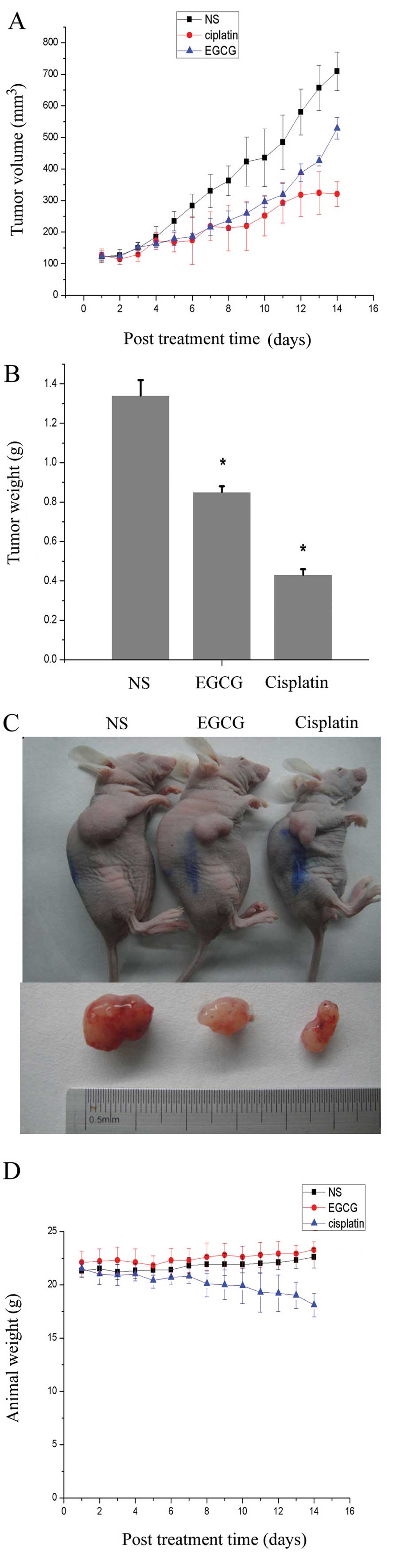
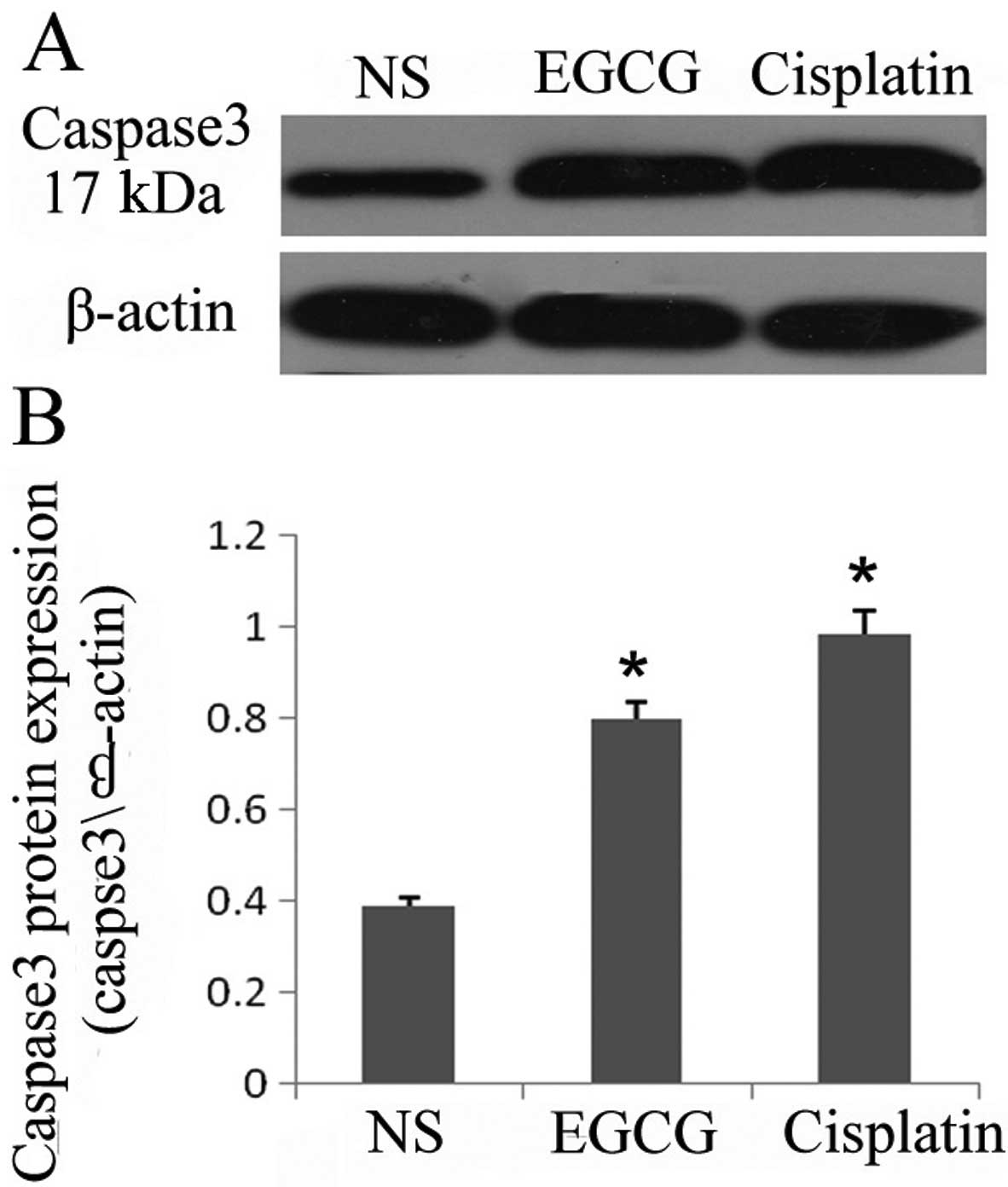
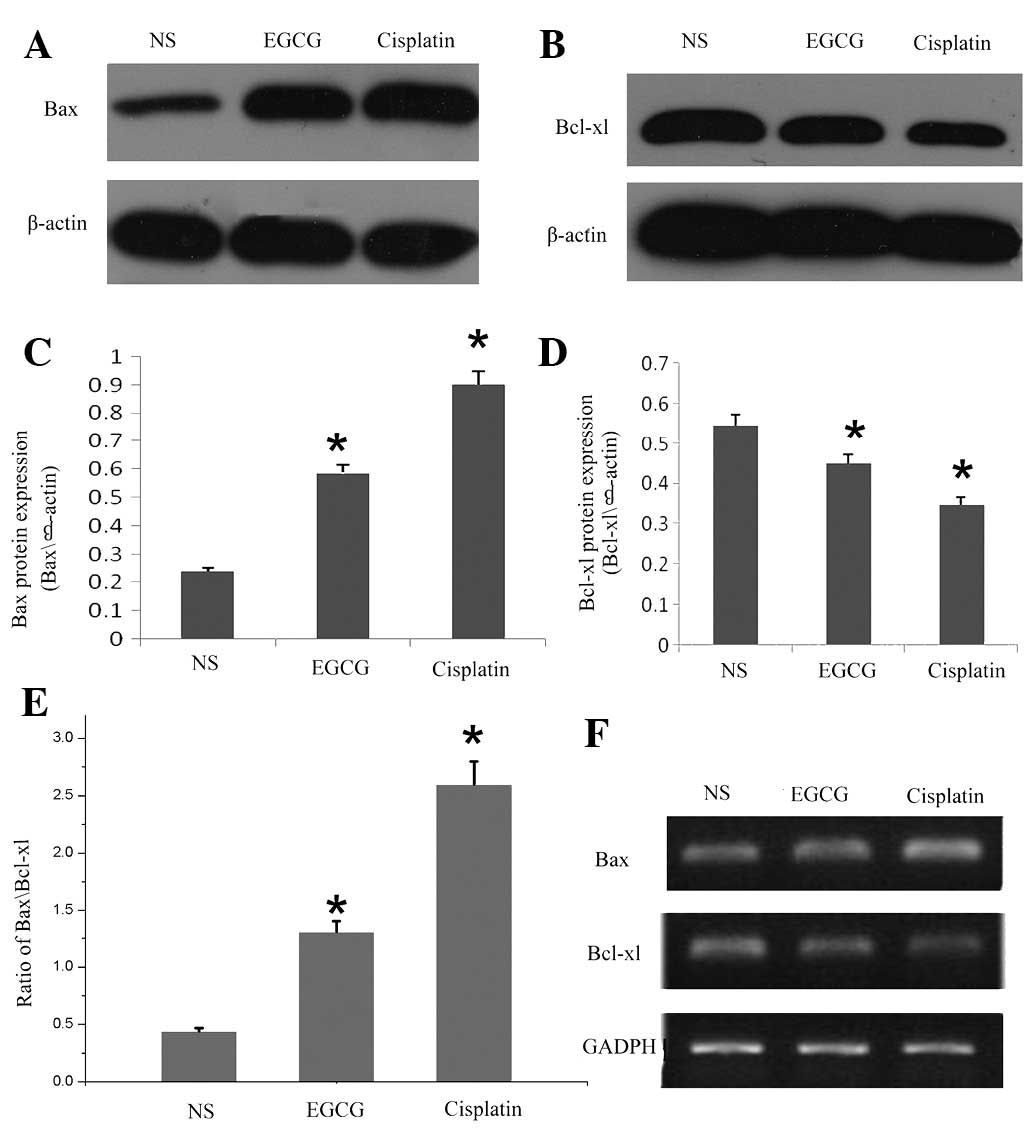
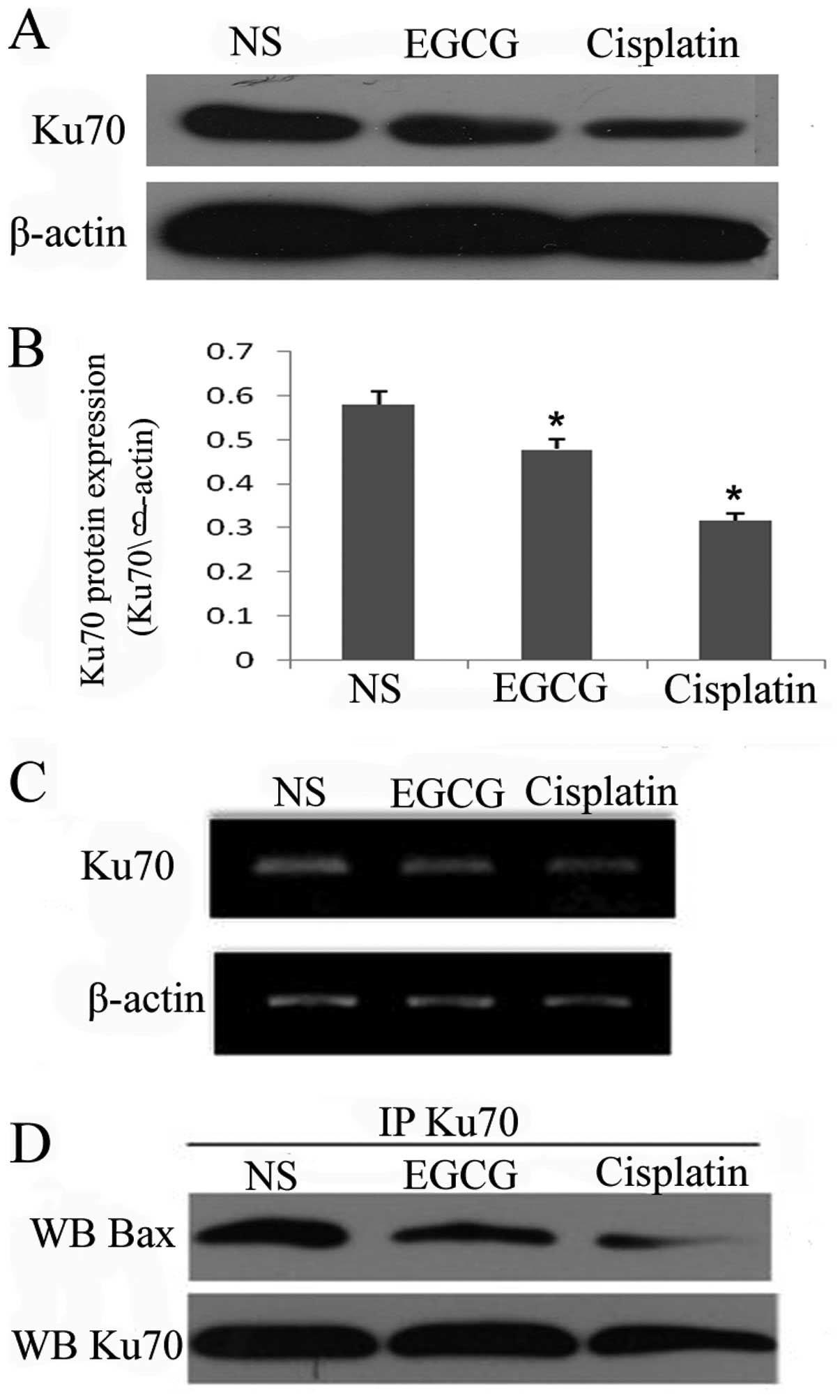
|
1
|
Lu YP, Lou YR, Xie JG, et al: Topical
applications of caffeine or (−)-epigallocatechin gallate (EGCG)
inhibit carcinogenesis and selectively increase apoptosis in
UVB-induced skin tumors in mice. Proc Natl Acad Sci USA.
99:12455–12460. 2002.
|
|
2
|
Syed DN, Afaq F, Kweon MH, et al: Green
tea polyphenol EGCG suppresses cigarette smoke condensate-induced
NF-kappaB activation in normal human bronchial epithelial cells.
Oncogene. 26:673–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee MH, Han DW, Hyon SH and Park JC:
Apoptosis of human fibrosarcoma HT-1080 cells by
epigallocatechin-3-O-gallate via induction of p53 and caspases as
well as suppression of Bcl-2 and phosphorylated nuclear
factor-kappaB. Apoptosis. 16:75–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukamoto S, Hirotsu K, Kumazoe M, et al:
Green tea polyphenol EGCG induces lipid-raft clustering and
apoptotic cell death by activating protein kinase Cdelta and acid
sphingomyelinase through a 67 kDa laminin receptor in multiple
myeloma cells. Biochem J. 443:525–534. 2012. View Article : Google Scholar
|
|
5
|
Adachi S, Nagao T, Ingolfsson HI, et al:
The inhibitory effect of (−)-epigallocatechin gallate on activation
of the epidermal growth factor receptor is associated with altered
lipid order in HT29 colon cancer cells. Cancer Res. 67:6493–6501.
2007.
|
|
6
|
Zhang G, Wang Y, Zhang Y, et al:
Anti-cancer activities of tea epigallocatechin-3-gallate in breast
cancer patients under radiotherapy. Curr Mol Med. 12:163–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei LH, Kuo ML, Chen CA, et al:
Interleukin-6 promotes cervical tumor growth by VEGF-dependent
angiogenesis via a STAT3 pathway. Oncogene. 22:1517–1527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Hao MW, Dong K, Lin F, Ren JH and
Zhang HZ: Apoptosis induction effects of EGCG in laryngeal squamous
cell carcinoma cells through telomerase repression. Arch Pharm Res.
32:1263–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Featherstone C and Jackson SP: Ku, a DNA
repair protein with multiple cellular functions. Mutat Res.
434:3–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez JJ, Seveau S, Veiga E, Matsuyama
S and Cossart P: Ku70, a component of DNA-dependent protein kinase,
is a mammalian receptor for Rickettsia conorii. Cell.
123:1013–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kusano K, Johnson-Schlitz DM and Engels
WR: Sterility of Drosophila with mutations in the Bloom syndrome
gene -complementation by Ku70. Science. 291:2600–2602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pace P, Mosedale G, Hodskinson MR, Rosado
IV, Sivasubramaniam M and Patel KJ: Ku70 corrupts DNA repair in the
absence of the Fanconi anemia pathway. Science. 329:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du T, Caragounis A, Parker SJ, et al: A
potential copper-regulatory role for cytosolic expression of the
DNA repair protein XRCC5. Free Radic Biol Med. 51:2060–2072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tonotsuka N, Hosoi Y, Miyazaki S, et al:
Heterogeneous expression of DNA-dependent protein kinase in
esophageal cancer and normal epithelium. Int J Mol Med. 18:441–447.
2006.PubMed/NCBI
|
|
15
|
Hosoi Y, Watanabe T, Nakagawa K, et al:
Up-regulation of DNA-dependent protein kinase activity and Sp1 in
colorectalcancer. Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
16
|
Gullo C, Au M, Feng G and Teoh G: The
biology of Ku and its potential oncogenic role in cancer. Biochim
Biophys Acta. 1765:223–234. 2006.PubMed/NCBI
|
|
17
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sawada M, Hayes P and Matsuyama S:
Cytoprotective membrane-permeable peptides designed from the
Bax-binding domain of Ku70. Nat Cell Biol. 5:352–357. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baliga MS, Meleth S and Katiyar SK: Growth
inhibitory and antimetastatic effect of green tea polyphenols on
metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and
in vivo systems. Clin Cancer Res. 11:1918–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milligan SA, Burke P, Coleman DT, et al:
The green tea polyphenol EGCG potentiates the antiproliferative
activity of c-Met and epidermal growth factor receptor inhibitors
in non-small cell lung cancer cells. Clin Cancer Res. 15:4885–4894.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thakur VS, Ruhul AAR, Paul RK, et al:
p53-Dependent p21-mediated growth arrest pre-empts and protects
HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer
Lett. 296:225–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onoda C, Kuribayashi K, Nirasawa S, et al:
(−)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by down-regulating survivin expression. Int J Oncol.
38:1403–1408. 2011.
|
|
23
|
Li GX, Chen YK, Hou Z, et al:
Pro-oxidative activities and dose-response relationship of
(−)-epigallocatechin-3-gallate in the inhibition of lung cancer
cell growth: a comparative study in vivo and in vitro.
Carcinogenesis. 31:902–910. 2010.
|
|
24
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lessene G, Czabotar PE and Colman PM:
BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang HG, Jenabi JM, Liu XF, Reynolds CP,
Triche TJ and Sorensen PH: Inhibition of the insulin-like growth
factor I receptor by epigallocatechin gallate blocks proliferation
and induces the death of Ewing tumor cells. Mol Cancer Ther.
9:1396–1407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balasubramanian S, Adhikary G and Eckert
RL: The Bmi-1 polycomb protein antagonizes the
(−)-epigallocatechin-3-gallate-dependent suppression of skin cancer
cell survival. Carcinogenesis. 31:496–503. 2010.PubMed/NCBI
|
|
30
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weterings E and van GDC: The mechanism of
non-homologous end-joining: a synopsis of synapsis. DNA Repair
(Amst). 3:1425–1435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chou CH, Wang J, Knuth MW and Reeves WH:
Role of a major autoepitope in forming the DNA binding site of the
p70 (Ku) antigen. J Exp Med. 175:1677–1684. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X and Lieber MR: Protein-protein and
protein-DNA interaction regions within the DNA end-binding protein
Ku70-Ku86. Mol Cell Biol. 16:5186–5193. 1996.PubMed/NCBI
|
|
34
|
Wang J, Dong X, Myung K, Hendrickson EA
and Reeves WH: Identification of two domains of the p70 Ku protein
mediating dimerization with p80 and DNA binding. J Biol Chem.
273:842–848. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Dong X and Reeves WH: A model for
Ku heterodimer assembly and interaction with DNA. Implications for
the function of Ku antigen. J Biol Chem. 273:31068–31074. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soderlund LK, Queseth S, Fornander T and
Askmalm MS: Low expression of Ku70/80, but high expression of
DNA-PKcs, predict good response to radiotherapy in early breast
cancer. Int J Oncol. 37:1547–1554. 2010.PubMed/NCBI
|
|
37
|
Zhang F, Zhang T, Qu Y, et al:
Replication-dependent gamma-H2AX formation is involved in
docetaxel-induced apoptosis in NSCLC A549 cells. Oncol Rep.
24:1297–1305. 2010.PubMed/NCBI
|
|
38
|
Busser B, Sancey L, Josserand V, et al:
Amphiregulin promotes resistance to gefitinib in nonsmall cell lung
cancer cells by regulating Ku70 acetylation. Mol Ther. 18:536–543.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sawada M, Hayes P and Matsuyama S:
Cytoprotective membrane-permeable peptides designed from the
Bax-binding domainof Ku70. Nat Cell Biol. 5:352–357. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramanian C, Jarzembowski JA, Opipari AW
Jr, Castle VP and Kwok RP: HDAC6 deacetylates Ku70 and regulates
Ku70-Bax binding in neuroblastoma. Neoplasia. 13:726–734.
2011.PubMed/NCBI
|
|
41
|
Cohen HY, Lavu S, Bitterman KJ, et al:
Acetylation of the C terminus of Ku70 by CBP and PCAF controls
Bax-mediated apoptosis. Mol Cell. 13:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cohen HY, Miller C, Bitterman KJ, et al:
Calorie restriction promotes mammalian cell survival by inducing
the SIRT1 deacetylase. Science. 305:390–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Subramanian C, Opipari AW Jr, Bian X,
Castle VP and Kwok RP: Ku70 acetylation mediates neuroblastoma cell
death induced by histone deacetylase inhibitors. Proc Natl Acad Sci
USA. 102:4842–4847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Busser B, Sancey L, Josserand V, et al:
Amphiregulin promotes resistance to gefitinib in nonsmall cell lung
cancer cells by regulating Ku70 acetylation. Mol Ther. 18:536–543.
2010. View Article : Google Scholar : PubMed/NCBI
|