Introduction
Chronic myeloid leukemia (CML) is a
myeloproliferative disorder that is characterized by the
unregulated growth of myeloid leukemia cells in the bone marrow and
their accumulation in the blood (1). Epidemiologic data suggest that CML
incidence is one to two per 100,000 individuals, with 15–20% of
adult leukemia cases being CML (2). While CML is commonly treated with
tyrosine kinase inhibitors, an increase in drug resistance has been
reported along with adverse side effects (3).
Baicalein (BL) is a bioactive flavone derived from
the root of traditional Chinese medicine herb Scutellaria
baicalensis Georgi, with broad antitumor activity against
ovarian (4), prostate (5), breast (6), cervical (7) and lung (8) cancers. However, clinical application
of free BL has been limited by its extensive first-pass metabolism,
low aqueous solubility, poor bioavailability, short half-life
(t1/2, 10 min) and its easy oxidation (9–16).
About 76% of the dose was found to be circulating as its conjugated
metabolites even after intravenous administration of BL in rats
(10). Tian et al (17) demonstrated that the absolute
bioavailability of BL ranges from 13.1 to 23.0% when it was
administered via oral and intravenous routes in monkeys.
Nanostructured lipid carriers such as liposomes have been developed
to improve the stability and bioavailability of BL (11).
Liposomes have been used recently as popular
nanovesicles for administration of oral drugs because they have
good biocompatibility and biodegradability due to their similarity
in structure to the cell-surface phospholipid bilayer. They have
also been shown to display excellent drug loading rates, as well as
targeting and slow releasing actions, enhanced oral bioavailability
and long-circulating properties (18–25).
Despite these advantages, there are no studies in the literature
describing the use of liposomes to deliver BL to K562 cells or to
investigate the antitumor activities of free BL and liposomal BL on
these cells.
Previous investigations have shown that BL has
multiple biological activities, including anti-inflammatory
(26) anti-microbial (27) and antioxidant (28) properties. BL exerts an antitumor
effect by promoting the apoptosis or inhibiting the proliferation
of cancer cells (29–32) through multiple signalling pathways
including the cell proliferation pathway, the cell apoptosis and
caspase activation pathway, the tumor suppressor pathway and the
protein kinase pathway (33,34).
However, the exact mechanism of apoptosis and its related pathways
induced by BL is not yet fully understood.
In the present study, we evaluated different sizes
of liposome formulations for the delivery of BL. We further
investigated the cytotoxicity and pro-apoptotic effects of BL and
liposomal BL on CML K562 cells. The mechanism involved in this
process was also explored.
Materials and methods
Materials
Soy phosphatidylcholine (PC) was purchased from
Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Meth
oxypolyethyleneglycol-di-stearoyl-phosphatidylethanolamine
(DSPE-PEG2000, with mPEG MW2000 Da) was obtained from Genzyme
(Oxford, UK). Cholesterol (Chol), PBS, dialysis tubing, propidium
iodide (PI), RNase and BL were all purchased from Sigma-Aldrich
(UK). Methanol, dichloromethane, CyQUANT® Cell
Proliferation Assay kit and Annexin V-FITC/PI Apoptosis Detection
kit were both from Thermo Fisher Scientific (Loughborough, UK).
RPMI-1640, L-glutamine, penicillin-streptomycin and fetal bovine
serum (FBS) were all from Invitrogen Life Technologies (UK). The
CellTiter 96® AQueous One Solution Cell
Proliferation Assay (MTS) kit was purchased from Promega
(Southampton, UK).
Liposome preparation and
characterization
Three types of liposomes with different diameters
were prepared. Liposomes were composed of soy PC, cholesterol, and
methoxypolyethyleneglycol-di-stearoyl-phosphatidylethanolamine
(DSPE-PEG2000; Genzyme). Liposomes were prepared as described
elsewhere (35). Briefly, the
lipids were dissolved in methanol:dichloromethane 1:2 (v/v) at a
PC:Cholesterol:DSPE-PEG2000 molar ratio of 78.9:19.7:1.4 at room
temperature. BL was dissolved in the solvent with lipid mixture
when formulating the liposomes. Different lipid/BL mass ratios were
tested before settling on a fixed ratio of 10:1. The lipid mixtures
were deposited on the side wall of the rotary glass vial by
removing the solvent with nitrogen. The dried lipid films were
hydrated in 10 mM sodium phosphate buffer pH 7.4. This process led
to the spontaneous formation of pegylated liposomes. The liposomes
were then down-sized by passing through 0.1, 0.2 or 0.4 µm
polycarbonate membrane syringe filters (Whatman®;
Whatman, Inc., Clifton, NJ, USA) to produce lipo1, 2 and 3
suspensions, respectively. Free BL was removed by dialysis (14,000
Da cutoff membrane) against 10 mM sodium phosphate buffer pH 7.4
overnight. The size and ζ-potential of liposomes were measured by
dynamic light scattering on a Zetasizer-Nano ZS (Malvern
Instruments Ltd., Malvern, UK).
Cell culture
Human leukemia K562 cells were purchased from ATCC
(UK). Cells were cultured in RPMI-1640 media containing
10% fetal calf serum, 100 U/ml of penicillin, 100 mg/ml
streptomycin in 75 cm2 flasks. The cells were grown in a
humidified incubator containing 5% CO2 and 95% air at
37°C. Cells growing in the log phase and free from mycoplasma was
used in this study.
Cytotoxicity assay
K562 cells were cultured at a density of
6×104 cells/well in 96-well plates overnight and treated
with different concentrations of BL and control liposomes for 48 h.
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) solution (50 µl) from CellTiter 96®
AQueous One Solution Cell Proliferation Assay kit was
added to detect live cells in each well as per manufacturer's
instructions. Cells were incubated for 30 min at 37°C with 95% air
and 5% CO2. The absorbance of the solution was measured
at 490 nm by FLUOstar Omega (BMG Labtech, Aylesbury, UK). Each
treatment was conducted in triplicates. The cell viability was
expressed as a percentage of cell viability of liposome treated
cells relative to untreated controls.
Cell proliferation assay
Cell proliferation assays were conducted with the
CyQUANT® Direct Cell Proliferation Assay Kit (Thermo
Fisher Scientific) to avoid interference from the color of BL. K562
cells were cultured at a density of 5,000 cells/well in 96-well
plates overnight and treated with different concentrations of free
BL or liposomal BL (0.185–100 µM) for 48 h. CyQUANT®
Detection reagent (100 µl) was added to detect live cells in each
well as per manufacturer's instructions. Cells were incubated for
60 min at 37°C with 95% air and 5% CO2. The fluorescence
of the solution was recorded at 485 (ex)/520 (em) nm by FLUOstar
Omega (BMG Labtech). Cell viability was expressed as a percentage
of cell survival of treated cells relative to untreated
controls.
Cell cycle and cell apoptosis
analysis
Approximately 1×105 K562 cells/well in
96-well plates were incubated with 20 µM free BL or liposomal BL
for 48 h. The cells were centrifuged, resuspended in binding buffer
and treated with Annexin V-FITC and PI for 5 min before analysis
for cell apoptosis. For cell cycle analysis, the cells were
harvested, washed and fixed gently (drop by drop) in 70% ethanol
and kept at 4°C for 30 min. The cells were then resuspended in PBS
containing 40 µg/ml PI and 0.1 mg/ml RNase for cell cycle analysis.
After 30 min incubation at 37°C in the dark, 10,000 cells/sample
were analyzed were analysed for DNA content by flow cytometry on BD
FACSCalibur (BD Biosciences, Oxford, UK) and the cell cycle
distribution was determined using a CellQuest software
(Becton-Dickinson, Franklin Lakes, NJ, USA). The percentage of cell
population that had undergone apoptosis and those in different
phases of the cell cycle were assessed accordingly.
Intracellular reactive oxygen species
(ROS) assay
The measurement of intracellular ROS was determined
by the Cellular Reactive Oxygen Species Detection Assay kit (Abcam,
Cambridge, UK), which uses the cell permeant reagent
2′,7′-dichlorofluorescin diacetate (DCFDA), a fluorogenic dye that
measures ROS activity within the cell. Briefly, K562 cells were
cultured in complete phenol-red free RPMI medium at a density of
1.5×105 cells/well in 96-well plates and were treated
with 20 µM BL or liposomal BL for 48 h. After the incubation time,
the cell treatments were overlaid with 20 µM DCFDA (100 µl) and
incubated at 37°C for further 30 min in the dark. The fluorescence
of the treatments was detected at the excitation and emission
spectra of 485 and 520 nm, respectively, by FLUOstar Omega (BMG
Labtech).
Statistical analysis
Results were presented as mean ± standard deviation
(SD). Statistical significance was tested by paired t-test or
one-way ANOVA analysis. Differences between experimental groups
were considered significant when the P-value <0.05.
Results
Liposome preparation and
characterization
Three groups of liposome suspensions: lipo1 (100
nm), lipo2 (200 nm) and lipo3 (400 nm), were prepared to
investigate whether the size of the liposomes contributed to the
efficiency of BL encapsulation. BL/PC 1:10 (w/w) was dissolved in
the solvent with lipid mixture when formulating the liposomes.
Unincorporated BL was removed by dialysis and the concentration of
BL in the liposome was determined by dissolving the liposomes in
methanol. The absorbance of BL was recorded at 278 nm by FLUOstar
Omega (BMG Labtech). Phospholipid (PC) was detected in the
liposomes by the Stewart assay (36). Drug encapsulation efficiency was
determined by encapsulated BL divided by original BL corrected by
PC concentration. The final liposome samples were characterized and
the data was shown in Table I,
with the drug encapsulation efficiency from high to low: Lipo2 >
lipo1 > lipo3.
 | Table I.Characterisation of the
liposomes. |
Table I.
Characterisation of the
liposomes.
| Sample | Baicalein (mM) | PC (mg/ml) | Drug encapsulation
(%) |
|---|
| Control lipo1 (100
nm) | – | 18.6±1.2 | – |
| Lipo1-BL | 2.3±0.1 | 22.6±0.1 | 28 |
| Control lipo2 (200
nm) | – | 20.3±0.9 | – |
| Lipo2-BL | 2.5±0.2 | 20.7±0.9 | 32 |
| Control lipo3 (400
nm) | – | 18.0±1.3 | – |
| Lipo3-BL | 2.0±0.3 | 20.2±0.4 | 26 |
The size and zeta potential of liposomes were
determined by dynamic light scattering (Fig. 1A). Three groups of control
liposomes displayed average diameters of 125, 161 and 230 nm,
respectively, with polydispersity of 0.1 for lipo1 and lipo2, 0.2
for lipo3. The average diameters for lipo2 and lipo3 were below 200
and 400 nm, which may be due to the quality control of the filters
(37). The negative ζ-potential of
the liposomes was attributed to one of the liposome components
DSPE-PEG2000 which is negatively charged. The stability of
liposomes was investigated by comparing the size and zeta potential
changes over a one month storage period at 4°C in 10 mM phosphate
buffer pH 7.4 (Fig. 1B). The
results showed that lipo1 formulation was the most stable, with no
statistically significant changes in diameter or ζ-potential over
at least a one month period.
In vitro cytotoxicity of the
liposomes
Potential cytotoxicity of the liposomes was measured
from cell viability relative to control K562 cells treated with
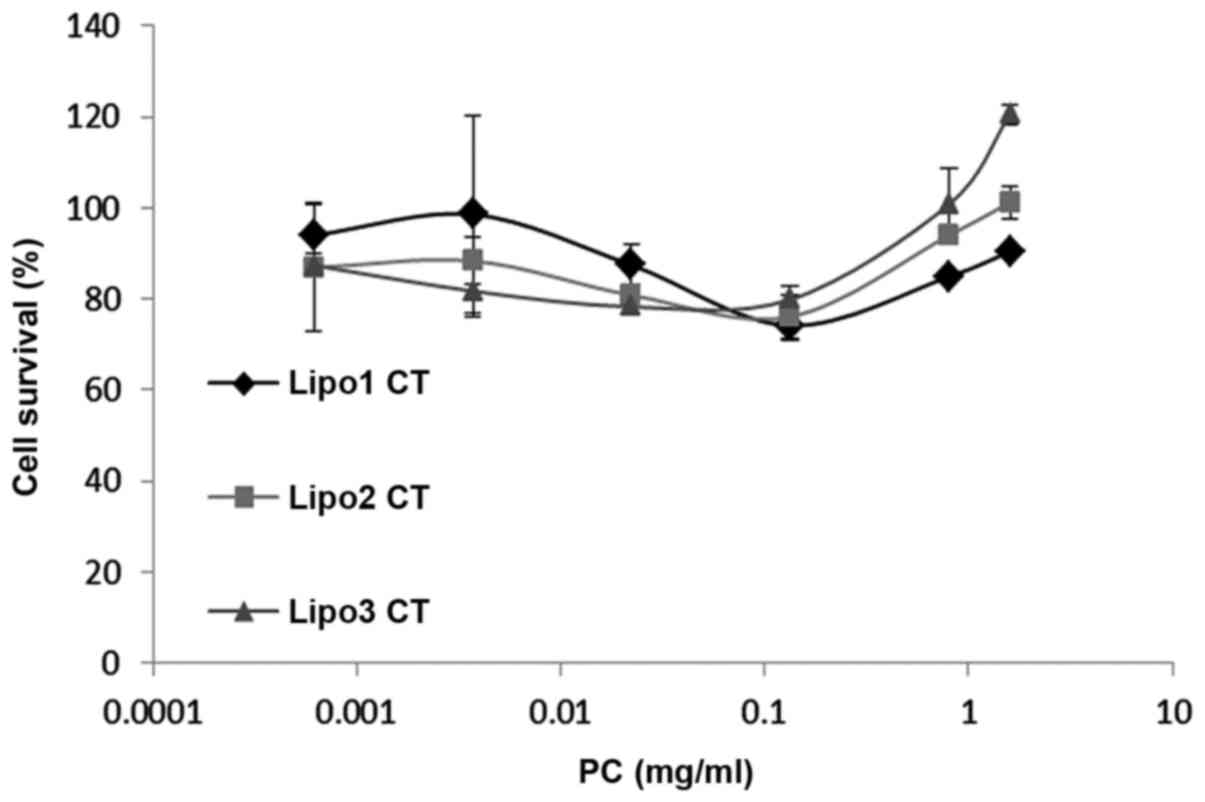
medium only. MTS assay demonstrated that cell viability was between
80 and 120% in relation to the control samples (Fig. 2). Statistical analysis showed no
evidence of liposome toxicity at phosphatidylcholine concentrations
up to 1.6 mg/ml.
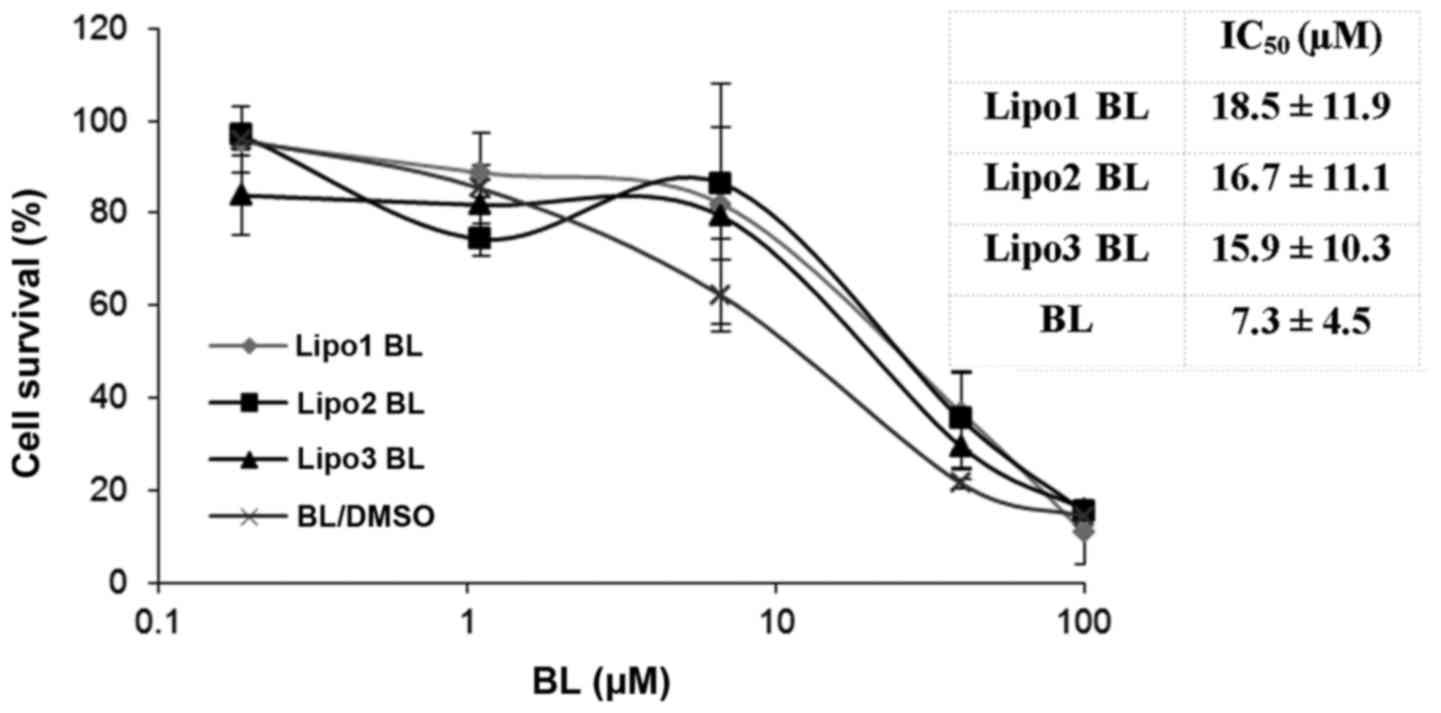
Liposomal BL inhibits proliferation of
K562 cells
The CyQUANT® Direct Cell Proliferation
Assay kit was used to evaluate the cytotoxicity of liposomal BL.
The cell proliferation assay was assessed after cells were treated
for 48 h with the encapsulated drug. Exposure to free and liposomal
BL inhibited leukemia cell line K562 growth in a
concentration-dependent manner (Fig.
3). The results showed that there was no significance
difference between the antiproliferative effects of liposomal BL
and those of free BL at equimolar concentrations. No inhibition
effects were observed from three groups of control liposomes, which
was consistent with the MTS assay showing no cytotoxicity from the
control liposomes.
Effect of BL on cell cycle progression
and cell death
K562 cells were exposed to three different sizes of
control liposomes and their relevant liposomal encapsulated BL for
48 h. Staining procedures using PI or Annexin V-FITC/PI were
conducted to evaluate the effect of BL preparations on cell cycle
and cell death, respectively, using a flow cytometer. Cells in each
sample from different phases (sub-G1, G1, S and G2/M) were
presented and their percentage in each phase was calculated and
shown in Table II; viable and
non-viable (necrotic, early apoptotic and late apoptotic) cells
were calculated and shown in Table
III. All the BL preparations showed significant increase
(P<0.005) in their populations in the sub-G1 phase and
significant decrease (P<0.01) in cell viability in comparison of
their relative control sample.
 | Table II.Percentage of cell populations in
different stages of the cell cycle following exposure to 20 µM BL
or liposomal baicalein for 48 h. |
Table II.
Percentage of cell populations in
different stages of the cell cycle following exposure to 20 µM BL
or liposomal baicalein for 48 h.
| Treatment
group | Sub-G1 phase | G1 phase | S phase | G2/M phase |
|---|
| Untreated
cells | 7.3±4.8 | 45.4±2.6 | 25.0±1.0 | 22.8±7.5 |
| Control lipo1 (100
nm) | 6.2±2.8 | 43.1±0.8 | 27.8±4.8 | 23.1±6.4 |
| Lipo1-BL |
17.9±4.9a | 41.7±1.5 | 21.6±3.4 | 19.4±2.7 |
| Control lipo2 (200
nm) | 4.8±1.3 | 46.3±3.0 | 25.0±0.9 | 23.4±5.6 |
| Lipo2-BL |
19.9±3.3a | 38.6±1.8 | 21.8±0.6 | 20.1±5.5 |
| Control lipo3 (400
nm) | 5.9±3.7 | 43.8±0.7 | 27.8±5.3 | 22.7±7.6 |
| Lipo3-BL |
16.3±2.4a | 39.9±2.1 | 25.0±1.3 | 20.0±0.7 |
| DMSO | 4.7±2.4 | 42.9±3.6 | 23.4±0.1 | 29.9±0.2 |
| Free BL (in
DMSO) |
23.3±11.5a | 36.5±8.1 | 25.9±1.9 | 15.3±5.4 |
 | Table III.Percentage of cell populations of
viable and non-viable cells exhibiting structural properties of
different cell death types following exposure to 20 µM BL or
liposomal baicalein for 48 h. |
Table III.
Percentage of cell populations of
viable and non-viable cells exhibiting structural properties of
different cell death types following exposure to 20 µM BL or
liposomal baicalein for 48 h.
|
|
| Non-viable
cells |
|---|
|
|
|
|
|---|
| Treatment
group | Viable cells | Necrosis | Late
apoptosis/secondary necrosis | Early
apoptosis |
|---|
| Untreated
cells | 84.3±8.0 | 1.4±0.5 | 9.5±5.8 | 4.9±1.6 |
| Control lipo1 (100
nm) | 84.5±3.7 | 1.1±0.1 | 8.8±3.7 | 5.6±0.1 |
| Lipo1-BL |
65.8±3.9a | 4.6±1.0 | 17.3±1.0 | 12.3±3.8 |
| Control lipo2 (200
nm) | 85.8±0.1 | 3.7±3.8 | 6.7±1.1 | 3.9±2.9 |
| Lipo2-BL |
65.1±2.2a | 4.0±0.9 | 16.4±1.9 | 14.5±5.0 |
| Control lipo3 (400
nm) | 83.1±3.3 | 1.2±0.6 | 10.2±3.2 | 5.6±0.6 |
| Lipo3-BL |
64.9±0.9a | 4.4±0.7 | 16.3±2.7 | 14.5±4.3 |
| DMSO | 85.2±2.7 | 3.1±3.3 | 7.4±2.1 | 4.2±3.8 |
| Free BL (in
DMSO) |
64.3±5.3a | 2.9±0.5 | 15.5±4.1 | 17.3±1.7 |
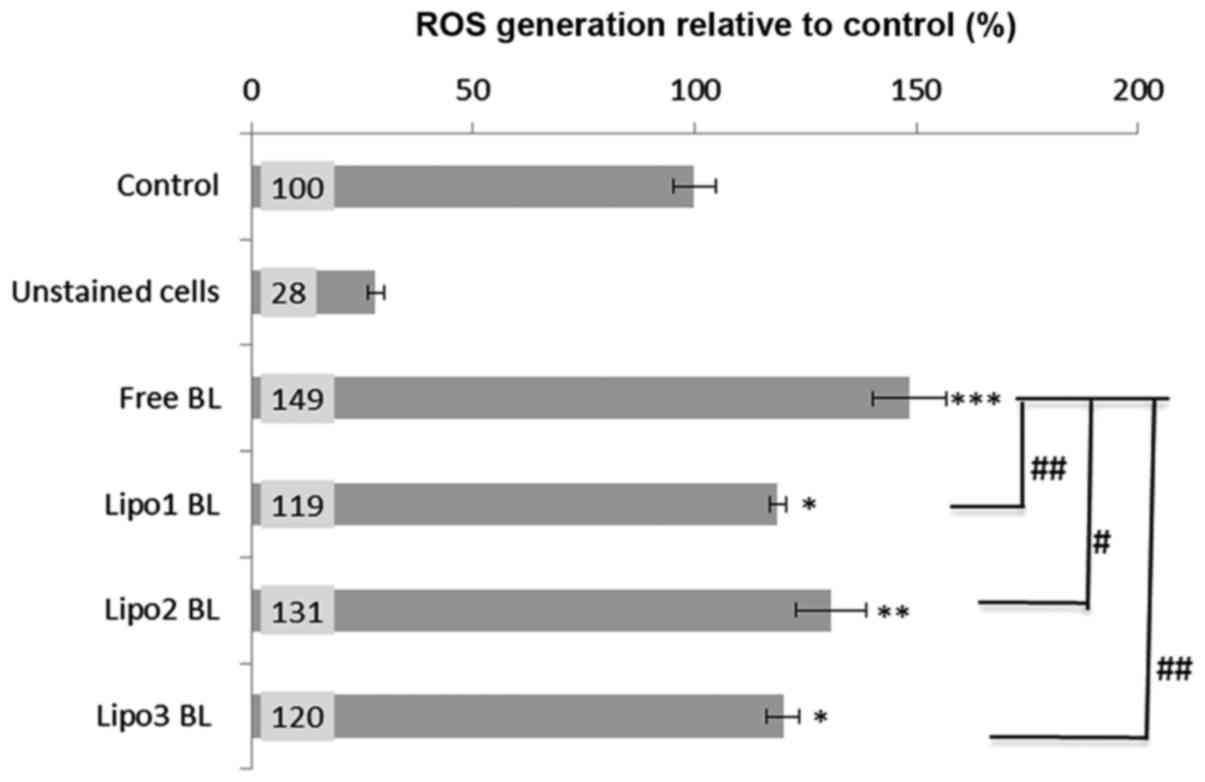
Intracellular ROS production
The cell permeant reagent DCFDA, a fluorogenic dye
that measures ROS activity within cells, was used to investigate
whether intracellular ROS production was involved in the mechanism
of apoptosis caused by BL. Analysis indicated that all BL
preparations induced significant ROS generation (as determined by
the fluorescence changes normalized as percentage increase over the
untreated cells control level). About 20–30% increase (P<0.05)
of ROS production was observed when K562 cells were treated with
liposome encapsulated BL, and 50% increase (P<0.001) following
treatment with non-encapsulated BL or free BL (Fig. 4). Moreover, there was a significant
difference in ROS induction between the treatments with liposomal
BL and free BL (Fig. 4).
Discussion
BL is a potent antitumor agent, but its poor
solubility in aqueous solution, instability and relative toxicity
affect its bioavailability and application in humans. In this
study, we prepared three groups of liposomal BL with different
sizes to enhance its solubility and stability and investigated
their ability to affect proliferation of CML K562 cells in
vitro. Earlier research has shown that uncoated liposomes were
quickly cleared from the blood by the reticuloendothelial system
(38). Therefore, liposomes coated
with polyethylene glycol (PEG), shown to increase oral
bioavailability of BL in vivo (11), were employed in this study.
A previous study has demonstrated that
internalization of liposomes by murine macrophages was similar with
liposome size of between 100 and 200 nm, but was 1.7 times higher
with liposomes larger than 400 nm, indicating that the degree of
internalization is positively related to the size of the liposome
(39). These researchers also
suggested that PEG coating significantly reduced endocytosis of
liposomes (39). However, others
have reported that immunoliposomes of ~100 nm in size may be ideal
for intraperitoneal and intravenous (i.p./i.v.) administration in
order to achieve high plasma concentration and subsequent tumor
targeting using tumor vasculature, whereas immunoliposomes of a
larger size (>300-400 nm) may be preferable for i.p.
administration and retention of the liposomes in the peritoneal
cavity for targeting tumors located in this site and surrounding
areas (40).
In this study, three groups of liposome formulations
with diameters of 100, 200 or 400 nm were evaluated for their
stability, drug encapsulation efficiency, cytotoxicity, and
pro-apoptotic effects on K562 cells. The results showed that the
100 nm liposome formulation was the most stable, with no
significant changes in diameter or zeta potential over at least a
one month period. The 200 nm liposome showed the highest drug
encapsulation efficiency. Liposomal BL displayed similar
cell-killing capabilities against the K562 cell line compared to
free BL, indicating that liposomes could be a potential platform
for BL delivery to K562 leukemia cells due to increased solubility
and stability.
Previous investigations have shown that BL exerts an
antitumor effect by promoting apoptosis or inhibiting the
proliferation of cancer cells (29–32)
through multiple signalling pathways (33,34).
For example, BL induces human osteosarcoma cell line MG-63 via
ROS-induced BNIPs expression (41)
and it also induces G1 arrest in oral cancer cells by enhancing the
degradation of cyclin D1 and activating AhR to decrease Rb
phosphorylation. We suspected that the anti-proliferative effect on
K562 of BL is associated with ROS generation and cell cycle arrest.
Therefore, in this study we investigated the molecular mechanisms
by evaluating the generation of ROS and through cell cycle
analysis. Significantly increased ROS production was observed when
K562 cells were treated with both encapsulated and non-encapsulated
BL compared to untreated control cells (Fig. 4). However, the results revealed
that there was no obvious cell cycle arrest observed after
treatment with free or liposomal BL (but showed an accumulation of
apoptotic cell in the sub-G1 phase) suggesting that BL induced K562
cell apoptosis is, in part, via ROS generation. Interestingly, more
intracellular ROS was produced from cells treated with free BL
compared with those treated with liposomal BL, although no
significant difference in cell apoptosis was observed. This
suggests that other mechanisms may also be involved in BL induced
K562 cell death.
ROS production occurs during normal metabolic
functions such as respiration (42) and the detoxification of
xenobiotics. ROS can cause cell death or lead to mutagenesis and
increased cell proliferation depending of its relative amount
compared to levels of endogenous antioxidants in the cells.
Elevated ROS levels in cells can cause genetic mutations which can
trigger programmed cell death via the mitochondrial (intrinsic)
pathway of apoptosis (43), but
may also result in normal cells becoming cancerous (42). Cancer cells increase their rate of
ROS production compared with normal cells to hyperactivate the cell
signalling pathways necessary for cellular transformation and
tumorigenesis. Meanwhile, they increase the antioxidant capacity to
maintain ROS homeostasis and evade cell death (42,44,45).
This altered redox environment of cancer cells may make them more
susceptible to ROS-manipulated therapies. It has been proposed that
a disproportional increase in intracellular ROS can induce cancer
cell cycle arrest, senescence and apoptosis. Apoptosis is linked to
an increase in mitochondrial oxidative stress that causes
cytochrome c release, an irrevocable event that leads to the
activation of caspases and cell death (46,47).
BL has been shown to trigger this apoptotic death program through
ROS-mediated mitochondrial dysfunction pathway in HL-60 cells
(48). This was further confirmed
by the other studies on human bladder cancer cells and osteosarcoma
cell line (41,49). Our results indicated the same
finding that BL induced cancer cell death by generation of ROS
or/and possibly by depletion of cells from antioxidant proteins
which protect cells from ROS-mediated apoptosis. The difference in
the ROS generation by free BL and liposomal BL suggests that other
signalling pathways could be involved in the liposome-BL induced
cell death. Further studies in the future are required to identify
other possible mechanisms of action of liposome-delivered BL
induction of myeloid leukemia cell death.
In this study, three groups of liposome formulations
with diameters of 100, 200 and 400 nm were synthesized. The results
demonstrated that the 100 nm liposome was the most stable
formulation and that liposomal BL had an equivalent cytotoxic
effect on K562 cells compared with free BL, indicating that
liposomes may be a potential route for the delivery of BL with
better solubility and stability. Enhanced intracellular ROS
generation was induced by all BL preparations, but no significant
cell cycle arrest was seen in any of the phases following liposomal
BL or free BL treatment, indicating that the mechanism involved in
K562 cell apoptosis following BL treatment was at least in part via
ROS generation. The findings are important as BL has been shown to
be potent in killing several cancer cell lines. However, the
hydrophobic nature of the drug hinders its application in
vivo. The results from this study are promising in
demonstrating the ability of liposome-encapsulated BL to exert
cytotoxicity on human chronic myeloid leukemic cells. However,
further studies are required to determine if the liposomal
formulation exerts selective cytotoxicity in leukemic cells
compared to normal cells and to evaluate further possible
mechanisms of action of the drug.
References
|
1
|
Wang Y, Wei S, Wang J, Fang Q and Chai Q:
Phenethyl isothiocyanate inhibits growth of human chronic myeloid
leukemia K562 cells via reactive oxygen species generation and
caspases. Mol Med Rep. 10:543–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melo JV and Barnes DJ: Chronic myeloid
leukaemia as a model of disease evolution in human cancer. Nat Rev
Cancer. 7:441–453. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owen HC, Appiah S, Hasan N, Ghali L,
Elayat G and Bell C: Phytochemical modulation of apoptosis and
autophagy: Strategies to overcome chemoresistance in leukemic stem
cells in the bone marrow microenvironment. Int Rev Neurobiol.
135:249–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan H, Xin S, Wang H, Ma J, Zhang H and
Wei H: Baicalein inhibits MMP-2 expression in human ovarian cancer
cells by suppressing the p38 MAPK-dependent NF-κB signaling
pathway. Anticancer Drugs. 26:649–656. 2015.PubMed/NCBI
|
|
5
|
Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng
X, Su J, Zhou Z, Xu Z, Nilsson S and Liu Z: Baicalein inhibits
prostate cancer cell growth and metastasis via the
caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 406:111–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang N, Ren D, Deng S and Yang X:
Differential effects of baicalein and its sulfated derivatives in
inhibiting proliferation of human breast cancer MCF-7 cells. Chem
Biol Interact. 221:99–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng Y, Guo C, Yang Y, Li F, Zhang Y,
Jiang B and Li Q: Baicalein induces apoptosis of human cervical
cancer HeLa cells in vitro. Mol Med Rep. 11:2129–2134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
9
|
He X, Pei L, Tong HH and Zheng Y:
Comparison of spray freeze drying and the solvent evaporation
method for preparing solid dispersions of baicalein with Pluronic
F68 to improve dissolution and oral bioavailability. AAPS
PharmSciTech. 12:104–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai MY, Hsiu SL, Tsai SY, Hou YC and Chao
PD: Comparison of metabolic pharmacokinetics of baicalin and
baicalein in rats. J Pharm Pharmacol. 55:205–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang J, Wu W, Liu Q and Chen S:
Long-circulating nanoliposomes (LCNs) sustained delivery of
baicalein (BAI) with desired oral bioavailability in vivo. Drug
Deliv. 20:319–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Oliveira MR, Nabavi SF, Habtemariam S,
Erdogan Orhan I, Daglia M and Nabavi SM: The effects of baicalein
and baicalin on mitochondrial function and dynamics: A review.
Pharmacol Res. 100:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Lin G, Chang Q and Zuo Z: Role of
intestinal first-pass metabolism of baicalein in its absorption
process. Pharm Res. 22:1050–1058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fong YK, Li CR, Wo SK, Wang S, Zhou L,
Zhang L, Lin G and Zuo Z: In vitro and in situ evaluation of
herb-drug interactions during intestinal metabolism and absorption
of baicalein. J Ethnopharmacol. 141:742–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seo MJ, Choi HS, Jeon HJ, Woo MS and Lee
BY: Baicalein inhibits lipid accumulation by regulating early
adipogenesis and m-TOR signaling. Food Chem Toxicol. 67:57–64.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY
and Chen CF: The effects of the cyclosporin A, a P-glycoprotein
inhibitor, on the pharmacokinetics of baicalein in the rat: A
microdialysis study. Br J Pharmacol. 137:1314–1320. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian S, He G, Song J, Wang S, Xin W, Zhang
D and Du G: Pharmacokinetic study of baicalein after oral
administration in monkeys. Fitoterapia. 83:532–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan ML, Choong PF and Dass CR: Recent
developments in liposomes, microparticles and nanoparticles for
protein and peptide drug delivery. Peptides. 31:184–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang YB, Tsai MJ, Wu PC, Tsai YH, Wu YH
and Fang JY: Elastic liposomes as carriers for oral delivery and
the brain distribution of (+)-catechin. J Drug Target. 19:709–718.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SJ, Choi SG, Davaa E and Park JS:
Encapsulation enhancement and stabilization of insulin in cationic
liposomes. Int J Pharm. 415:267–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song YK, Hyun SY, Kim HT, Kim CK and Oh
JM: Transdermal delivery of low molecular weight heparin loaded in
flexible liposomes with bioavailability enhancement: Comparison
with ethosomes. J Microencapsul. 28:151–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cansell M, Nacka F and Combe N: Marine
lipid-based liposomes increase in vivo FA bioavailability. Lipids.
38:551–559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan P, Lu Y, Qi J, Niu M, Lian R, Hu F
and Wu W: Enhanced oral bioavailability of cyclosporine A by
liposomes containing a bile salt. Int J Nanomedicine. 6:965–974.
2011.PubMed/NCBI
|
|
24
|
Isacchi B, Arrigucci S, La Marca G,
Bergonzi MC, Vannucchi MG, Novelli A and Bilia AR: Conventional and
long-circulating liposomes of artemisinin: Preparation,
characterization, and pharmacokinetic profile in mice. J Liposome
Res. 21:237–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vural I, Sarisozen C and Olmez SS:
Chitosan coated furosemide liposomes for improved bioavailability.
J Biomed Nanotechnol. 7:426–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HP, Son KH, Chang HW and Kang SS:
Anti-inflammatory plant flavonoids and cellular action mechanisms.
J Pharmacol Sci. 96:229–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Y, Joerger R and Wu C: Study of the
chemical composition and antimicrobial activities of ethanolic
extracts from roots of Scutellaria baicalensis Georgi. J Agric Food
Chem. 59:10934–10942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shieh DE, Liu LT and Lin CC: Antioxidant
and free radical scavenging effects of baicalein, baicalin and
wogonin. Anticancer Res. 20:2861–2865. 2000.PubMed/NCBI
|
|
29
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J
and Zhou J: Exploring therapeutic potentials of baicalin and its
aglycone baicalein for hematological malignancies. Cancer Lett.
354:5–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng YH, Li LA, Lin P, Cheng LC, Hung CH,
Chang NW and Lin C: Baicalein induces G1 arrest in oral cancer
cells by enhancing the degradation of cyclin D1 and activating AhR
to decrease Rb phosphorylation. Toxicol Appl Pharmacol.
263:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma GZ, Liu CH, Wei B, Qiao J, Lu T, Wei
HC, Chen HD and He CD: Baicalein inhibits DMBA/TPA-induced skin
tumorigenesis in mice by modulating proliferation, apoptosis, and
inflammation. Inflammation. 36:457–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chow JM, Shen SC, Wu CY and Chen YC:
12-o-Tetradecanoylphorbol 13-acetate prevents baicalein-induced
apoptosis via activation of protein kinase C and JNKs in human
leukemia cells. Apoptosis. 11:1999–2011. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JH, Li YC, Ip SW, Hsu SC, Chang NW,
Tang NY, Yu CS, Chou ST, Lin SS, Lino CC, et al: The role of
Ca2+ in baicalein-induced apoptosis in human breast
MDA-MB-231 cancer cells through mitochondria- and
caspase-3-dependent pathway. Anticancer Res. 28:1701–1711.
PubMed/NCBI
|
|
35
|
Wang X, Li D, Ghali L, Xia R, Munoz LP,
Garelick H, Bell C and Wen X: Therapeutic potential of delivering
arsenic trioxide into HPV infected cervical cancer cells using
liposomal nanotechnology. Nanoscale Res Lett. 11:942016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SX, Michiels J, Ariën KK, New R,
Vanham G and Roitt I: Inhibition of HIV virus by neutralizing Vhh
attached to dual functional liposomes encapsulating dapivirine.
Nanoscale Res Lett. 11:3502016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berger N, Sachse A, Bender J, Schubert R
and Brandl M: Filter extrusion of liposomes using different
devices: Comparison of liposome size, encapsulation efficiency and
process characteristics. Int J Pharm. 223:55–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clayton R, Ohagen A, Nicol F, Del Vecchio
AM, Jonckers TH, Goethals O, Van Loock M, Michiels L, Grigsby J, Xu
Z, et al: Sustained and specific in vitro inhibition of HIV-1
replication by a protease inhibitor encapsulated in gp120-targeted
liposomes. Antiviral Res. 84:142–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JS, Hwang SY and Lee EK: Imaging-based
analysis of liposome internalization to macrophage cells: Effects
of liposome size and surface modification with PEG moiety. Colloids
Surf B Biointerfaces. 136:786–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng J, Iyer A, Seo Y, Broaddus C, Liu B,
VanBrocklin H and He J: Effects of size and targeting ligand on
biodistribution of liposome nanoparticles in tumor mice. Soc Nucl
Med Annu Meet Abstr. 54:13392013.
|
|
41
|
Ye F, Wang H, Zhang L, Zou Y, Han H and
Huang J: Baicalein induces human osteosarcoma cell line MG-63
apoptosis via ROS-induced BNIP3 expression. Tumor Biol.
36:4731–4740. 2015. View Article : Google Scholar
|
|
42
|
Reczek CR and Chandel NS: The two faces of
reactive oxygen species in cancer. Annu Rev Cancer Biol. 1:79–98.
2017. View Article : Google Scholar
|
|
43
|
Galadari S, Rahman A, Pallichankandy S and
Thayyullathil F: Reactive oxygen species and cancer paradox: To
promote or to suppress? Free Radic Biol Med. 104:144–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nieborowska-Skorska M, Kopinski PK, Ray R,
Hoser G, Ngaba D, Flis S, Cramer K, Reddy MM, Koptyra M, Penserga
T, et al: Rac2-MRC-cIII-generated ROS cause genomic instability in
chronic myeloid leukemia stem cells and primitive progenitors.
Blood. 119:4253–4263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cadenas E: Mitochondrial free radical
production and cell signaling. Mol Aspects Med. 25:17–26. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Yu Y, Hashimoto F, Sakata Y, Fujii
M and Hou DX: Baicalein induces apoptosis through ROS-mediated
mitochondrial dysfunction pathway in HL-60 cells. Int J Mol Med.
14:627–632. 2004.PubMed/NCBI
|
|
49
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|