Introduction
Lung cancer is a common cause of cancer mortality
(1,2). Lung cancer has been associated with a
32% decrease in pre-illness weight and poor survival of patients
(3). Thus, establishing
tumor-bearing models in order to prevent lung cancer and slow the
course of the disease is crucial for cancer research.
Animal models have been developed to study the
pathophysiology of this disease and the effects of therapy. Lewis
lung carcinoma (LLC) was isolated from the epidermoid carcinoma of
the lung in mice. It is regarded as an essential tumor model in
studies of metastasis (4,5), vessel formation (6), and effects of therapy (7). A tumor-bearing model of mice with LLC
exhibits a greater metastatic potential than a tumor-bearing model
alone (8,9). LLC cells have been inoculated into
various sites, including the tail vein (5), muscle (10), subcutaneous tissue (9,11),
lungs (9), intrathorax and
intrabronchus (12). LLC-bearing
mice have been used in experimental models of lung tumor (13), brain metastasis (14) and cachexia (15). In the study by Li et al mice
were implanted with LLC cells in various sites, and the results
were compared. These authors found that intrabronchial implantation
yielded slow-growing tumors, but no distant metastasis.
Additionally, mice with intrathoracic implantation succumbed more
rapidly than those with intrabronchial implantation. Intrathoracic
and intrabronchial implantation were not considered favorable
tumorigenic and metastatic models (12). Recently, Liu et al compared
intrapulmonary and subcutaneous models of lung cancer using LLC
cells and found a higher rate of tumor formation, stronger transfer
characteristics and a shorter survival time in the intrapulmonary
than in the subcutaneous model (9).
However, the lack of distant metastasis suggests that
intrapulmonary inoculation is unsuitable for use in metastatic
study. In the orthotopic model, it is not possible to evaluate the
time-related change in tumor growth until the point of sacrifice.
Harlos et al reported the subcutaneous and intramuscular
models of lung cancer. Their results showed that there is no
difference in tumor growth in mice receiving the subcutaneous and
intramuscular inoculation (16).
However, they observed that intramuscular inoculation with cancer
cells may damage skeletal muscle and decrease activity during tumor
formation in mice. It may also affect the outcome measurement of
muscle mass in cancer cachexia. To assess the effect of tumor
growth and cancer cachexia, the subcutaneous inoculation model was
considered appropriate.
Tumor growth and metastasis require angiogenesis,
which is regulated by various factors, including the vascular
endothelial growth factor (VEGF) (17). VEGF overexpression is an indicator
of poor prognosis in patients with non-small and small cell lung
cancer (18). Shimanuki et
al measured the serum VEGF levels preoperatively in patients
with non-small cell lung cancer and revealed a correlation with
microvessel density in the resected tumor. Their results showed
that the average overall survival and disease-free survival time
were significantly longer when the VEGF levels were lower (19). VEGF also plays a significant role in
the rapid growth of micrometastasis in the lungs (5). To understand the tumor growth, biology
of metastases and cancer cachexia, an animal model with a
subcutaneous inoculation of LLC cells was used in the present
study. The aim of this study was to describe the features of Lewis
lung cancer (LLC) in mice and compare the serum VEGF-A levels with
that of normal control mice.
Materials and methods
Animals, cell culture and study
guidelines
A total of 22 8-week-old, healthy C57BL/6 male mice
were purchased from the National Animal Center (Taipei, Taiwan).
The animals were housed in cages under a 12-h light/dark cycle at
the Laboratory Animal Center of the National Taiwan University
(Taipei, Taiwan) and were provided with food and water ad
libitum. LLC cells were cultured in tissue culture flasks
containing DMEM supplemented with 10% fetal bovine serum (FBS), 2
mM glutamine, 100 mg/dl streptomycin, and 100 U/ml penicillin, and
were maintained at 37°C in a humidified atmosphere containing 5%
CO2 (7).
The mice were given one week to acclimatize to their
environment before any treatment was administered, and then divided
into two groups. Sixteen mice were inoculated subcutaneously with
5×105 LLC cells in 0.1 ml PBS and 0.1 ml Matrigel in the
back. The remaining 6 mice were used as normal controls and were
injected with PBS and Matrigel devoid of tumor cells.
Blood samples (150 μl) were drawn prior to
inoculation, and on days 7, 21 and 35 post-inoculation to measure
the serum VEGF-A levels. Body weight was measured 3 times a week.
After 5 weeks, the surviving mice were sacrificed by CO2
asphyxiation. This study was approved by the National Taiwan
University College of Medicine and the College of Public Health
Institutional Animal Care and Use Committee (IACUC).
Assessment of tumor volume and
metastasis
The tumor volume [V=L (longest diameter) ×
W2 (shortest diameter) × 0.5] was measured 3 times a
week. Following sacrifice, the tumors were excised and weighed. The
lungs and liver were harvested and the surface nodules were counted
to evaluate the metastatic spread of the tumor. The tissues were
fixed in 4% paraformaldehyde and stained with hematoxylin and eosin
(20,21).
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples (150 μl) were drawn from mice by
submandibular venipuncture, collected using Eppendorf tubes,
clotted overnight at 4°C, and centrifuged for 20 min at 2,000 × g.
The serum was collected and stored at −80°C. The levels of serum
VEGF-A were determined using a commercially available ELISA kit
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions (22).
Statistical analysis
The statistical software package SPSS 16.0 (SPSS
Inc., Chicago, IL, USA) was used to perform data analyses. Data
analysis included the calculation of descriptive statistics (means
± SEM). The Kaplan-Meier analysis was performed for survival
analysis. The significance of differences between groups was
assessed using the Mann-Whitney U test. Generalized estimating
equations (GEE) were also used to test the significance of
differences in tumor effects on body weight and serum VEGF-A
concentration at each measurement point. The α-value was set at
0.05.
Results
Survival rate
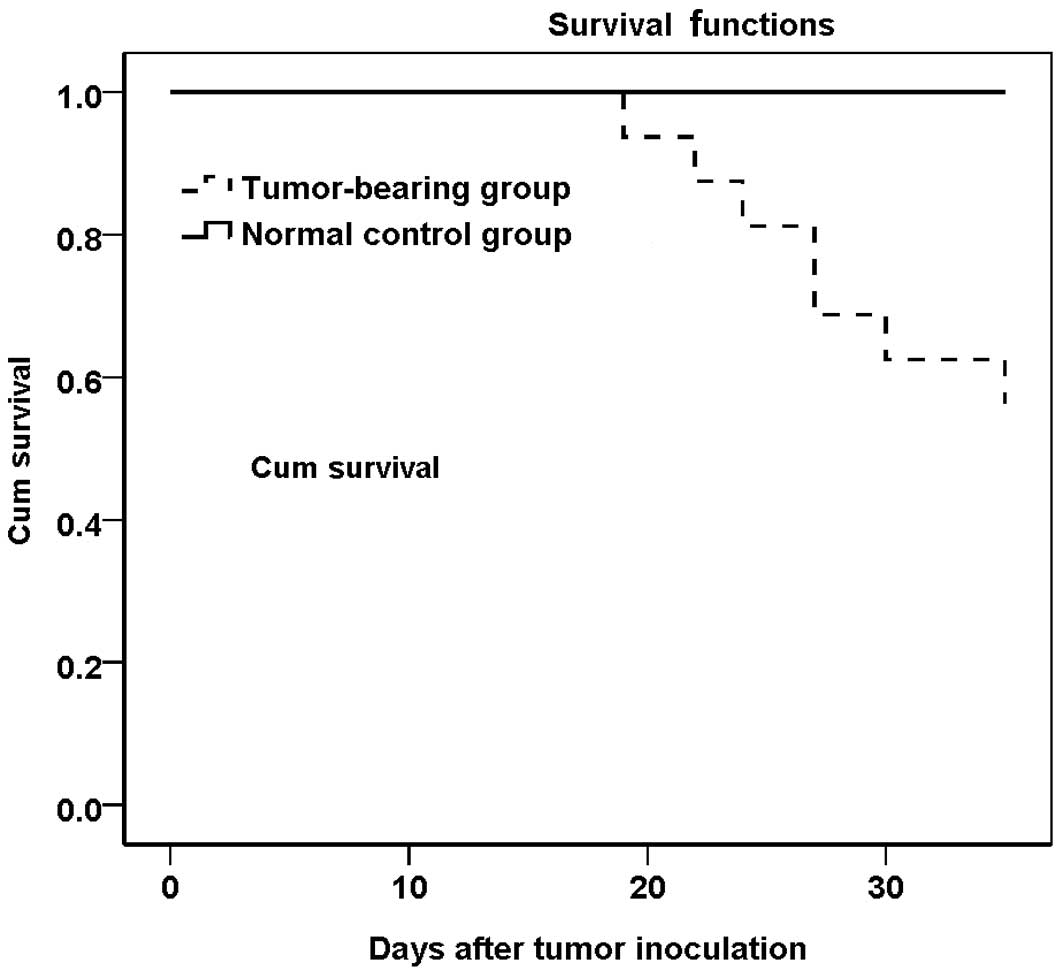
The survival rate was 56.3% (9/16) in the
tumor-bearing group on day 35 post-tumor inoculation (Fig. 1).
Body weight
The mean body weight was not significantly different
between the tumor-bearing (n=16) and normal control (n=6) groups
prior to tumor inoculation (23.88±0.36 and 23.41±0.51 g,
respectively). GEE analysis revealed a significant time effect
(P<0.01) and a group-time interaction effect (P<0.01) on body
weight, but no group effect was found (P=0.368). The total weight
gain was higher in the tumor-bearing mice than in the control mice
[3.94±0.74 (n=9) vs. 1.93±0.47 g (n=6), P=0.03] on day 35
post-inoculation; however, following tumor resection, total weight
gain was found to be lower (0.24±0.45 vs. 1.93±0.47 g, P=0.01)
(Table I). Seven mice in the
tumor-bearing group died prior to the end of the experiment (35
days post-tumor inoculation) with a mean body weight of 1.05±0.08
times the mean body weight of the normal control group. In two
mice, the body weight decreased prior to their death.
 | Table ICharacteristics in tumor-bearing and
normal control groups. |
Table I
Characteristics in tumor-bearing and
normal control groups.
| Tumor-bearing
(n=9) | Normal control
(n=6) |
|---|
| Body weighta (g) | 27.54 (0.81) | 25.34 (0.88)d |
| Tumor weight (g) | 3.70 (0.83) | NA |
| Carcassb (g) | 23.84 (0.51) | 25.34 (0.88) |
| Body weight
gainc (g) | 0.24 (0.45) | 1.93 (0.47)d |
| Average gastrocnemius
weight (g) | 0.1315 (0.0066) | 0.1308 (0.0069) |
| Lung weight (g) | 0.1568 (0.0076) | 0.1326 (0.0126) |
| Liver weight (g) | 1.4779 (0.0466) | 1.1640
(0.0485)d |
| Nodules in liver | 2.22 (1.20) | NA |
| Nodules in lung | 6.33 (2.51) | NA |
Tumor growth and metastasis
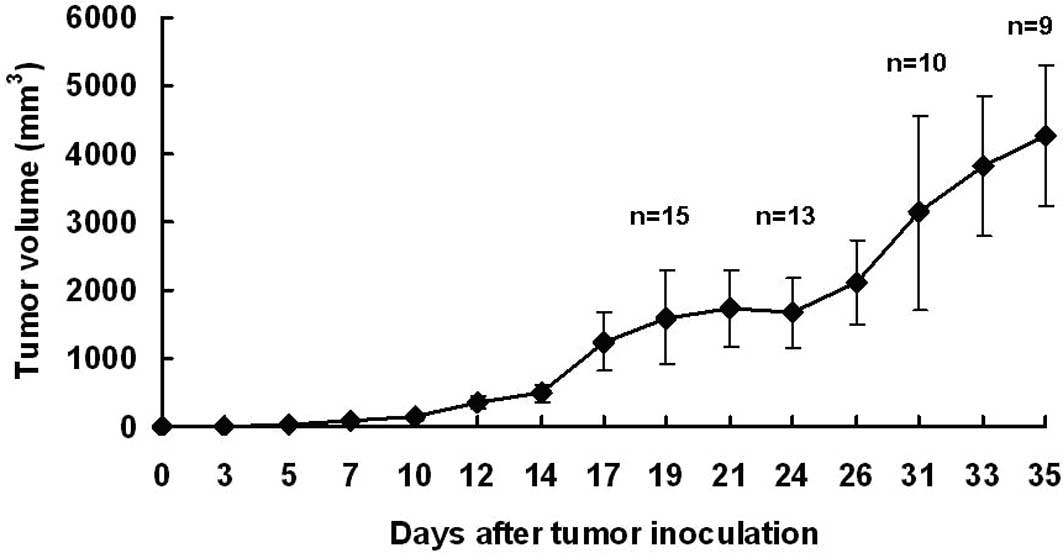
Primary tumor formation was detected 5–7 days after
tumor inoculation, and all mice in the tumor-bearing group
developed tumors. At the end of the experiment, the tumor-bearing
mice had a mean tumor volume of 4264.69±1038.32 mm3
(Fig. 2). After exposing the skin,
the primary tumor was large, vascularized, soft and roughly
spherical in shape. The mean tumor weight was 3.70±0.83 g.
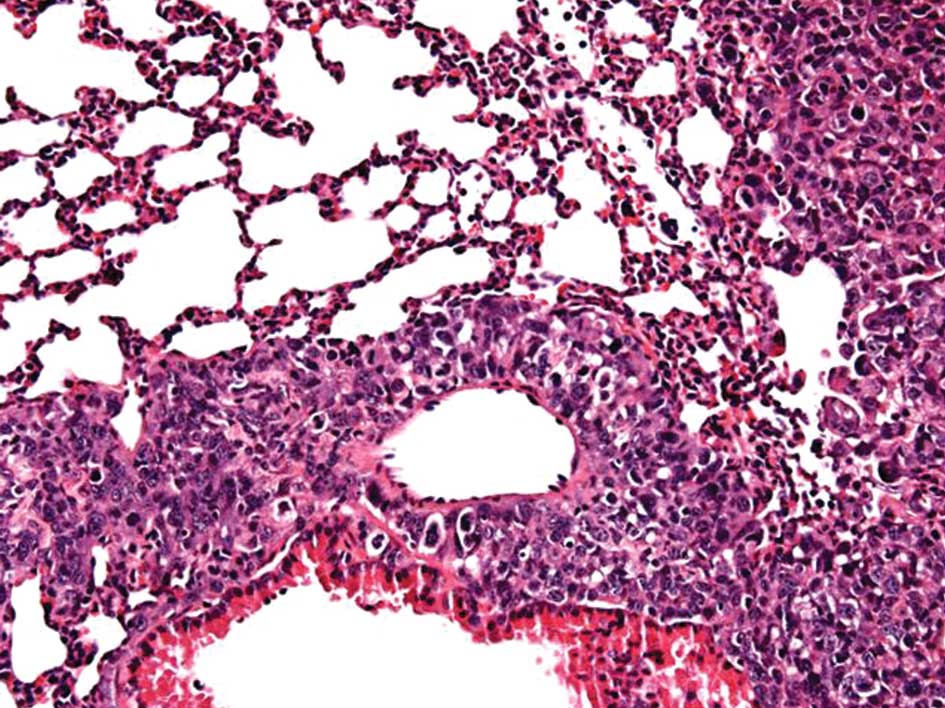
A number of tumor nodules were found in the lungs
and liver (Fig. 3). The mean number
of nodules in the lungs and liver was 6.33 (range 0–20) and 2.22
(range 0–11), respectively (Table
I). The liver weight was higher in the tumor-bearing group than
in the control group (P<0.01) and correlated with the primary
tumor weight (r=0.667, P<0.05). The lung weight was higher in
the tumor-bearing group than in the control group, but no
significant difference was found (P=0.077). The histological
analysis revealed metastasis in the liver and lungs (Fig. 4).
Muscle wasting
No significant differences were found in the average
gastrocnemius muscle weight of the two hindlimbs [0.1315±0.0066 g
(n=9) for the tumor-bearing group vs. 0.1308±0.0069 g (n=5) for the
normal control group], indicating the absence of cancer-induced
wasting of the gastrocnemius muscle.
Serum VEGF-A
GEE equations revealed a significant group effect
(P=0.009), but no time effect (P=0.623) or group-time interaction
(P=0.779). The serum levels of VEGF-A were significantly higher in
the tumor-bearing group (Table
II).
 | Table IISerum VEGF-A expression according to
generalized estimating equation analyses. |
Table II
Serum VEGF-A expression according to
generalized estimating equation analyses.
| Before
inoculation | 7 days
post-inoculation | 21 days
post-inoculation | 35 days
post-inoculation |
|---|
| Tumor-bearing | 188.50±37.30 | 104.56±7.29 | 123.34±23.61 | 111.69±6.93 |
| Normal control | 130.37±20.75 | 88.15±8.41 | 89.32±3.82 | 92.06±14.87 |
Discussion
This study demonstrated that the tumor formation
rate was 100% and that the primary tumor grew significantly
following the subcutaneous inoculation of LLC cells. The
tumor-bearing mice developed significant metastasis in the liver
and lungs, and an increased liver weight was evident. The
tumor-bearing mice gained less body weight following tumor
resection and the serum VEGF-A levels were increased.
The lungs and liver are the most common sites for
the occurrence of metastasis (23).
In the present study, metastasis to the liver and lungs was
considerable and the liver weight in tumor-bearing mice was higher
than that of the control mice. These results are supported by the
study of Argilés et al (10). The different metastatic
specificities may be associated with different extracellular matrix
molecules (24). The lung weight in
tumor-bearing mice was not significantly different from that in the
control mice; this was also found in a study by O’Reilly et
al (4).
In tumor-bearing mice, metastasis in the liver and
lungs and the liver weight were found to increase significantly. At
the same time, body weight gain following tumor resection was lower
than that observed in the control group, which demonstrated that
cancer cachexia was present. The body weight loss in tumor-bearing
mice compared with the control mice in this study may be due to
loss of body fat. Our hypothesis is supported by the findings of
Argilés et al that inoculation of LLC cells results in
decreased adipose tissue mass (10).
Argilés et al revealed marked skeletal muscle
wasting following the injection of LLC cells into hindlimb muscle
(10,25). The skeletal muscle wasting in the
intramuscular implantation was due to increasing protein
degradation (25) and apoptosis
(26). However, the gastrocnemius
muscle weight did not decrease in the present study. In addition to
the different sites of inoculation. As shown in a previous study,
resistance exercise attenuates extensor digitorum longus muscle
wasting following inoculation in mice bearing colon-26
adenocarcinoma (27). We suggest
that the lower extremity muscles received more loading with the
subcutaneous inoculation on the back, which may account for the
different findings in our study. However, identifying the main
factor involved in skeletal muscle wasting in cancer cachexia, and
whether the mechanism of muscle wasting changes or not in different
sites of inoculation is crucial.
VEGF increases endothelial cell permeability and
enhances endothelial cell migration and proliferation (28), rendering it crucial for tumor growth
and angiogenesis (29). VEGF-A
binds the two receptor tyrosine kinases, (RTK) VEGFR1 (Flt-1) and
VEGFR2 (Flk-1/KDR), and transduces signals for angiogenesis. Maniwa
et al reported that micrometastasis was enhanced following
the intraperitoneal injection of VEGF, demonstrating the
significance of VEGF in micrometastatic development (5). Another study reported that anti-VEGF
monoclonal antibody therapy significantly attenuated
micrometastatic tumor growth (30).
These results confirm the significance of VEGF in tumor metastasis.
To study the effects VEGF have on cancer and therapy, we
established a tumor-bearing model and examined the VEGF
concentration with LLC. Similar to patients with hematological
malignancies (31), the
tumor-bearing mice in our study had significantly higher serum
VEGF-A levels than their healthy counterparts. Vicioso et al
demonstrated a positive correlation between the serum VEGF levels
and tumor size in primary breast cancer (32). However, the serum VEGF165 levels did
not correlate with tumor size in HCC (33). Similarly, the serum VEGF-A levels
did not correlate with tumor volume or tumor weight on day 35
post-tumor cell inoculation in our study. VEGF may be secreted from
the tumor, platelets and muscle contraction (19,34).
Poon et al demonstrated that the serum VEGF165
levels/platelet count ratio correlated significantly with tumor
cytosolic VEGF165 (33). Although
the platelet count and the amount of VEGF in the tumor was not
measured, we assume that the tumor is the main source of VEGF.
Furthermore, O’Reilly reported that metastatic growth was
suppressed by a circulating angiostatin (angiogenesis inhibitor)
when a primary tumor was present (4). Other angiogenic or anti-angiogenic
factors should also be considered in future studies.
In conclusion, our tumor-bearing mice had obvious
metastases in the liver and lungs, a lower body-weight gain and
higher VEGF-A levels as compared to the control mice. This animal
model may be employed to study cancer pathophysiology, metastasis
and the effects of intervention.
Acknowledgements
The authors thank the Behavior Core Laboratory of
the Neurobiology and Cognitive Science Center, National Taiwan
University, for technical and operational support.
References
|
1
|
Parkin M, Bray F, Ferlay J and Pisani P:
Global Cancer Statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Department of Health EY R.O.C. (Taiwan).
Statistics of causes of death. Department of Health, Executive
Yuan, R.O.C. (Taiwan); 2008
|
|
3
|
Tisdale MJ: Mechanisms of cancer cachexia.
Physiol Rev. 89:381–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Reilly MS, Holmgren L, Shing Y, et al:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.PubMed/NCBI
|
|
5
|
Maniwa Y, Okada M, Ishii N and Kiyooka K:
Vascular endothelial growth factor increased by pulmonary surgery
accelerates the growth of micrometastases in metastatic lung
cancer. Chest. 114:1668–1675. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savai R, Wolf JC, Greschus S, et al:
Analysis of tumor vessel supply in Lewis lung carcinoma in mice by
fluorescent microsphere distribution and imaging with micro-and
flat-panel computed tomography. Am J Pathol. 167:937–946. 2005.
View Article : Google Scholar
|
|
7
|
Chen HC: Studies on the inhibitory
mechanism of angiogenesis inhibitor, endostatin. Department of
Biological Sciences, National Sun Yat-Sen University; pp.
1082000
|
|
8
|
Rashidi B, Yang M, Jiang P, et al: A
highly metastatic Lewis lung carcinoma orthotopic green fluorescent
protein model. Clin Exp Metastasis. 18:57–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Wu Z, Zuo S, Zhou Y, Chen Y and
Wang X: Establishment of orthotopic Lewis lung cancer model in
mouse. Chin J Lung Cancer. 13:42–47. 2010.PubMed/NCBI
|
|
10
|
Argilés JM, Figueras M, Ametller E, et al:
Effects of CRF2R agonist on tumor growth and cachexia in mice
implanted with Lewis lung carcinoma cells. Muscle Nerve.
37:190–195. 2008.PubMed/NCBI
|
|
11
|
Lippman MM, Laster WR, Abbott BJ, Venditti
J and Baratta M: Antitumor activity of macromomycin B (NSC 170105)
against murine leukemias, melanoma, and lung carcinoma. Cancer Res.
35:939–945. 1975.PubMed/NCBI
|
|
12
|
Li LM, Shin DM and Fidler IJ:
Intrabronchial implantation of the Lewis lung tumor cell does not
favor tumorigenicity and metastasis. Invasion Metastasis.
10:129–141. 1990.PubMed/NCBI
|
|
13
|
Savai R, Langheinrich AC, Schermuly RT, et
al: Evaluation of angiogenesis using micro-computed tomography in a
xenograft mouse model of lung cancer. Neoplasia. 11:48–56.
2009.PubMed/NCBI
|
|
14
|
Zhang Z, Hatori T and Nonaka H: An
experimental model of brain metastasis of lung carcinoma.
Neuropathology. 28:24–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Argiles JM, Figueras M, Ametller E, et al:
Effects of CRF2R agonist on tumor growth and cachexia in mice
implanted with Lewis lung carcinoma cells. Muscle Nerve.
37:190–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harlos JP and Weiss L: Differences in the
peripheries of Lewis lung tumor cells growing in different sites in
the mouse. Int J Cancer. 32:745–750. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan P, Wang J, Lv XL, et al: Prognostic
value of vascular endothelial growth factor expression in patients
with lung cancer: A systematic review with meta-analysis. J Thorac
Oncol. 4:1094–1103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimanuki Y, Takahashi K, Cui R, et al:
Role of serum vascular endothelial growth factor in the prediction
of angiogenesis and prognosis for non-small cell lung cancer. Lung.
183:29–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Dong X, Xu Z, et al: Endostatin gene
therapy enhances the efficacy of paclitaxel to suppress breast
cancers and metastases in mice. J Biomed Sci. 15:99–109. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maillard CM, Bouquet C, Petitjean MM, et
al: Reduction of brain metastases in plasminogen activator
inhibitor-1-deficient mice with transgenic ocular tumors.
Carcinogenesis. 29:2236–2242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hotz HG, Hines OJ, Masood R, et al: VEGF
antisense therapy inhibits tumor growth and improves survival in
experimental pancreatic cancer. Surgery. 137:192–199. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited–the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535.
2011.
|
|
24
|
Chung DC, Zetter BR and Brodt P: Lewis
lung carcinoma variants with differing metastatic specificities
adhere preferentially to different defined extracellular matrix
molecules. Invasion Metastasis. 8:103–117. 1988.
|
|
25
|
Llovera M, Garcia-Martinez C,
Lopez-Soriano J, et al: Protein turnover in skeletal muscle of
tumour-bearing transgenic mice overexpressing the soluble TNF
receptor-1. Cancer Lett. 130:19–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Royen M, Carbo N, Busquets S, et al:
DNA fragmentation occurs in skeletal muscle during tumor growth: A
link with cancer cachexia? Biochem Biophys Res Commun. 270:533–537.
2000.PubMed/NCBI
|
|
27
|
Al-Majid S and McCarthy DO: Resistance
exercise training attenuates wasting of the extensor digitorum
longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res
Nurs. 2:155–166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta MK and Qin RY: Mechanism and its
regulation of tumor-induced angiogenesis. World J Gastroenterol.
9:1144–1155. 2003.PubMed/NCBI
|
|
29
|
Wicki A and Christofori G: The angiogenic
switch in tumorigenesis. Tumor angiogenesis. Marme D and Fusenig N:
Springer Berlin; Heidelberg, New York: pp. 67–88. 2008, View Article : Google Scholar
|
|
30
|
Austin K, Sookhai S, Wang J, Pedmond K,
Kirwan W and Pedmond H: Anti-angiogenic therapy attenuates
micrometastatic tumour growth in a Lewis lung carcinoma model. Ir J
Med Sci. 170:112001. View Article : Google Scholar
|
|
31
|
Erdem F, Gündogdu M and Kiziltunç A: Serum
vascular endothelial growth factor level in Patients with
hematological malignancies. Eur J Gen Med. 3:116–120. 2006.
|
|
32
|
Vicioso L, Gonzalez FJ, Alvarez M, et al:
Elevated serum levels of vascular endothelial growth factor are
associated with tumor-associated macrophages in primary breast
cancer. Am J Clin Pathol. 125:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poon RT, Lau CP, Cheung ST and Yu WC:
Quantitative correlation of serum levels and tumor expression of
vascular endothelial growth factor in patients with hepatocellular
carcinoma. Cancer Res. 63:6121–6126. 2003.PubMed/NCBI
|
|
34
|
Kivela R, Silvennoinen M, Lehti M, Jalava
S, Vihko V and Kainulainen H: Exercise-induced expression of
angiogenic growth factors in skeletal muscle and in capillaries of
healthy and diabetic mice. Cardiovasc Diabetol. 7:132008.
View Article : Google Scholar : PubMed/NCBI
|