Introduction
Green tea, the second most popular beverage
worldwide, has potential chemotherapeutic effects against a wide
range of malignancies. Epidemiological and rodent carcinogenesis
studies have provided evidence that green tea, particularly its
major constituent, epigallocatechin-3-gallate (EGCG) has various
anticancer effects, including inhibition of carcinogen-induced
mutagenesis (1,2), induction of cell cycle arrest
(3), induction of apoptosis
(4), inhibition of growth
factor-mediated proliferation (5),
inhibition of transformation (6),
inhibition of angiogenesis (7) and
inhibition of telomerase activity (8). To date, there have been no studies
examining Ku70 in lung cancer; however, such studies may lead to
new approaches in the treatment of lung cancer.
Ku70 was first characterized as part of the
Ku70/Ku80 heterodimer that is essential for the repair of DNA
double-strand breaks (DSBs) by the nonhomologous end-joining (NHEJ)
pathway and rearrangement of antibody and T cell receptor genes via
V(D) J recombination (9). It has
been associated with numerous diseases, including Rickettsia
conorii infection (10),
sterility (11), Fanconi anemia
(12) and cancer (13). It is a highly versatile regulatory
protein that has been implicated in several nuclear processes,
including DNA repair, telomere maintenance and apoptosis.
Accordingly, Ku70 is considered to play a vital role in the
maintenance of chromosomal integrity and cell survival. A study has
suggested that there is a positive correlation between Ku70 and the
development of cancer (14),
indicating that Ku70 is an important candidate target for
anti-cancer drug development. Specifically, further studies have
suggested that a delicate balance exists in Ku70 expression, with
overexperssion of Ku70 leading to genomic instability and
tumorigenesis (15,16).
Another study demonstrated that Ku70 is able to
suppress apoptosis by sequestering Bax from the mitochondria in
cancer tissues (17). In contrast,
when Ku70 is released from Bax, it allows Bax to translocate to the
mitochondria and trigger cytochrome C release, leading to
caspase-dependent apoptosis. These studies suggest that Ku70
degradation is a necessary step in the activatation of Bax-mediated
apoptosis. Further analysis revealed that Bax inhibition peptide
(BIP), which is comprised of five amino acids designed from the
Bax-binding domain of Ku70, is able to inhibit Bax-mediated
apoptosis and suppress the mitochondrial translocation of Bax
(18). Pretreatment of Hela cells
with BIP peptides for 1 h was sufficient to provide protection from
staurosporine (STS)-and ultraviolet C (UVC)-induced apoptosis
(18).
However, the exact role of Ku70 in the anticancer
mechanism of EGCG remains unknown. In vivo animals are
considered a gold standard in chemotherapy studies, as they provide
clear indications on the pharmacological and therapeutic effects of
chemotherapy agents, which may then be extrapolated to humans, as
examined in this study.
Materials and methods
Chemicals and antibodies
Purified EGCG (>95% pure) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The following antibodies
against various proteins were obtained; Ku70 mouse monoclonal
antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax
rabbit polyclonal antibodies (Cell Signaling Technology, Beverly,
MA, USA), Bcl-xl (Proteintech Group, Chicago, IL, USA), cleaved
caspase 3 (Assay Technology Inc., Livermore, CA, USA),
pan-acetylated-lysine (Santa Cruz Biotechnology) and β-actin
(Sigma-Aldrich). Goat anti-rabbit IgG-horseradish peroxidase (HRP)
conjugates and goat anti-mouse IgG-HRP conjugates were purchased
from Sigma-Aldrich.
Cell lines and cell culture
The human lung cancer A549 cell line was purchased
from the Cell Center of Central South University, Hunan, China. The
cells were cultured as a monolayer in 10% fetal bovine serum
(FBS)-supplemented RPMI-1640 containing 100 units/ml penicillin and
100 Ag/ml streptomycin and were maintained in a humidified
atmosphere of 95% air and 5% CO2 at 37°C.
Animal experiments
A total of 24, 4- to 6-week-old, female BALB/c mice
were kept in groups of six per cage and provided with food and
water ad libitum. The animals were acclimatized for 1 week
before use and maintained throughout at standard conditions: 24±2°C
temperature, 50±10% relative humidity and 12-h light/12-h dark
cycle.
A549 cells were detached from the culture dishes by
trypsinization and then collected, washed and resuspended in
RPMI-1640. To establish A549 tumor xenografts, mice were injected
s.c. with 1–2×106 A549 cells in the right flanks. The
mice were then randomly divided into three groups, each consisting
of 8 animals. Animals in group I (control) received normal saline
at 10 ml/kg, i.p. daily for 2 weeks; group II received cisplatin at
4 mg/kg, i.p. Q4dX3; and group III received EGCG at 50 mg/kg, i.p.
daily for 2 weeks. Body weight was recorded each day throughout the
study. Once xenografts started growing, their sizes were measured
each day using Vernier calipers. The tumor volume was calculated
using the formula: Volume = π/6 × length × width2. When
the tumors reached a volume of 100 mm3, the animals were
administered different drugs. At the termination of the experiment,
animals were sacrificed, and the tumors were surgically removed,
weighed and then stored at −80°C for further biochemical
analysis.
All animals were housed and handled according to
Central South University Institutional Animal Care and Use
Committee guidelines and animal work was approved by the
appropriate committee. All experiments were performed according to
institutional guidelines and approved by the Ethics Committees of
our hospital and conducted in accordance with the ethical
guidelines of the Declaration of Helsinki.
Western blot analysis and
immunoprecipitation
Western blot analysis was conducted to determine the
expression of different proteins. Tumor tissues were collected at
the termination of the experiment, minced, homogenized with
homogenizer in ice-cold lysis buffer and lysed with ice-cold NP-40
lysis buffer for 30 min, then centrifuged at 14,000 × g for 20 min
at 4°C. The supernatant was collected and either used immediately
or stored at −80°C to examine the expression of different proteins
using western blot analysis and immunoprecipitation. Protein
concentration was determined using a BCA protein assay kit
according to the manufacturer’s instructions.
Western blot analysis was conducted to analyze the
expression of various proteins following the manufacturer’s
instructions. Briefly, aliquots of equal amounts of protein (40–80
μg) from the tumor lysates were subjected to 12% SDS-PAGE
electrophoresis and transferred to PVDF membranes. The membranes
were blocked with blocking buffer by incubating for 1 h at room
temperature, then probed overnight with the desired primary
antibody at 4°C. Following washing, the membranes were incubated
with the respective HRP-conjugated secondary antibody for 1 h at
room temperature. After further washing, protein expression was
detected by enhanced chemiluminescence detection systems (Thermo
Scientific, Waltham, MA, USA).
For immunoprecipitation of Ku70, 1 mg protein was
precleared by incubation with protein A/G Sepharose beads (Santa
Cruz Biotechnology). The supernatant was incubated with
agarose-conjugated anti-Ku70 antibody, followed by three washes in
1% Triton in PBS. The immunocomplex was separated by SDS-PAGE and
proteins were detected with anti-pan-acetyllysine (panAc-K)
antibody or anti-Bax antibody.
Reverse transcription (RT)-PCR
RT-PCR analysis was conducted to determine the
expression of different mRNA. Tumor mRNA from different groups was
extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). RT-PCR was
performed with primer pairs for Bax: forward, 5′-AGCTCT
GAGCAGATCATGAAG-3′ and reverse, 5′-GGTGGACGC ATCCTGAG-3′; for
Bcl-xl: forward, 5′-ACTGTGCGT GGAAAGCGTAG-3′ and reverse,
5′-AAAAGTATCCCA GCCGCC-3′; for Ku70: forward, 5′-TCTTGGCTGTGG
TGTTCTATGGT-3′ and reverse, 5′-GAGTGAGTAGTC AGATCCGTGGC-3′; for
GADPH: forward, 5′-AGAAGG CTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCAT CCACAGTCTTC-3′. The standard PCR conditions were: 95°C
for 15 min and then 35 cycles at 95°C for 30 sec, 55°C for 30 sec
and 72°C for 30 sec.
Statistical analysis
Data are presented as the means and standard
deviations (SDs). Groups were compared by a one-way analysis of
variance (ANOVA). P<0.05 was considered to indicate a
statistically significant difference.
Results
EGCG inhibits in vivo growth of A549 lung
cancer cells
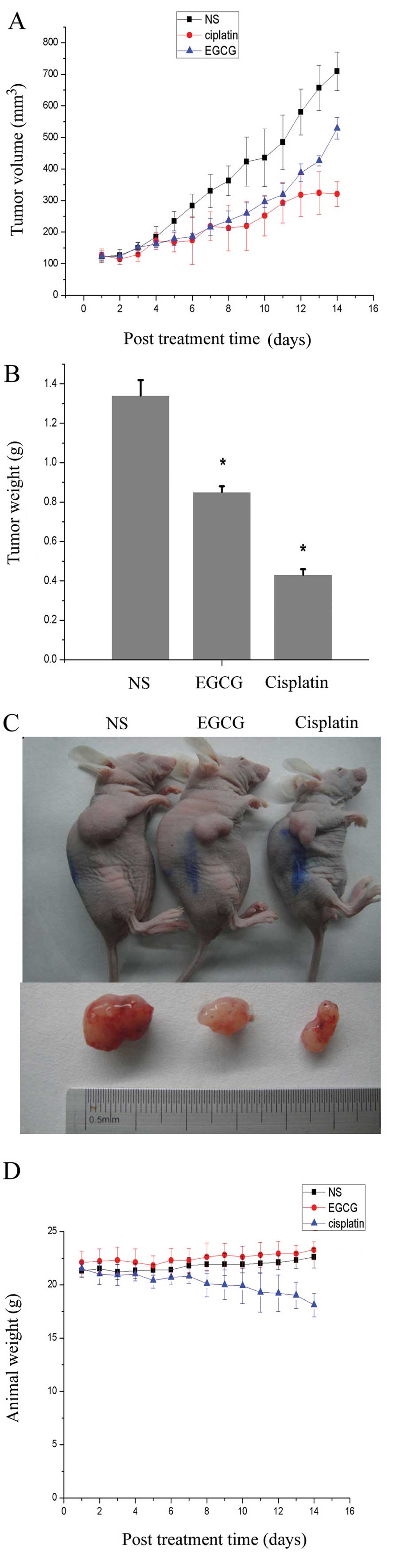
The rate of tumor growth of A549 cells, evaluated by
measuring tumor volume at regular intervals, was decreased in
animals administered EGCG and cisplatin, compared with NS control
animals (Fig. 1A). Administration
of EGCG resulted in a 37% inhibition in tumor weight when recorded
at the termination of the experiment, while the inhibition in the
cisplatin group was 50% (Fig. 1B).
As observed in Fig. 1C, EGCG
inhibited the tumor growth; however, the efficiency was lower than
cisplatin, the classic chemotherapy agent of major cancers.
However, due to the toxicity of drugs, the weight of the animals
was decreased, and the average weight of the EGCG treatment animals
was 23.3 g, equal to the NS control group, while the cisplatin
treatment animals had a lower weight of only 18.1 g (Fig. 1D). It was also demonstrated that the
toxicity of EGCG was milder than cisplatin.
EGCG inhibits the surrogate markers of
proliferation and apoptosis (caspase 3) in A549-induced tumors in
BALB/c mice
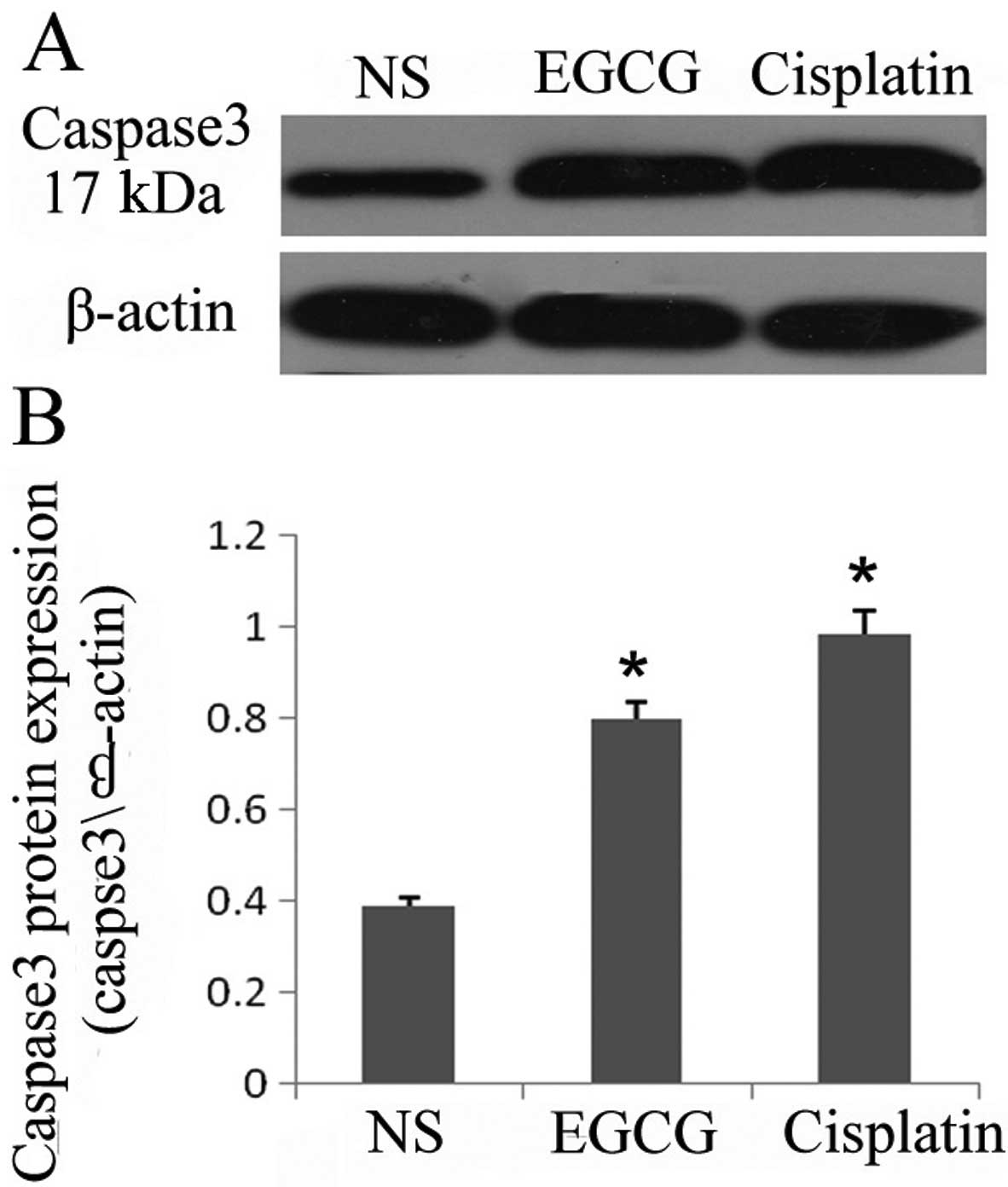
As treatment of EGCG inhibited the cell
proliferation and viability in the in vivo system, we
examined the effect of EGCG on markers of cell proliferation by
assessing the protein expression of activated caspase 3 in the
tumors. Cleaved caspase 3 is considered a hallmark of apoptosis. As
determined by western blot analysis, the level of cleaved caspase 3
in the tumors was markedly increased in EGCG- and cisplatin-treated
animals compared with the NS-treated animals (Fig. 2A and B). The expression of the basal
level of caspase 3 was not detectable in the tumors as the
antibodies that were used only recognized cleaved caspase 3. These
observations support the evidence that administration of EGCG
inhibited tumor growth possibly through the induction of apoptosis
in A549 tumor cells. The administration of EGCG may affect the
expression of activated caspase 3 in tumor-bearing mice.
EGCG downregulates the expression of
Bcl-xl and upregulates the expression of Bax mRNA and protein in
tumors in vivo
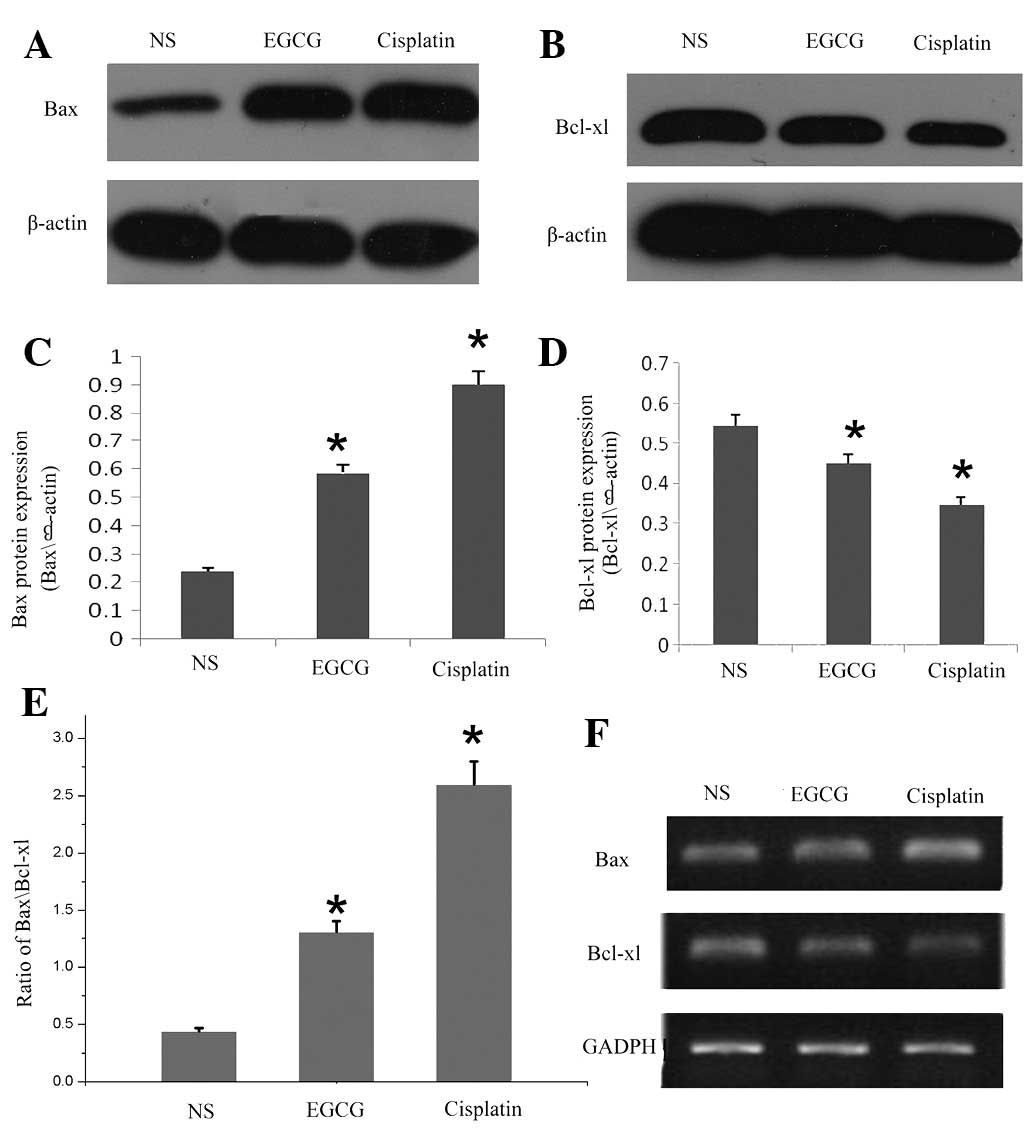
Furthermore, we examined the effect of EGCG on the
apoptotic protein involved in mitochondrial disruption pathway in
in vivo tumor development. As shown in Fig. 3A, B, C and D, western blot analysis
revealed that treatment with EGCG downregulated the expression of
antiapoptotic protein Bcl-xl, but increased the expression of
proapoptotic protein Bax. As shown in Fig. 3E, the increase in the ratio of
Bax/Bcl-xl in in vivo tumors suggested that the
susceptibility of tumor growth was blocked or inhibited in
EGCG-treated BALB/c mice. In addition, using RT-PCR, we also
assessed the effect of EGCG on the mRNA expression of Bax and
Bcl-xl during apoptosis. As indicated by the western blot result,
EGCG induced a decrease in the expression of mRNA coding for Bcl-xl
and increased Bax mRNA (Fig.
3F).
EGCG downregulates the expression of Ku70
mRNA and protein and interrupts the binding of Ku70 and Bax
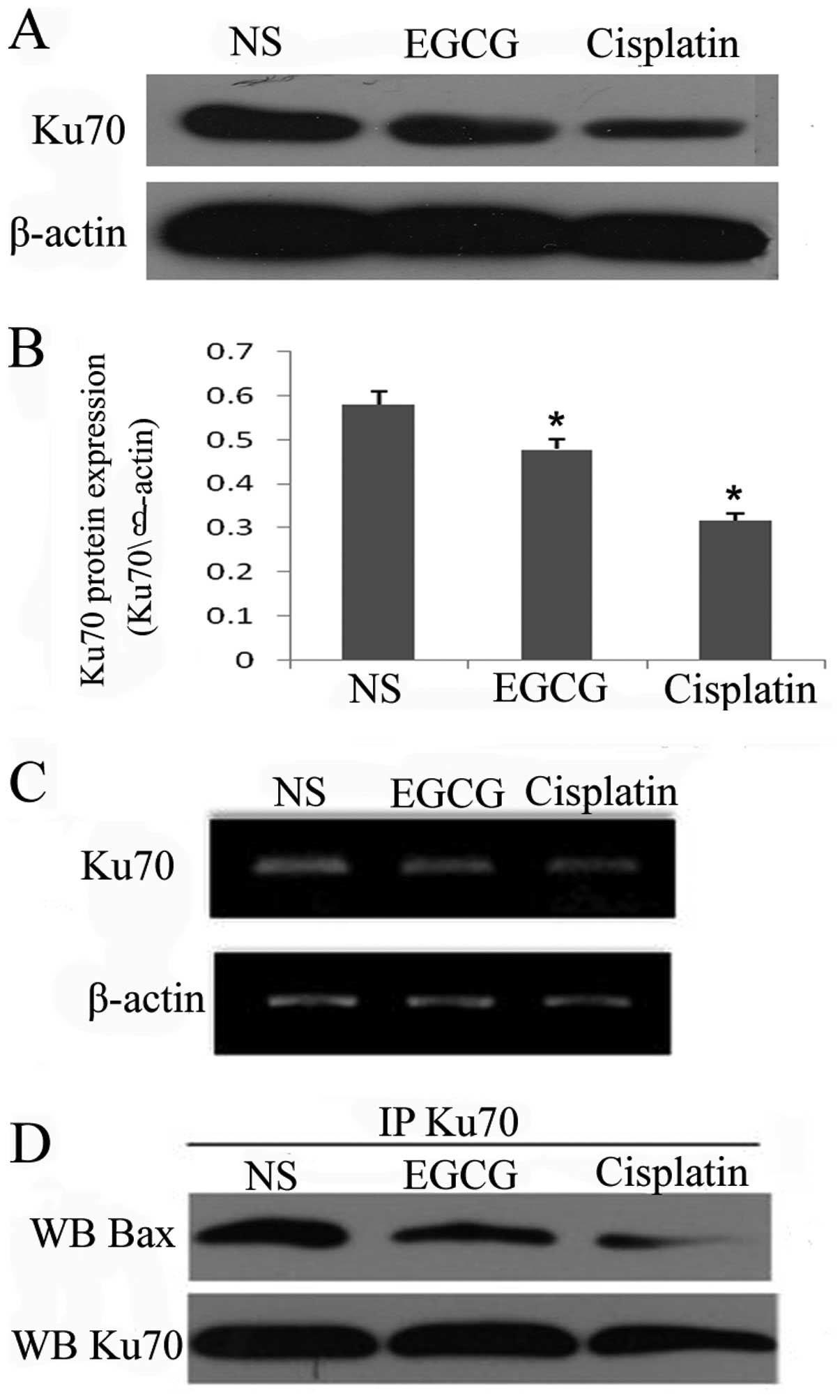
Ku70 is a multifunctional protein playing roles in
DNA repair and cell survival and it has been demonstrated to
inhibit Bax-mediated cell death by binding Bax. As mentioned above,
EGCG was able to upregulate the expression of Bax protein and mRNA,
therefore, we examined the effect of EGCG on Ku70. Western blot
(Fig. 4A and B) and RT-PCR
(Fig. 4C) analysis revealed that
EGCG treatment is able to decrease the expression of Ku70 mRNA and
protein, similar to cisplatin. To determine whether the Bax-Ku70
interaction was affected by EGCG treatment, the protein was
co-immunoprecipitated with Bax antibody. Notably, Ku70-Bax
complexes decreased in the EGCG and cisplatin groups compared with
the NS group (Fig. 4D). These
results suggest that EGCG is able to decrease the expression of
Ku70 and interrupt the binding between Bax and Ku70.
Discussion
In this study, we identified that EGCG, the major
polyphenolic agent present in green tea, inhibited the growth of
lung cancer A549 cells in vivo, which was consistent with
previous studies indicating that EGCG inhibited growth and
apoptosis in human lung, colon, gastric, prostate and mammary
carcinoma (19–23). At present, a number of agents are
used to clinically treat carcinoma, including cisplatin,
gemcitabine and paclitaxel. However, most of these agents have
numerous toxicities and side-effects, including bone marrow
depression, loss of weight, gastrointestinal reaction and hepatic
function failure, which may cause great agony to patients and have
a negative impact on their daily life. In animals, loss of weight
is the best marker to evaluate the toxicities and side-effects of
an agent. In our study, mice treated with cisplatin had lower
weight due to the toxicities. Inversely, in the EGCG-treated group,
the weight of the animals was equal to the control group. The
present study also revealed that EGCG had less toxicities and
side-effects than the existing anticancer agents in human normal
cells, while it was able to induce apoptosis and arrest the cell
cycle in human carcinoma cells (24). Due to its wide range of
pharmacological properties and reduced toxicities and side-effects,
EGCG is one of the most promising chemotherapy agents for
cancer.
Many of the molecular alterations that accompany
carcinogenesis lead to uncontrolled proliferation and the ability
of transformed cells to evade apoptosis. Apoptosis is a genetically
controlled mechanism of cell death involved in the regulation of
tissue homeostasis. The Bcl-2 protein family plays an important
role in the control of apoptosis (25,26).
Bcl-2 and Bcl-xl are the prototypes of this family and inhibit the
induction of apoptosis, while Bax and Bad are proapoptotic. High
concentrations of Bcl-2 or Bcl-xl affect cell susceptibility to the
induction of apoptosis by altering the ratio of death promoters to
suppressors, providing tumor cells with a survival advantage and
permitting expansion of transformed cells harboring mutations
within their genome. The Bcl-xl protein and other family members
locate at the intracellular organelles, including the endoplasmic
reticulum and the outer mitochondrial and nuclear membranes, where
they modulate responses to diverse death stimuli (27). In Ewing family tumor (EFT) cells,
apoptosis by EGCG correlated with altered expression of Bcl-2
family proteins, including increased expression of proapoptotic Bax
and decreased expression of prosurvival Bcl-2, Bcl-xl and Mcl-1
proteins (28). In addition, EGCG
enhanced apoptosis as demonstrated by an increase in the Bax:Bcl-xl
ratio, cleavage of procaspases 3, 8 and 9 and poly (adenosine
diphosphate ribose) polymerase and accumulation of subG1 cells in
skin cancer cells (29). In our
study, we detected the protein and mRNA expression of the Bcl
family. Treatment with EGCG resulted in downregulation of the
expression of the antiapoptotic protein Bcl-xl, while it increased
the expression of proapoptotic protein Bax. The ratio of Bax/Bcl-xl
in lung cancer increased compared with the control group, and EGCG
increased the expression of activated caspase 3 in tumor-bearing
mice. In the human head and neck squamous cell carcinoma cell line,
EGCG also induced apoptosis via a mitochondrial pathway, revealing
that EGCG caused a decrease in the Bcl-2 and Bcl-xl proteins, as
well as an increase in Bax protein and activation of caspase 9
(3). Together, these results
suggest that EGCG induces carcinoma cell apoptosis via the
mitochondrial pathway.
As mentioned above, EGCG is able to regulate a
number of molecules involved in cell apoptosis and cell cycle
arrest, including Bax-mediated apoptosis. The mechanism of how Bax
is kept inactive remained unclear until 2003, when yeast-based
functional screening of Bax inhibitors from mammalian cDNA
libraries identified Ku70 as a new Bax suppressor. Bax-mediated
apoptosis was suppressed by overexpression of Ku70 in mammalian
cells, but enhanced by downregulation of Ku70 (30). Ku70 forms a heterodimeric Ku protein
complex with Ku80, which represents a crucial component of the NHEJ
DNA double-strand break (DSB) repair machinery (31). In cooperation with Ku80, Ku70 binds
and bridges two proximal broken DNA ends, which facilitates DNA
end-joining through a cascade of reactions that involve
DNA-dependent protein kinase and DNA ligase IV. Ku70 contains two
DNA-binding domains at NH2 and COOH termini, both of which are
required for the high affinity to DNA (32–35).
In early breast cancer patients, low expression of Ku70/Ku80
predicts a good response to radiotherapy (36). Wortmannin pretreatment of A549 cells
causes increased apoptosis induced by docetaxel, due to the
inhibition of the DNA repair process by wortmannin, and
downregulation of DNA repair proteins, including Ku70 (37).
In the present study, for the first time, we reveal
that the induction of apoptosis by EGCG may be caused by the
down-regulation of Ku70. Knockdown of Ku70 has been previously
found to enhance gefitinib-induced death in NSCLC cells (38). In addition, recent evidence
indicates that Ku70 interacts with Bax, and that the carboxyl
terminus of Ku70 and the amino terminus of Bax are required for
this interaction (30), and the
binding of Ku70 and Bax suppresses its apoptotic translocation to
the mitochondria (39). In
tumorigenic neuroblastic cell models of NB, the disruption of Ku70
binding to Bax caused activated Bax to translocate from the cytosol
to the mitochondria and trigger cell death (40). In order to explore the effect of
EGCG on the interaction between Ku70 and Bax,
co-immunoprecipitation experiments of Ku70 and Bax were performed.
Our data also demonstrated that EGCG is able to disrupt the
interaction between Ku70 and Bax.
However, the molecular mechanisms of how EGCG
regulates the expression of Ku70 was not explored in this study.
Recent studies demonstrate that the activity of Ku70 might be
regulated at both the transcriptional and post-translational levels
in response to apoptotic stimuli. Particularly, it is noteworthy
that Ku70 is targeted for acetylation and deacetylation by histone
acetyltransferases and histone deacetylase (HDAC), respectively,
in vivo(41–43). Evidence indicates that increased
acetylation levels of Ku70, as a result of HDAC inhibition,
abolishes its ability to bind Bax and suppress Bax-mediated
apoptosis (41–44). The question of whether EGCG
regulates the expression of Ku70 via regulating the balance between
acetylation and deacetylation of Ku70 requires investigation in
future studies.
In conclusion, in the present study, we have
demonstrated that EGCG is able to inhibit the growth of lung cancer
A549 cells in vivo. The possible molecular mechanism
indicated is that EGCG treatment may interrupt the binding of Ku70
and Bax, resulting in the upregulation of Bax and a parallel
downregulation of Bcl-xl, which might ultimately initiate the
activation of the caspase cascade leading to apoptosis. These
results provide new insights into the anticancer effects of EGCG.
The mechanism for the regulation of Ku70 is currently being
investigated in our laboratory.
Abbreviations:
|
EGCG
|
epigallocatechin-3-gallate;
|
|
NHEJ
|
nonhomologous end-joining;
|
|
BIP
|
Bax-inhibition peptide;
|
|
FBS
|
fetal bovine serum;
|
|
IP
|
immunoprecipitation;
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction;
|
|
ANOVA
|
one-way analysis of variance;
|
|
DSB
|
double-strand break
|
|
HDAC
|
histone deacetylase
|
Acknowledgements
This study was supported by grants
from the Hunan Province Doctoral Student Innovation Project (No.
CX2011B060).
References
|
1
|
Lu YP, Lou YR, Xie JG, et al: Topical
applications of caffeine or (−)-epigallocatechin gallate (EGCG)
inhibit carcinogenesis and selectively increase apoptosis in
UVB-induced skin tumors in mice. Proc Natl Acad Sci USA.
99:12455–12460. 2002.
|
|
2
|
Syed DN, Afaq F, Kweon MH, et al: Green
tea polyphenol EGCG suppresses cigarette smoke condensate-induced
NF-kappaB activation in normal human bronchial epithelial cells.
Oncogene. 26:673–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee MH, Han DW, Hyon SH and Park JC:
Apoptosis of human fibrosarcoma HT-1080 cells by
epigallocatechin-3-O-gallate via induction of p53 and caspases as
well as suppression of Bcl-2 and phosphorylated nuclear
factor-kappaB. Apoptosis. 16:75–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukamoto S, Hirotsu K, Kumazoe M, et al:
Green tea polyphenol EGCG induces lipid-raft clustering and
apoptotic cell death by activating protein kinase Cdelta and acid
sphingomyelinase through a 67 kDa laminin receptor in multiple
myeloma cells. Biochem J. 443:525–534. 2012. View Article : Google Scholar
|
|
5
|
Adachi S, Nagao T, Ingolfsson HI, et al:
The inhibitory effect of (−)-epigallocatechin gallate on activation
of the epidermal growth factor receptor is associated with altered
lipid order in HT29 colon cancer cells. Cancer Res. 67:6493–6501.
2007.
|
|
6
|
Zhang G, Wang Y, Zhang Y, et al:
Anti-cancer activities of tea epigallocatechin-3-gallate in breast
cancer patients under radiotherapy. Curr Mol Med. 12:163–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei LH, Kuo ML, Chen CA, et al:
Interleukin-6 promotes cervical tumor growth by VEGF-dependent
angiogenesis via a STAT3 pathway. Oncogene. 22:1517–1527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Hao MW, Dong K, Lin F, Ren JH and
Zhang HZ: Apoptosis induction effects of EGCG in laryngeal squamous
cell carcinoma cells through telomerase repression. Arch Pharm Res.
32:1263–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Featherstone C and Jackson SP: Ku, a DNA
repair protein with multiple cellular functions. Mutat Res.
434:3–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez JJ, Seveau S, Veiga E, Matsuyama
S and Cossart P: Ku70, a component of DNA-dependent protein kinase,
is a mammalian receptor for Rickettsia conorii. Cell.
123:1013–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kusano K, Johnson-Schlitz DM and Engels
WR: Sterility of Drosophila with mutations in the Bloom syndrome
gene -complementation by Ku70. Science. 291:2600–2602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pace P, Mosedale G, Hodskinson MR, Rosado
IV, Sivasubramaniam M and Patel KJ: Ku70 corrupts DNA repair in the
absence of the Fanconi anemia pathway. Science. 329:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du T, Caragounis A, Parker SJ, et al: A
potential copper-regulatory role for cytosolic expression of the
DNA repair protein XRCC5. Free Radic Biol Med. 51:2060–2072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tonotsuka N, Hosoi Y, Miyazaki S, et al:
Heterogeneous expression of DNA-dependent protein kinase in
esophageal cancer and normal epithelium. Int J Mol Med. 18:441–447.
2006.PubMed/NCBI
|
|
15
|
Hosoi Y, Watanabe T, Nakagawa K, et al:
Up-regulation of DNA-dependent protein kinase activity and Sp1 in
colorectalcancer. Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
16
|
Gullo C, Au M, Feng G and Teoh G: The
biology of Ku and its potential oncogenic role in cancer. Biochim
Biophys Acta. 1765:223–234. 2006.PubMed/NCBI
|
|
17
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sawada M, Hayes P and Matsuyama S:
Cytoprotective membrane-permeable peptides designed from the
Bax-binding domain of Ku70. Nat Cell Biol. 5:352–357. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baliga MS, Meleth S and Katiyar SK: Growth
inhibitory and antimetastatic effect of green tea polyphenols on
metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and
in vivo systems. Clin Cancer Res. 11:1918–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milligan SA, Burke P, Coleman DT, et al:
The green tea polyphenol EGCG potentiates the antiproliferative
activity of c-Met and epidermal growth factor receptor inhibitors
in non-small cell lung cancer cells. Clin Cancer Res. 15:4885–4894.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thakur VS, Ruhul AAR, Paul RK, et al:
p53-Dependent p21-mediated growth arrest pre-empts and protects
HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer
Lett. 296:225–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onoda C, Kuribayashi K, Nirasawa S, et al:
(−)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by down-regulating survivin expression. Int J Oncol.
38:1403–1408. 2011.
|
|
23
|
Li GX, Chen YK, Hou Z, et al:
Pro-oxidative activities and dose-response relationship of
(−)-epigallocatechin-3-gallate in the inhibition of lung cancer
cell growth: a comparative study in vivo and in vitro.
Carcinogenesis. 31:902–910. 2010.
|
|
24
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lessene G, Czabotar PE and Colman PM:
BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang HG, Jenabi JM, Liu XF, Reynolds CP,
Triche TJ and Sorensen PH: Inhibition of the insulin-like growth
factor I receptor by epigallocatechin gallate blocks proliferation
and induces the death of Ewing tumor cells. Mol Cancer Ther.
9:1396–1407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balasubramanian S, Adhikary G and Eckert
RL: The Bmi-1 polycomb protein antagonizes the
(−)-epigallocatechin-3-gallate-dependent suppression of skin cancer
cell survival. Carcinogenesis. 31:496–503. 2010.PubMed/NCBI
|
|
30
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weterings E and van GDC: The mechanism of
non-homologous end-joining: a synopsis of synapsis. DNA Repair
(Amst). 3:1425–1435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chou CH, Wang J, Knuth MW and Reeves WH:
Role of a major autoepitope in forming the DNA binding site of the
p70 (Ku) antigen. J Exp Med. 175:1677–1684. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X and Lieber MR: Protein-protein and
protein-DNA interaction regions within the DNA end-binding protein
Ku70-Ku86. Mol Cell Biol. 16:5186–5193. 1996.PubMed/NCBI
|
|
34
|
Wang J, Dong X, Myung K, Hendrickson EA
and Reeves WH: Identification of two domains of the p70 Ku protein
mediating dimerization with p80 and DNA binding. J Biol Chem.
273:842–848. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Dong X and Reeves WH: A model for
Ku heterodimer assembly and interaction with DNA. Implications for
the function of Ku antigen. J Biol Chem. 273:31068–31074. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soderlund LK, Queseth S, Fornander T and
Askmalm MS: Low expression of Ku70/80, but high expression of
DNA-PKcs, predict good response to radiotherapy in early breast
cancer. Int J Oncol. 37:1547–1554. 2010.PubMed/NCBI
|
|
37
|
Zhang F, Zhang T, Qu Y, et al:
Replication-dependent gamma-H2AX formation is involved in
docetaxel-induced apoptosis in NSCLC A549 cells. Oncol Rep.
24:1297–1305. 2010.PubMed/NCBI
|
|
38
|
Busser B, Sancey L, Josserand V, et al:
Amphiregulin promotes resistance to gefitinib in nonsmall cell lung
cancer cells by regulating Ku70 acetylation. Mol Ther. 18:536–543.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sawada M, Hayes P and Matsuyama S:
Cytoprotective membrane-permeable peptides designed from the
Bax-binding domainof Ku70. Nat Cell Biol. 5:352–357. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramanian C, Jarzembowski JA, Opipari AW
Jr, Castle VP and Kwok RP: HDAC6 deacetylates Ku70 and regulates
Ku70-Bax binding in neuroblastoma. Neoplasia. 13:726–734.
2011.PubMed/NCBI
|
|
41
|
Cohen HY, Lavu S, Bitterman KJ, et al:
Acetylation of the C terminus of Ku70 by CBP and PCAF controls
Bax-mediated apoptosis. Mol Cell. 13:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cohen HY, Miller C, Bitterman KJ, et al:
Calorie restriction promotes mammalian cell survival by inducing
the SIRT1 deacetylase. Science. 305:390–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Subramanian C, Opipari AW Jr, Bian X,
Castle VP and Kwok RP: Ku70 acetylation mediates neuroblastoma cell
death induced by histone deacetylase inhibitors. Proc Natl Acad Sci
USA. 102:4842–4847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Busser B, Sancey L, Josserand V, et al:
Amphiregulin promotes resistance to gefitinib in nonsmall cell lung
cancer cells by regulating Ku70 acetylation. Mol Ther. 18:536–543.
2010. View Article : Google Scholar : PubMed/NCBI
|