontitis on the invasiveness
and metastatic ability of OSCC are limited. Inflammatory mediators
such as IL-6 and IL-8 modulate the invasion and metastasis of other
types of cancers such as lung adenocarcinoma, as well as breast and
ovarian cancers (12–14). With multiple bacterial pathogens
associated with periodontitis, OSCC patients with this inflammatory
disease are continuously exposed to a plethora of periodontopathic
bacteria including Porphyromonas gingivalis, Aggregatibacter
actinomycetemcomitans, and Prevotella intermedia (15,16).
These bacterial pathogens may affect the biological behavior of
oral cancer through the modulation of inflammatory mediators and
invasion-related molecules. In the present study, we investigated a
possible link between chronic periodontitis and the aggressiveness
of oral cancer cells by infecting YD10B OSCC cells with P.
gingivalis, one of the major periodontal pathogens.
Acetylshikonin, a derivative of shikonin isolated
from the roots of Lithospermum erythrorhizon, the purple
gromwell and Chinese herbal medicine, has been shown to possess
anti-inflammatory as well as antibacterial properties (17). Few studies have been undertaken on
the effects of acetylshikonin on cancers (18–20).
In particular, its role in modulating the biological behavior of
cancer cells such as OSCC is largely unknown. In the present study,
we examined the possible utility of acetylshikonin, a traditional
anti-inflammatory agent, in the suppression of the invasiveness of
P. gingivalis-infected YD10B OSCC cells. We also examined
the potential molecular mechanisms that underlie P.
gingivalis-induced changes in the invasive characteristics of
YD10B cells, including the altered expression of P.
gingivalis-induced matrix metalloproteinases (MMPs) and a
relevant cytokine.
Materials and methods
Cancer cells and bacterial
cultures
The human oral squamous cell carcinoma (OSCC) cell
line, YD10B, from Professor JI Yook (College of Dentistry, Yonsei
University, South Korea) was used. YD10B OSCC cells were grown in a
3:1 mixture of Dulbecco's Modified Eagle's Medium and Ham's
nutrient mixture F12 (Hyclone, Logan, UT, USA) that contained 10%
fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO, USA).
The cells were maintained at 37°C in a 5% CO2 humidified
incubator. The P. gingivalis strain 381 were anaerobically
cultured in GAM broth (Nissui, Tokyo, Japan) that contained 5 mg/ml
hemin and 5 µg/ml vitamin K.
Infection of OSCC cells with P.
gingivalis
YD10B cells were cocultured with live P.
gingivalis strain 381 at a multiplicity of infection (MOI) of
1:100 at 37°C. After 2 h, the cells were washed with
phosphate-buffered saline (PBS) and then cultured in new fresh
media until harvest or the next infection. As a control, YD10B
cells were subjected to the same media change and PBS wash but
without any bacterial infection.
Reagents and chemicals
Human recombinant interleukin-8 (IL-8) was purchased
from Peprotech (London, UK). Acetylshikonin was kindly gifted by
Prof. Young Whan Choi (Department of Horticultural Bioscience,
Pusan National University, South Korea). Acetylshikonin was
dissolved in dimethylsulfoxide (DMSO) at a stock concentration of 4
mM, stored at −20°C, and diluted before use. All chemicals and
reagents were purchased from Sigma (St. Louis, MO, USA), unless
otherwise specified.
Flow cytometry analysis
Cells were incubated with FITC-conjugated antibodies
against CD44 (BD Pharmingen, Franklin Lakes, NJ, USA) and
APC-conjugated CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany)
for 25 min at 4°C in the dark. The stained cells were immediately
analyzed using a flow cytometer (FC500; Beckman-Coulter Cytomics,
San Jose, CA, USA) equipped with an argon laser at the excitation
wavelength of 488 and 633 nm. The results shown were based on the
analysis of 20,000 cells.
Western blot analysis
Cell lysates were analyzed using antibodies against
the following molecules: Cytokeratin 13 (BD Biosciences, San Jose,
CA, USA); α-smooth muscle actin (SMA), twist, and β-actin (Santa
Cruz Biotechnology, Dallas, TX, USA); vimentin and slug (Cell
Signaling Technology, Danvers, MA, USA).
In vitro invasion assay
Cells were seeded on the upper sides of 24-well
Transwell polycarbonate filters (8 µm pore size, Costar, Cambridge,
MA, USA) coated with 40 µl of matrigel (BD Biosciences) at a 1:2
dilution in serum-free medium. The upper chambers contained 1%
serum DMEM/F12 medium, whereas the lower wells were filled with
medium that contained 10% FBS. After 30 h, cells on the inside of
the inserts were removed with cotton tips, and invading cells on
the outside of the inserts were visualized after hematoxylin/eosin
staining. The filters were mounted on glass slides, and the number
of invading cells were counted using a Photoshop counting program
(Adobe Systems, San Jose, CA, USA).
Multiplex bead (Luminex) assay
The concentration of MMP-1, MMP-2, MMP-9 and MMP-10
in supernatants of YD10B OSCC cells were measured using a Milliplex
Map Human MMP Magnetic Bead Panel 2 kit (Millipore, Billerica, MA,
USA) with a Luminex 200 system (Luminex, Austin, TX, USA). Briefly,
beads coupled with anti-MMP-1, MMP-2, MMP-9, and MMP-10 antibodies
were diluted in blocking buffer. The standards and samples that
contained all MMPs were prepared in blocking buffer and then
incubated with a bead solution for 2 h at room temperature. Each
well was supplied with the primary antibody mixture. A
streptavidin-phycoerythrin mixture was then added to each well, and
the beads were resuspended in PBS. The Luminex 200 platform coupled
with BioRad Bio-Plex software (BioRad, Hercules, CA, USA) was used
to measure MMP levels.
Gelatin zymography
P. gingivalis-infected or IL-8 treated YD10B
cells were incubated in serum-free DMEM/F-12 medium for 24, 48, or
72 h. Conditioned media were collected, and electrophoresed in a
10% SDS-polyacrylamide gel electrophoresis (PAGE) gel containing
0.2% gelatin (w/v) for gelatin zymography. After electrophoresis,
the gel was washed twice with a solution containing 2.5% (v/v)
Triton X-100 for 30 min at room temperature. Then, the gel was
incubated under a shaking condition with a reaction buffer for
enzymatic reaction, containing 1% sodium azide (NaN3),
10 mM calcium chloride (CaCl2), and 40 mM
Tris-hydrochloride (pH 8.0), for 16 h at 37°C. Finally, the gel was
stained with 0.25% (w/v) Coomassie blue and destained with 5%
acetic acid and 2.5% methanol at room temperature.
IL-8 enzyme linked immunosorbent
assay
The conditioned medium of YD10B cells was harvested
and analyzed for the presence of IL-8. Enzyme-linked immunoassay
(ELISA) MAX™ Deluxe sets (Biolegend Inc., San Diego, CA,
USA) were used according to the manufacturer's instructions.
Briefly, 96-well plates were coated with a primary capture antibody
specific for IL-8 in carbonate/bicarbonate buffer overnight. The
samples were incubated in plates for 2 h, and a
streptavidin-conjugated secondary antibody specific for IL-8 was
added. The wells were then incubated with a peroxidase substrate
for 30 min, and a 1 M H2SO4 solution was
subsequently added to stop the reaction.
siRNA transfection
IL-8 siRNA (Bioneer Co., Ltd., Daejeon, South Korea)
was utilized with the following sequence: CCAAGGGCCAAGAGAAUAUTT.
Cells were transfected with 100 nM of IL-8 siRNA or scrambled
control siRNA (Bioneer Co., Ltd.) using DharmaFECT transfection
reagent (Dharmacon-ThermoScientific, Waltham, MA, USA).
Cell viability assay
The viability of YD10B cells was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were plated in 96-well plates, incubated overnight to
reach approximately 80% confluence, and then treated with varying
concentrations of acetylshikonin (0–2 µM) for 24 or 48 h. Cells
grown in medium containing an equivalent amount of DMSO without
acetylshikonin were used as a control. Then, media were removed,
and 100 µl of MTT (5 mg/ml) was added to each well. The plates were
further incubated for 4 h at 37°C. The resulting formazan crystals
were solubilized in 200 µl of DMSO. The absorbance of colored
solutions at 570 nm was quantified using a PerkinElmer Victor-3
spectrophotometer (Perkin-Elmer, Waltham, MA, USA).
Statistical analysis
Data were analyzed with Student's t-tests for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
P. gingivalis-infected YD10B OSCC
cells displayed increased invasiveness as well as
epithelial-mesenchymal transition-like changes
To verify the invasion of the bacteria into YD10B
cells and their subsequent existence within the cells, the presence
of 16S rRNA from P. gingivalis within the cells was
analyzed. P. gingivalis 16S rRNA levels were selectively
detected only in P. gingivalis-infected cells (data not
shown). Our previous study demonstrated that prolonged and repeated
infection with P. gingivalis twice a week for 5 weeks
enhanced the invasiveness of Ca9-22 OSCC cells through the
acquisition of cancer stemness as well as epithelial-mesenchymal
transition (EMT) characteristics (21), suggesting chronic periodontitis is
one of the most important contributing factors in the metastatic
progression of oral cancers. In the present study, we observed that
sustained short-term infection with P. gingivalis induced
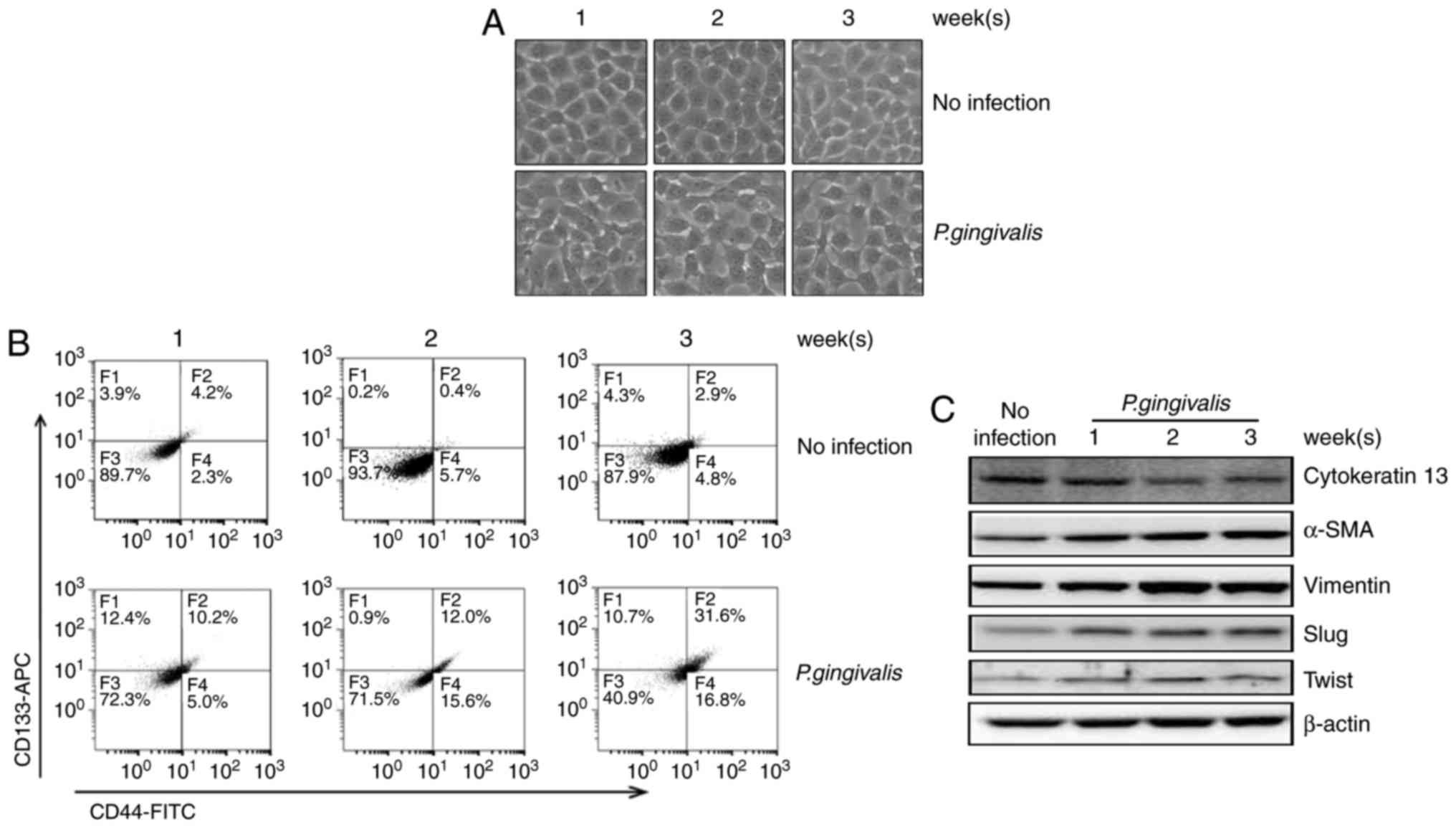
morphologic changes in YD10B OSCC cells, such as the loss of
adhesiveness and a polygonal shape, compared with the absence of
morphologic changes in non-infected controls (Fig. 1A). P. gingivalis-infected
cells also showed increased expression of both CD44 and CD133,
representative indicators for cancer stemness (Fig. 1B) (22,23).
Additional changes in the expression profile of various EMT markers
were detected, including up-regulation of α-SMA and vimentin,
down-regulation of cytokeratin 13, as well as the up-regulation of
EMT-related transcriptional factors, such as slug and twist
(Fig. 1C). These findings together
suggest that P. gingivalis, a major pathogen causing chronic
periodontitis, can induce a transitional change in YD10B OSCC cells
to a mesenchymal phenotype.
P. gingivalis increases expression of
matrix metalloproteases in YD10B OSCC cells
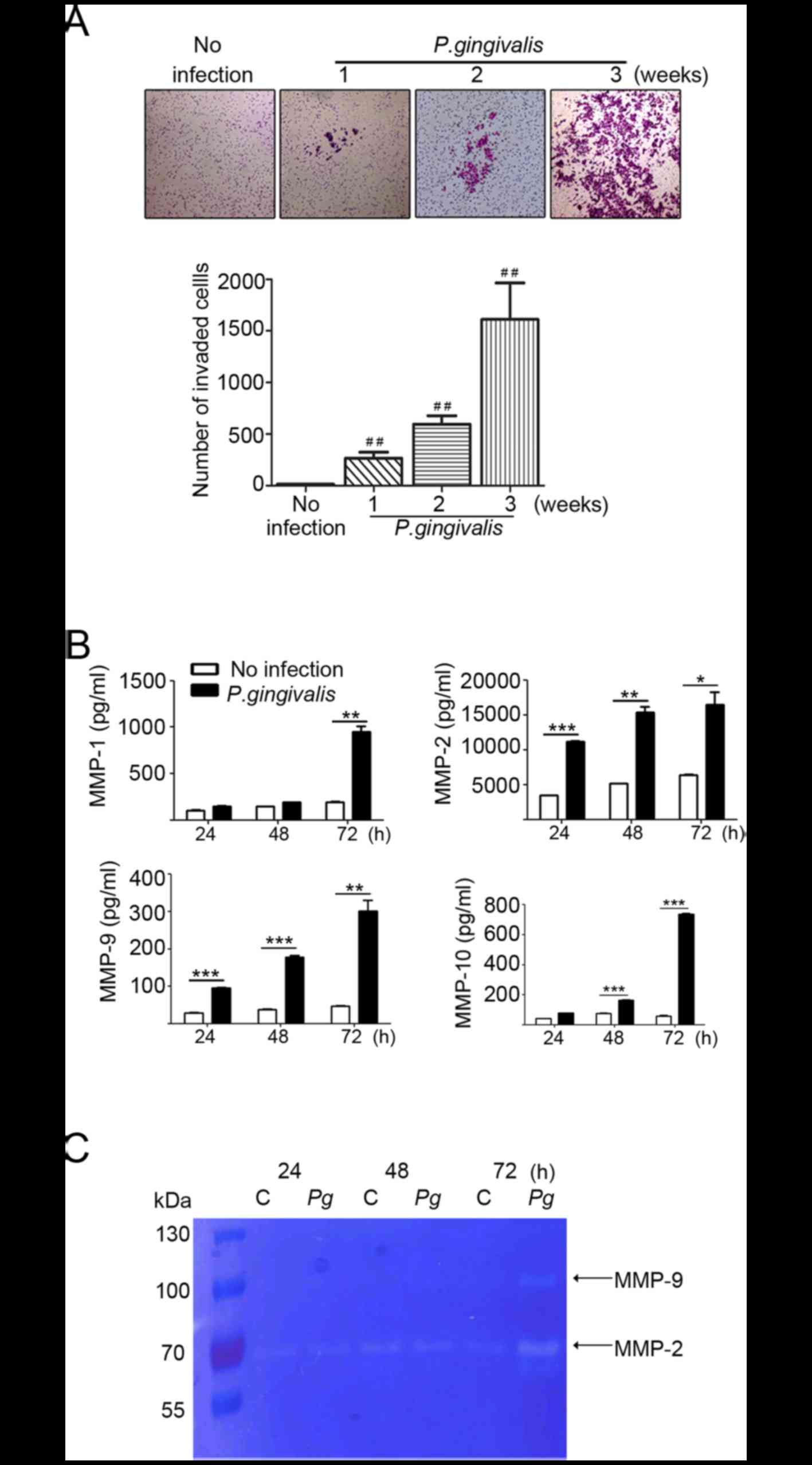
When the invasiveness of P.
gingivalis-infected YD10 B OSCC cells was examined, OSCC cells
chronically infected with P. gingivalis exhibited higher
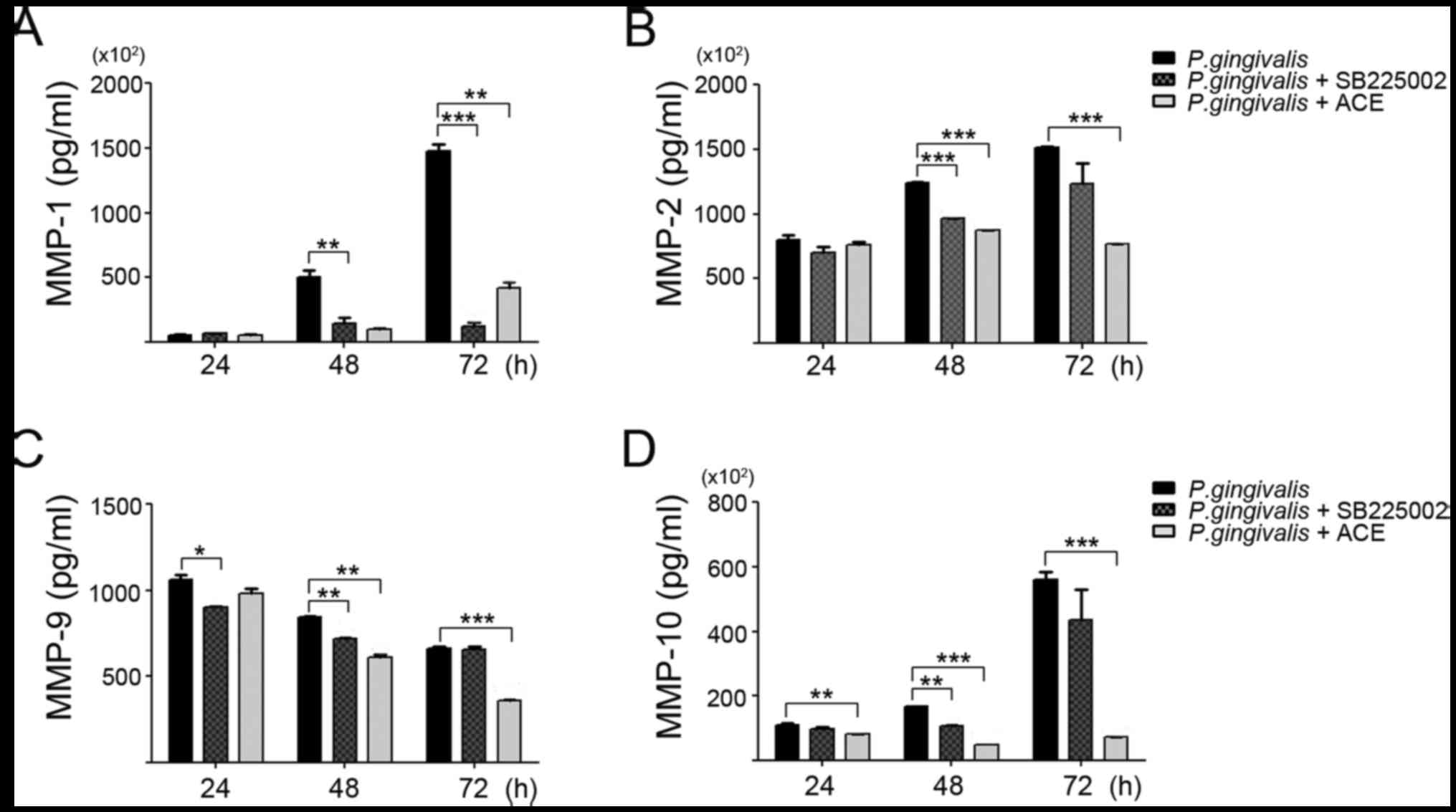
invasive properties compared with non-infected cells (Fig. 2A). Matrix metalloproteinases (MMPs)
are one of the major effectors in the invasion of cancer cells into
neighboring tissues. The enhanced expression of MMPs has been
observed in various types of cancer, including colorectal (24), esophageal (25) and lung (26) cancer. We analyzed MMP-1, −2, −9 and
−10 levels using a multiplex bead assay. Compared with non-infected
cells, levels of MMP-1, −2, −9, and −10 were substantially
increased in a time-dependent manner after P. gingivalis
infection. The levels of MMPs were maximal at 72 h post-infection
(Fig. 2B). As shown in Fig. 2C, increased expression of MMP-2 and
MMP-9 was further confirmed in the supernatant from P.
gingivalis-infected YD10B OSCC cells using gelatin zymography.
These findings suggest that P. gingivalis contributes to the
increase in invasiveness of YD10B by elevating the level of MMPs.
However, further study is needed to define the mechanism that
mediates increases of MMPS by P. gingivalis.
Increased invasiveness of P.
gingivalis-infected OSCC cells is modulated by IL-8-dependent MMP
release
Another important feature of P. gingivalis is
its ability to secrete extracellular virulence factors, such as
fimbriae, endotoxins, and gingipains. These factors stimulate
neighboring epithelial cells to produce various cytokines,
including interleukins (ILs) and tumor necrosis factor-alpha
(TNF-α), which contribute to inflammatory responses (27–29).
Considering the potential link between cancer and bacteria-induced
inflammation, the existence of a causal relationship between P.
gingivalis and oral cancer progression is strongly
expected.
Multiple studies have reported that cytokines play a
major role in the production of MMPs (30–32).
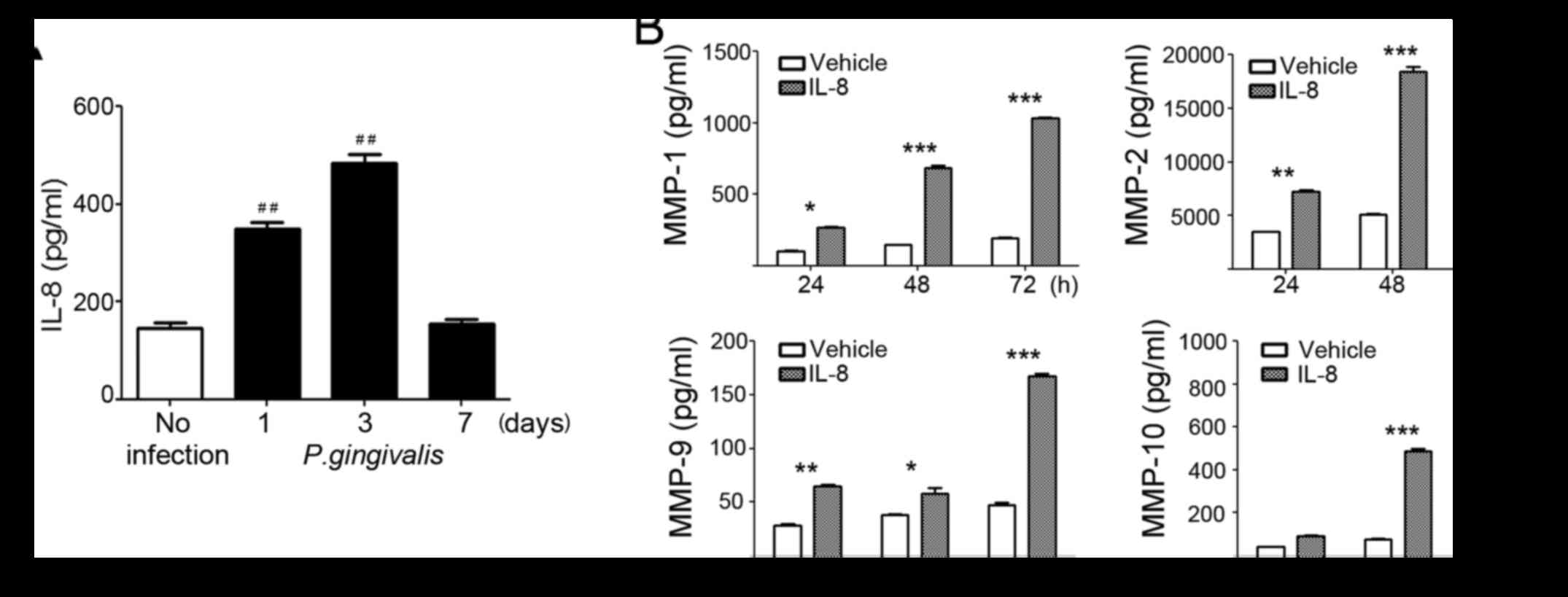
P. gingivalis induces an inflammatory cytokine response in
mammalian cells due to its virulence factors. We found that P.
gingivalis induced the release of IL-8, with a maximal increase
observed 72 h post-infection (Fig.
3A). However, basal levels of other cytokines, such as
interferon-γ, TNF-α, RANTES, IL-1β, IL-6, and IL-10, were very low
and not increased by P. gingivalis infection (data not
shown). Consistent with the increase of MMPs in P.
gingivalis-infected cells, the conditioned media of YD10B cells
treated with 100 nM of recombinant IL-8 treatment contained
significantly increased levels of MMPs, in a time-dependent manner,
compared to vehicle-treated cells (Fig. 3B). The effects of IL-8 on MMP
release and the invasive ability of P. gingivalis-infected
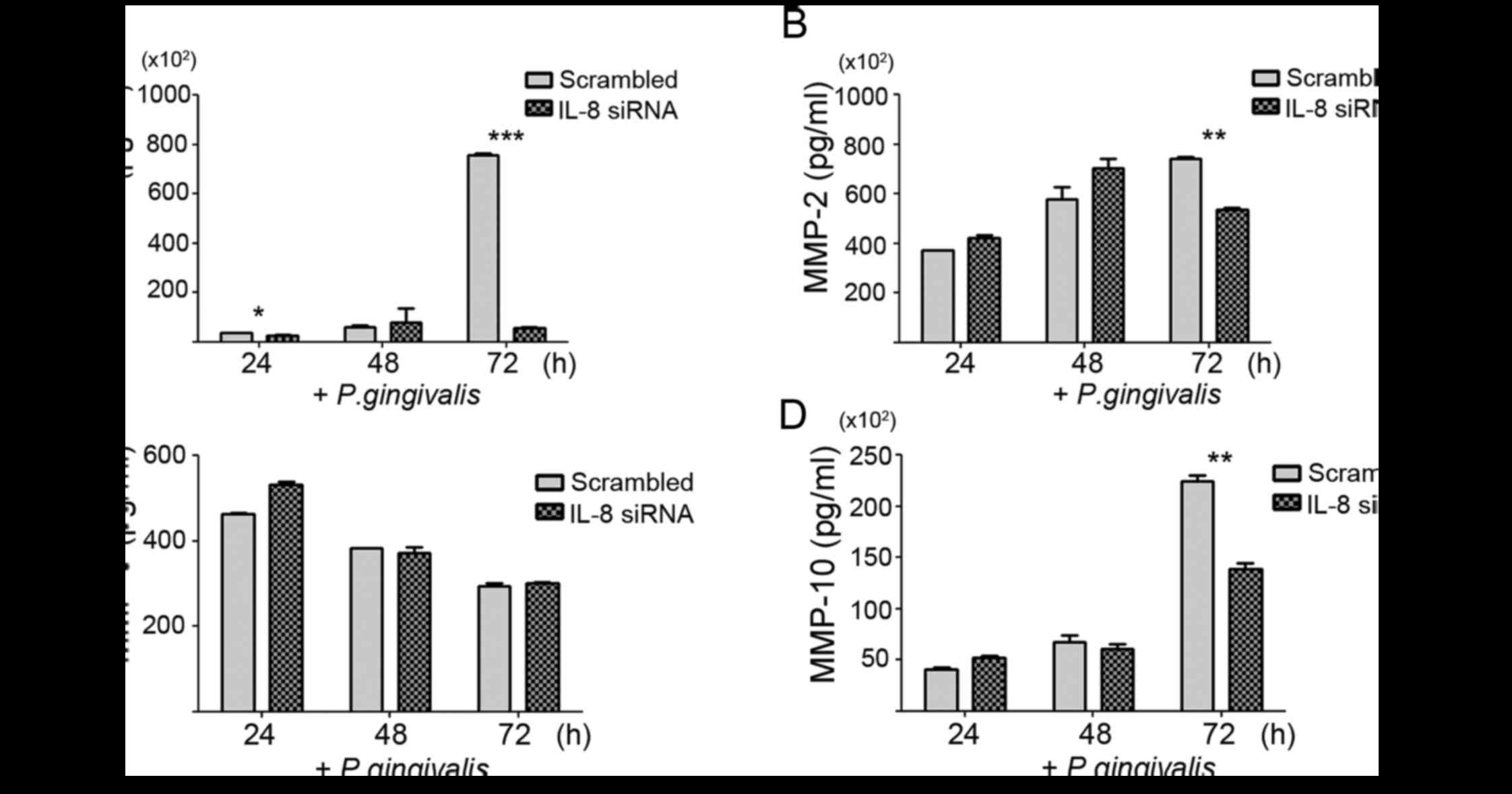
YD10B cells were further investigated using RNA interference. We
confirmed the reduction of IL-8 mRNA and protein by siRNA using
real-time PCR and ELISA, respectively (data not shown). Knockdown
of IL-8 substantially decreased MMP-1, −2, and −10 release 72 h
post-infection, but a significant change in the MMP-9 level was not
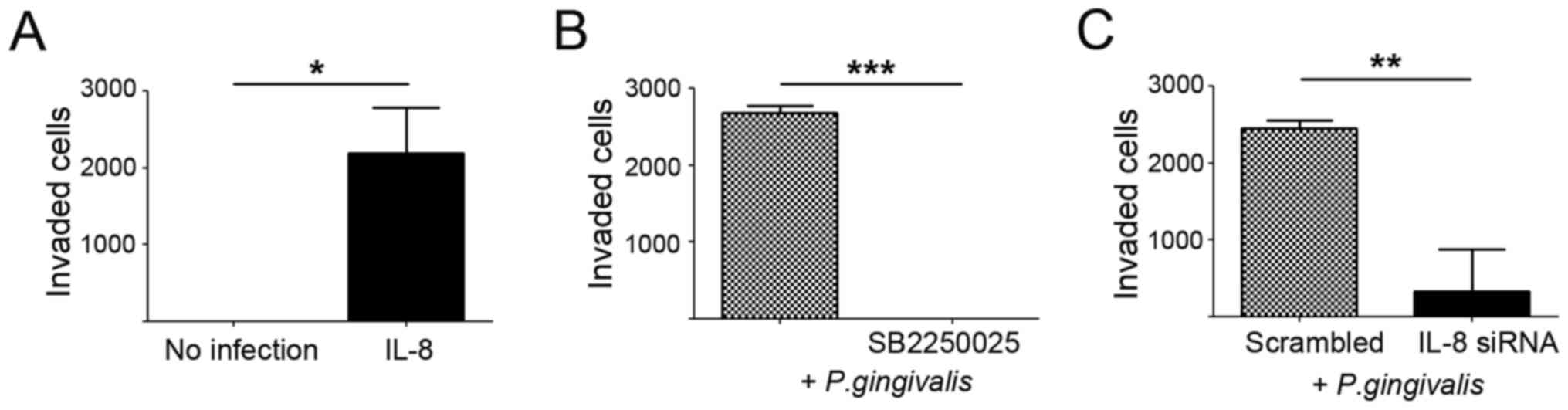
noted (Fig. 4). We also observed
significant suppression of the invasive ability of P.
gingivalis-infected YD10B cells following an siRNA approach
against IL-8, as well as after treatment with SB2250025, an
inhibitor of the IL-8 receptor. In contrast, IL-8 treatment
substantially enhanced the invasiveness of YD10B cells compared
with vehicle-treated control cells (Fig. 5). These findings strongly support
the notion that the IL-8-induced upregulation of MMPs in P.
gingivalis-infected YB10B cells underlies the increased
invasiveness of YD10B OSCC cells.
Acetylshikonin inhibits the invasion
of P. gingivalis-infected OSCC cells by decreasing release of both
IL-8 and MMPs
Prior to observing the influence of acetylshikonin
on the invasive ability of P. gingivalis-infected YD10B OSCC
cells, we firstly investigated the toxicity of acetylshikonin to
YD10B cells using an MTT assay. Cells were exposed to various
concentrations (0 to 2 µM) of acetylshikonin for 24 or 48 h
(Fig. 6A). Since acetylshikonin up
to a 1 µM concentration showed no toxicity to cells, a 0.5 µM
concentration was used in subsequent experiments. Acetylshikonin
substantially suppressed the IL-8 release that was induced by P.
gingivalis infection (Fig.
6B). MMP-1, −2, −9, and −10 were significantly reduced with 0.5
µM of acetylshikonin treatment in P. gingivalis-infected
YD10B cells. The suppressive effect of acetylshikonin on MMP
release was more potent than the IL-8 receptor inhibitor, SB225002
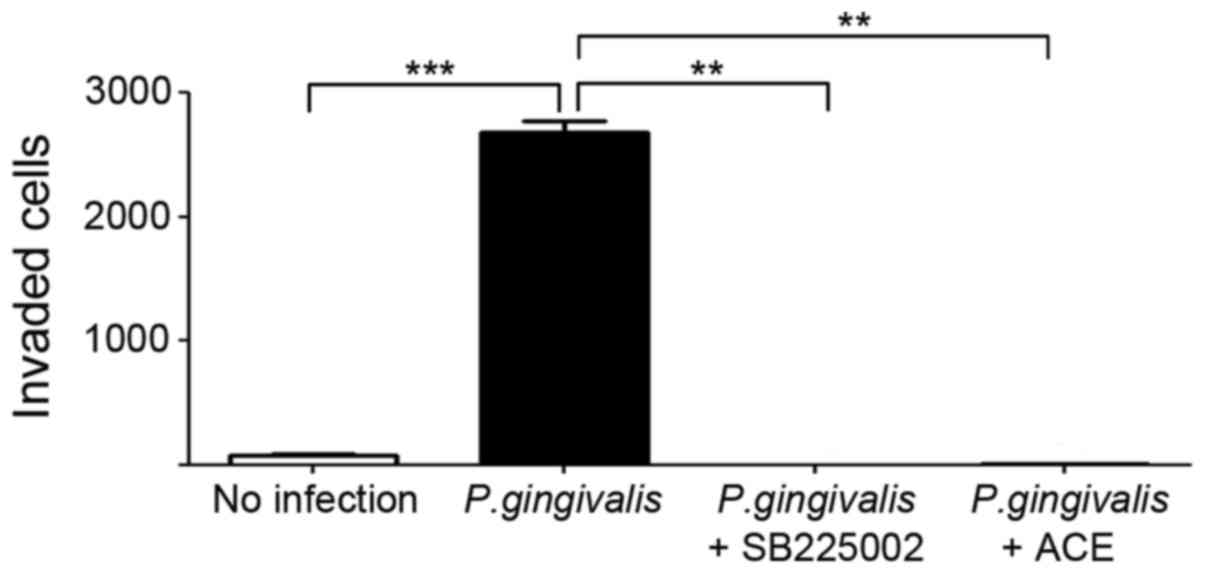
(Fig. 7). In addition,
acetylshikonin of the same concentration potently suppressed the
increased invasive ability of OSCC cells induced by P.
gingivalis infection (Fig.
8).
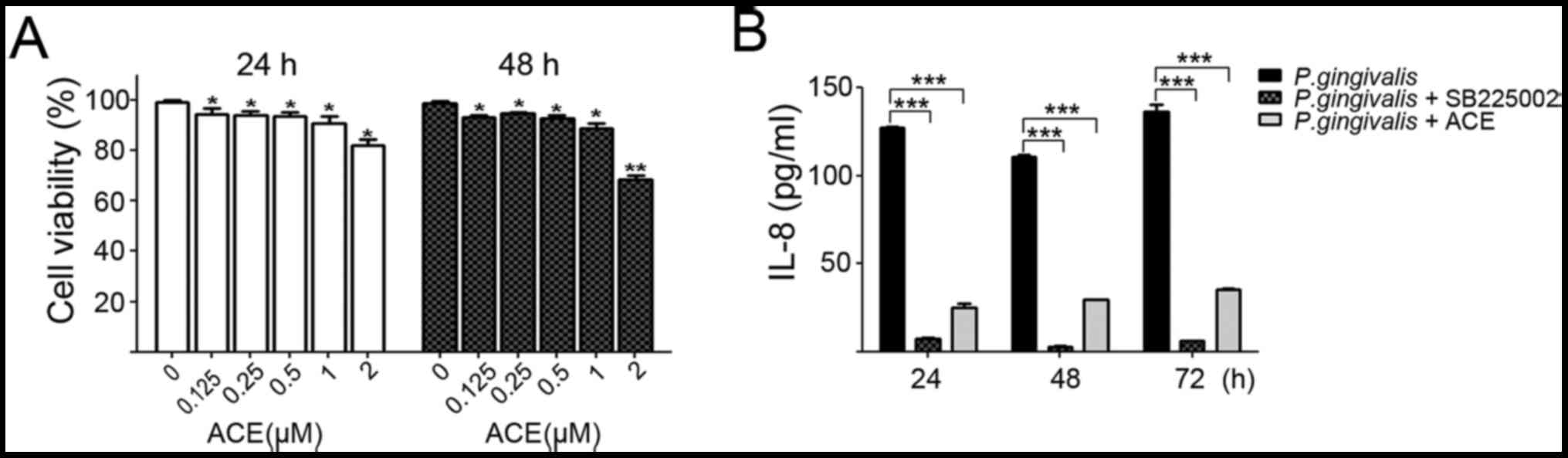 | Figure 6.Acetylshikonin inhibited secretion of
IL-8 from P. gingivalis-infected OSCC cells. (A) Cells were
grown in 96-well plates and treated with 0, 0.125, 0.25, 0.5, 1 or
2 µM of acetylshikonin for 24 or 48 h. Dimethyl sulfoxide (DMSO)
was used as a vehicle control. Cell viability was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. (B) P. gingivalis-infected cells were treated with
100 nM of SB225002, an IL-8 receptor inhibitor, or 0.5 µM of
acetylshikonin for 24, 48, or 72 h. The amount of IL-8 in the
supernatant was subsequently determined using ELISA. Statistical
significance was assessed by paired t test. *P<0.05,
**P<0.01, compared with vehicle-treated control cells;
***P<0.001, compared with P. gingivalis-infection group
as indicated. 1. ACE, acetylshikonin. |
Discussion
While previous studies have focused on the role of
pathogens in carcinogenesis, more recent studies have described the
effect of microbial infection on the biologic behavior of cancer
cells. A number of studies have demonstrated that microbial
pathogens, including Epstein-Barr virus, hepatitis B and C virus,
and Citrobacter rodentium, contribute to the transition of
cancers into a more aggressive form, and that they may play a role
in the induction of EMT in cancer cells associated with tumor
invasion and metastasis (33–36).
From recent studies on periodontal pathogens, a link between
periodontal pathogens and EMT in oral cancer cells has been
suggested (21,37). In the present study, P.
gingivalis-infected YD10B OSCC cells displayed a morphologic
transformation into a mesenchymal cell-like shape. In addition,
additional characteristics of EMT have been detected, including
epithelial marker repression, and the upregulation of mesenchymal
markers as well as the stem cell markers, CD44 and CD133. While the
interpretation of this result may be restricted to a single cell
line used, these findings are consistent with our previous studies
demonstrating an effect of P. gingivalis on Ca9-22, another
OSCC cell line (21). Regardless
of OSCC cell lines used, P. gingivalis-infected OSCC cells
in both studies exhibited enhanced invasive properties,
characteristic of EMT. All these findings strongly support the idea
that P. gingivalis may induce EMT in the progression of oral
cancer.
P. gingivalis can induce cytokine production
in not only immune cells but also in cells from periodontal tissue
(27,29). Previous reports have demonstrated
that P. gingivalis-infected oral cancer cells secrete
cytokines and chemokines, especially IL-6 and IL-8 (21,38).
Few studies exist describing the effect of IL-8 on the invasion and
metastasis of cancer cells while cytokine studies have mainly
focused on IL-6 (9,12–14).
Recently, a role for IL-8 in the migratory and invasive abilities
of human ovarian and breast cancer cells has been shown (39). The mechanism involved in the IL-8
promoted invasion of cancer cells has been further clarified by a
study showing that invasion and metastasis of bladder cancer can be
increased by an IL-8/MMP axis (40). MMPs are known as important
molecules in the process of periodontal tissue destruction, as well
as in tumor invasion, by playing a key role in the degradation of
extracellular matrix proteins. The current finding of increased
MMP-1, −2, −9, and −10 in P. gingivalis-infected YD10B OSCC
cells is in line with our previous reports demonstrating the
activation of similar sets of MMPs in other OSCC cell lines
(21,38). Our results also indicate the
IL-8-induced enhancement of MMPs as a common molecular pathway.
Considering that IL-8 is one of the most important
contributors to the enhancement of aggressive behaviors by P.
gingivalis-infected oral cancer cells, we postulate that
anti-inflammatory agents capable of modulating the IL-8 pathway can
effectively suppress the invasion of OSCC cells surrounded by
microbial pathogens. Recently, natural compounds such as flavonoids
have become the focus of attention as anticancer agents and/or
adjuncts to chemotherapeutic reagents, although the emphasis is as
their utilization as chemopreventive agents rather than for
therapeutic benefit (41,42). Of numerous flavonoids, we
demonstrated, in a previous study, that acetylshikonin, a flavonoid
with an anti-inflammatory potential, has anti-tumor properties
after inducing apoptotic cell death on Ca9-22 OSCC cells at a low
concentration (1 µM), with little to no toxicity to normal cells
(18). However, YD10B OSCC cells
in the present study were little damaged by acetylshikonin at low
concentrations. Instead, herein, we demonstrated that
acetylshikonin significantly suppressed IL-8 induction and MMP
release as well as the invasive ability of P.
gingivalis-infected YD10B OSCC cells, with no direct toxic
effect. These data suggest that acetylshikonin may be a good
candidate as an adjuvant chemotherapeutic reagent that suppresses
the invasion and metastasis of oral cancer cells, especially
against OSCC cells chronically infected with periodontal pathogens
that otherwise have the potential to be transformed into more
aggressive populations. Further in vitro and in vivo
studies are required to clarify the suppressive role of
acetylshikonin and to broaden our understanding of relevant
molecular pathways in aggressive P. gingivalis-infected oral
cancer cells.
In summary, we present data to further confirm the
hypothesis that P. gingivalis, a major pathogen responsible
for chronic periodontitis, induces the development of aggressive
behaviors in host YD10B oral cancer cells via the IL-8 dependent
release of MMPs. More importantly, acetylshikonin, a flavonoid with
an anti-inflammatory potential, effectively prevented P.
gingivalis-infected YD10B OSCC cells from transforming into
more aggressive populations, presumably via the suppression of IL-8
and MMP release. Our results may help to provide a useful platform
for developing pharmaceutical adjuvants that will aid traditional
therapeutic agents in curing chronic inflammation-associated oral
cancer as well as in chemoprevention.
Acknowledgements
The present study was supported by Dental Research
Institute (grant no. PNUDH DRI-2015-02), Pusan National University
Dental Hospital.
Glossary
Abbreviations
Abbreviations:
|
IL-8
|
interleukin-8
|
|
MMP
|
matrix metalloproteinase
|
|
OSCC
|
oral squamous cell carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Katto J and Mahlknecht U: Epigenetic
regulation of cellular adhesion in cancer. Carcinogenesis.
32:1414–1418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, Lin PC and Zhou BP: Inflammation
fuels tumor progress and metastasis. Curr Pharm Des. 21:3032–3040.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Song X, Mohri Y and Qiao L: Role
of inflammation and tumor microenvironment in the development of
gastrointestinal cancers: What induced pluripotent stem cells can
do? Curr Stem Cell Res Ther. 10:245–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Wang L, Pappan L, Galliher-Beckley A
and Shi J: IL-1β promotes stemness and invasiveness of colon cancer
cells through Zeb1 activation. Mol Cancer. 11:872012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang D, Fu L, Sun H, Guo L and DuBois RN:
Prostaglandin E2 promotes colorectal cancer stem cell expansion and
metastasis in mice. Gastroenterology. 149:1884–1895. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
St John MA: Inflammatory mediators drive
metastasis and drug resistance in head and neck squamous cell
carcinoma. Laryngoscope. 125 Suppl 3:S1–S11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eke PI, Dye BA, Wei L, Thornton-Evans GO
and Genco RJ: CDC periodontal disease surveillance workgroup: James
beck (university of north carolina, chapel hill, usa), gordon
douglass (past president, american academy of periodontology), roy
page (university of washin: Prevalence of periodontitis in adults
in the united states: 2009 and 2010. J Dent Res. 91:914–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Natarajan E and Eisenberg E: Contemporary
concepts in the diagnosis of oral cancer and precancer. Dent Clin
North Am. 55:63–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dehai C, Bo P, Qiang T, Lihua S, Fang L,
Shi J, Jingyan C, Yan Y, Guangbin W and Zhenjun Y: Enhanced
invasion of lung adenocarcinoma cells after co-culture with
THP-1-derived macrophages via the induction of EMT by IL-6. Immunol
Lett. 160:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim S, Lee J, Jeon M, Lee JE and Nam SJ:
MEK-dependent IL-8 induction regulates the invasiveness of
triple-negative breast cancer cells. Tumour Biol. 37:4991–4999.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X
and Xu RC: Interleukin-6 signaling regulates anchorage-independent
growth, proliferation, adhesion and invasion in human ovarian
cancer cells. Cytokine. 59:228–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abiko Y, Sato T, Mayanagi G and Takahashi
N: Profiling of subgingival plaque biofilm microflora from
periodontally healthy subjects and from subjects with periodontitis
using quantitative real-time PCR. J Periodontal Res. 45:389–395.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff L, Dahlen G and Aeppli D: Bacteria
as risk markers for periodontitis. J Periodontol. 65:498–510. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim DJ, Lee JH, Park HR and Choi YW:
Acetylshikonin inhibits growth of oral squamous cell carcinoma by
inducing apoptosis. Arch Oral Biol. 70:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Lee HJ, Magesh V, Nam D, Lee EO,
Ahn KS, Jung MH, Ahn KS, Kim DK, Kim JY and Kim SH: Shikonin,
acetylshikonin and isobutyroylshikonin inhibit VEGF-induced
angiogenesis and suppress tumor growth in lewis lung
carcinoma-bearing mice. Yakugaku Zasshi. 128:1681–1688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon J, Koh SS, Malilas W, Cho IR,
Kaewpiboon C, Kaowinn S, Lee K, Jhun BH, Choi YW and Chung YH:
Acetylshikonin induces apoptosis of hepatitis B virus X
protein-expressing human hepatocellular carcinoma cells via
endoplasmic reticulum stress. Eur J Pharmacol. 735:132–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha NH, Woo BH, Kim DJ, Ha ES, Choi JI, Kim
SJ, Park BS, Lee JH and Park HR: Prolonged and repetitive exposure
to porphyromonas gingivalis increases aggressiveness of oral cancer
cells by promoting acquisition of cancer stem cell properties.
Tumour Biol. 36:9947–9960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okamoto A, Chikamatsu K, Sakakura K,
Hatsushika K, Takahashi G and Masuyama K: Expansion and
characterization of cancer stem-like cells in squamous cell
carcinoma of the head and neck. Oral Oncol. 45:633–639. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murray GI, Duncan ME, O'Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu ZD, Li JY, Li M, Gu J, Shi XT, Ke Y and
Chen KN: Matrix metalloproteinases expression correlates with
survival in patients with esophageal squamous cell carcinoma. Am J
Gastroenterol. 100:1835–1843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong L, Wu D, Zou J, Chen J, Chen L, Chen
Y, Ni C and Yuan H: Prognostic impact of serum and tissue MMP-9 in
non-small cell lung cancer: A systematic review and meta-analysis.
Oncotarget. 7:18458–18468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bodet C, Chandad F and Grenier D:
Modulation of cytokine production by porphyromonas gingivalis in a
macrophage and epithelial cell co-culture model. Microbes Infect.
7:448–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandros J, Karlsson C, Lappin DF, Madianos
PN, Kinane DF and Papapanou PN: Cytokine responses of oral
epithelial cells to porphyromonas gingivalis infection. J Dent Res.
79:1808–1814. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao JJ, Feng XP, Zhang XL and Le KY:
Effect of porphyromonas gingivalis and lactobacillus acidophilus on
secretion of IL1B, IL6 and IL8 by gingival epithelial cells.
Inflammation. 35:1330–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beklen A, Ainola M, Hukkanen M, Gürgan C,
Sorsa T and Konttinen YT: MMPs, IL-1 and TNF are regulated by IL-17
in periodontitis. J Dent Res. 86:347–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franco C, Patricia HR, Timo S, Claudia B
and Marcela H: Matrix metalloproteinases as regulators of
periodontal inflammation. Int J Mol Sci. 18:E4402017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mauviel A: Cytokine regulation of
metalloproteinase gene expression. J Cell Biochem. 53:288–295.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teng J, Wang X, Xu Z and Tang N:
HBx-dependent activation of twist mediates STAT3 control of
epithelium-mesenchymal transition of liver cells. J Cell Biochem.
114:1097–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iqbal J, McRae S, Banaudha K, Mai T and
Waris G: Mechanism of hepatitis C virus (HCV)-induced osteopontin
and its role in epithelial to mesenchymal transition of
hepatocytes. J Biol Chem. 288:36994–37009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horikawa T, Yoshizaki T, Kondo S, Furukawa
M, Kaizaki Y and Pagano JS: Epstein-barr virus latent membrane
protein 1 induces snail and epithelial-mesenchymal transition in
metastatic nasopharyngeal carcinoma. Br J Cancer. 104:1160–1167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chandrakesan P, Roy B, Jakkula LU, Ahmed
I, Ramamoorthy P, Tawfik O, Papineni R, Houchen C, Anant S and Umar
S: Utility of a bacterial infection model to study
epithelial-mesenchymal transition, mesenchymal-epithelial
transition or tumorigenesis. Oncogene. 33:2639–2654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sztukowska MN, Ojo A, Ahmed S, Carenbauer
AL, Wang Q, Shumway B, Jenkinson HF, Wang H, Darling DS and Lamont
RJ: Porphyromonas gingivalis initiates a mesenchymal-like
transition through ZEB1 in gingival epithelial cells. Cell
Microbiol. 18:844–858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ha NH, Park DG, Woo BH, Kim DJ, Choi JI,
Park BS, Kim YD, Lee JH and Park HR: Porphyromonas gingivalis
increases the invasiveness of oral cancer cells by upregulating
IL-8 and MMPs. Cytokine. 86:64–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li
LZ and Meng XY: Interleukin-8 secretion by ovarian cancer cells
increases anchorage-independent growth, proliferation, angiogenic
potential, adhesion and invasion. Cytokine. 59:145–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L and
Chang C: Tumor microenvironment B cells increase bladder cancer
metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs
signals. Oncotarget. 6:26065–26078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
George VC, Dellaire G and Rupasinghe HPV:
Plant flavonoids in cancer chemoprevention: Role in genome
stability. J Nutr Biochem. 45:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Anticancer efficacy of polyphenols and their combinations.
Nutrients. 8:E5522016. View Article : Google Scholar : PubMed/NCBI
|