Introduction
Intravenous leiomyomatosis (IVL) is a rare benign
smooth muscle tumor, which is characterized by its origin in the
uterus and intrapelvic or extra-pelvic extension along the venous
system (1). This growth pattern may
present clinically as low-grade malignant potential. Since it was
first reported in 1896 by Hirschfeld (2), ~700 cases have been reported worldwide
(3). Typically, IVL is diagnosed in
women of reproductive-age with a history of uterine leiomyomas, and
it is considered to be one of the more unusual extrauterine
leiomyomas. Other similar unique growth patterns are found in
benign metastasizing leiomyoma (BML), leiomyomatosis peritonealis
disseminata, parasitic leiomyoma and retroperitoneal growth
(4). Due to the lack of obvious
symptoms in the early stages and no effective diagnostic approach,
it is usually challenging to make a diagnosis prior to surgery.
When the tumor is confined within the pelvis (commonly found in the
uterus and rarely found invading the pelvic veins and vena cava),
abdominal or pelvic pain associated with a pelvic mass is the most
common symptom, which is similar to that in patients with uterine
leiomyomas (5). Severe symptoms
such as shortness of breath, chest tightness, pulmonary embolism
and even shock may occur in patients with IVL if the right side of
the heart or the pulmonary arteries are involved (6). The main treatment option for IVL is a
complete tumor resection. However, when the mass clearly invades
the extrauterine venous system, patients are at risk of
intraoperative hemorrhage or collateral damage and there is an
increased mortality rate. Moreover, the overall recurrence rate of
IVL has been reported to be 10–31% (7,8).
Recently, the molecular and clinicopathological characterization of
IVL has been discussed in the literature. In the present review,
the clinical manifestations, pathogenesis, pathological
characteristics, treatment and prognosis of IVL are summarized. The
present review aims to assist clinicians to delineate the clinical
spectrum of this disease and provide a meaningful reference for its
diagnosis and treatment.
Etiology
To explain the pathogenesis of IVL, two possible
principal theories have been proposed. One theory considers that
the smooth muscle cells in the vessel wall are the origin of IVL.
Several cases have demonstrated that primary leiomyoma exists in
large veins outside the uterine vein, such as the jugular vein, the
popliteal vein, the common iliac vein and the subclavian vein
(9–11). The number of cases of primary and
male IVL is much lower compared with that of female patients with
IVL and a history of uterine leiomyoma. The other theory proposes
that the benign uterine leiomyoma invades the uterus or
para-uterine veins. Both theories have supporting evidence,
although most of the published literature currently supports the
second hypothesis (11).
The majority of patients with IVL have a history of
uterine leiomyoma or have undergone myomectomy or hysterectomy.
Additionally, pathological and imaging examinations have shown a
physical connection between the base of the tumor and the wall of
the uterus (12). Furthermore,
there is positive expression of progesterone receptor (PR) and
estrogen receptor (ER) in IVL tumor cells, resembling the
pathogenesis of uterine myoma. By contrast, there are low ER or PR
expression levels in endothelial and subendothelial cells. This
feature is consistent with the theory that IVL originates from
uterine leiomyomas (13). In
addition, several studies have reported the comparison of the gene
expression between IVL and uterine leiomyomas (14–16). A
study on the molecular cytogenetics of IVL revealed a similar
chromosomal mechanism in IVL compared with that in typical uterine
leiomyoma. Generally, structural chromosomal abnormalities were
reported in ~50% of the cases of uterine leiomyomas, and
spontaneous chromosome rearrangements accounted for ~20% of these
abnormalities, which were mainly manifested as rearrangements of
12q14-15 (17). This chromosomal
region targets the gene that encodes high-mobility group AT-hook 2
(18), which is considered to be
associated with the self-renewal ability of stem cells.
Additionally, this chromosomal rearrangement has been observed in
IVL, underlying the association between the two entities. Another
study demonstrated the genome-wide copy number alterations in a
large series of 28 IVLs from 26 patients (2 of the cases were
recurrences). Apart from der(14)t(12;14), chromosome alterations
are reported in 1p, 22q, 2q, 1q, 13q, 3q and 10q, involving genes
implicated in mesenchymal tumors (19). Furthermore, apart from sharing
various molecular cytogenetic characteristics with uterine
leiomyoma, IVL demonstrates similar expression profiles of
leiomyosarcoma in hierarchical cluster analysis, which could be
associated with the intermediate, quasi-malignant behavior of IVL
(14).
A genome-wide investigation of 9 cases with IVL used
oligonucleotide array comparative genomic hybridization. The result
showed that all 9 cases of IVL harbored a wild-type mediator
complex subunit 12 (MED12) gene at codon 44, while MED12 mutations
were observed in up to 80% of uterine leiomyoma cases in another
study (20,21). A similar result was reported in the
study by Wang et al (22),
which used Sanger sequencing to detect the mutation status of MED12
gene exon 2 in 9 cases of IVL (22). This revealed two novel MED12
mutations in IVL, which were different from that of uterine
leiomyoma. No mutation at MED12 gene exon 2 was revealed in the
remaining 7 cases of IVL. These results suggest that the MED12 gene
may exhibit a different pathogenesis between IVL and uterine
leiomyoma. Another previous study compared the molecular
association between IVL and uterine myoma, and demonstrated similar
dysregulated gene networks in the two diseases by identifying
differentially expressed genes (DEGs) using RNA sequencing
(RNA-seq) (15). Further results
from Gene Ontology analysis in this study revealed that these DEGs
were associated with cell adhesion, hormone stimulus and the
extracellular matrix. However, elevated homeobox A13 (HOXA13) gene
expression levels were shown in IVL, which could serve as a
biomarker to differentiate uterine myoma from IVL (15). Another study identified DEGs between
IVL and leiomyoma using RNA-seq analysis with reverse
transcription-quantitative PCR validation. Upregulated
anti-apoptosis-related genes BCL2A1 and CDKN2A, and the
downregulated angiogenesis-related gene CXCL8 were found in IVL,
partly demonstrating the possible molecular mechanism of the
differences between IVL and uterine leiomyoma (16).
In addition to genomic and transcriptomic analysis,
a recent study compared IVL and other smooth muscle tumors (uterine
leiomyoma, soft tissue leiomyoma and BML) at the protein level
using proteomic profiling analyses (23). A group of co-regulated splicing
factors were found in IVL, which could be associated with the
unique clinical behavior of the disease. One of these enriched
clusters in IVL was shown to participate in the high expression of
a number of important proteins, involving nascent protein
(SRP-dependent cotranslational protein targeting to membrane),
proteins involved in viral transcription and small GTPases
mediating signal transduction (23). Furthermore, this study also found
that IVL is more similar to BML at the proteomic level compared
with uterine leiomyoma. This is consistent with a previous array
comparative genomic hybridization analysis, which identified the
recurrent copy number alterations in IVL overlap those found in BML
(19).
Overall, these comparative gene expression studies
demonstrate the difference and similarities between IVL and other
smooth muscle tumors at multiple levels. Given the rarity of IVL,
most studies have a limited number of cases; however, these data
are required for an improved understanding of the pathogenesis of
IVL. The similar cytogenetic and protein expression features
support the theory that IVL originates from a pre-existing uterine
leiomyoma. The unique alterations in IVL, including distinct MED12
mutations, downregulated expression levels of the
angiogenesis-related gene CXCL8 and elevated expression levels of
the HOXA13 gene and anti-apoptosis-related genes, are the focus for
exploring the molecular mechanism of IVL progression. These studies
have revealed that the unique molecular profile of IVL partially
overlaps with uterine leiomyoma and uterine leiomyosarcoma, similar
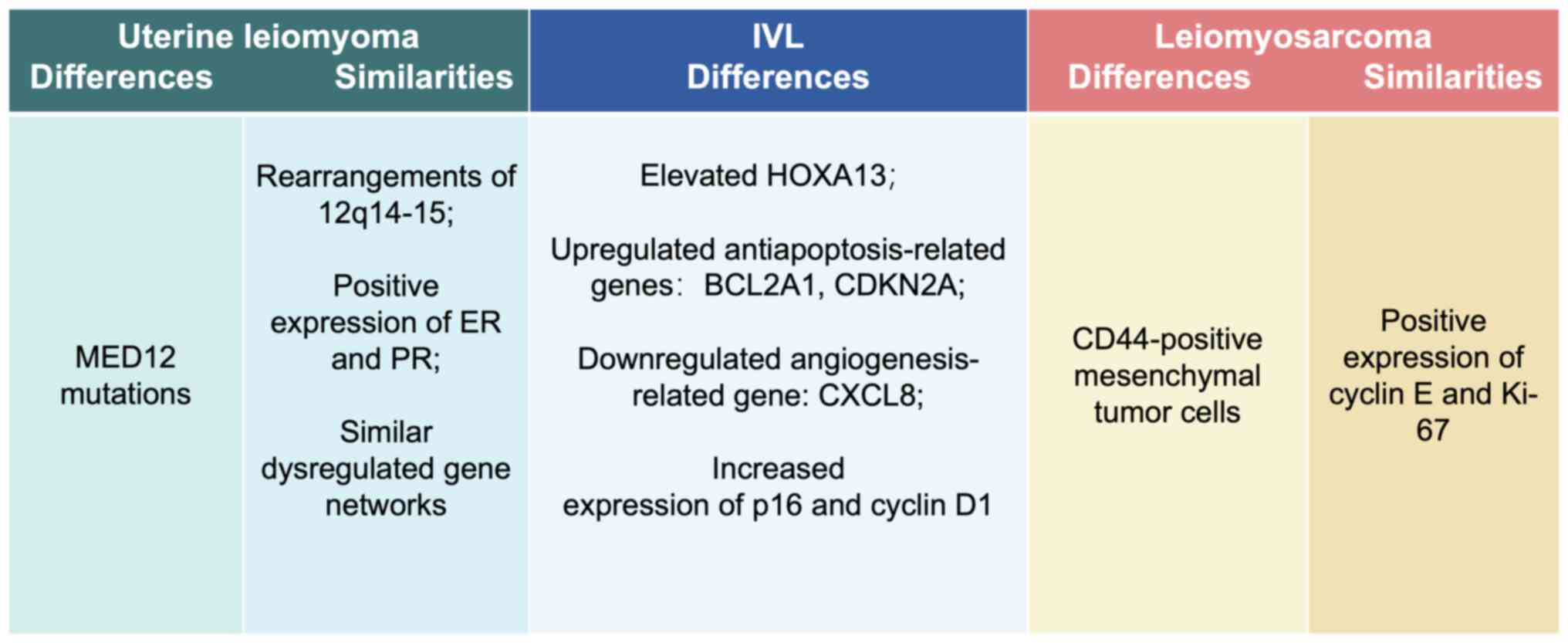
to their intermediate clinical behavior (4,16,19,22,23).
Future work investigating these DEGs will provide further insight
into the possible pathogenesis of IVL.
Clinical manifestations
IVL occurs mainly in premenopausal women with a
previous reproductive history and occasionally in postmenopausal
women. The age at diagnosis ranges from 21–81 years, with a median
age of 46 years (24). The clinical
manifestations of IVL are usually variable and non-specific, which
mainly depends on the tumor size and the organ involved (3). In most cases, IVL is confined within
the pelvis, and patients in this stage are usually asymptomatic or
demonstrate a number of symptoms caused by pelvic masses, such as
abdominal distention, unexplained pelvic pain, abnormal vaginal
bleeding, hypermenorrhea and menostaxis (25). Urinary tract obstruction could occur
when the tumor compresses the ureter (26). All these common presenting
complaints are similar to those of uterine leiomyomas. As the tumor
grows out of the pelvic cavity, reaching the renal vein and
inferior vena cava, patients experience edema and heaviness of the
lower extremities (27). Acute
Budd-Chiari syndrome, with shortness of breath, bilateral diffuse
lower extremities, ascites, edema and hepatomegaly, has been
reported in several patients as a result of tumor thrombosis
(28). When the tumor invades the
right atrium or the pulmonary artery, it is known as
intravenous-cardiac leiomyomatosis (IVCL). IVCL accounts for 10–30%
of IVL cases, and the patient may suffer from chest discomfort,
palpitations, dyspnea, syncope, and in severe cases, develop
congestive heart failure, pulmonary embolism or even sudden
mortality (24). Two pathways have
been reported for IVL extending into the inferior vena cava and
right atrium. The first access is via the uterine veins to the
internal iliac veins to the common iliac veins to the inferior vena
cava to the right side of the heart (29). A case study reported that the right
iliac vein was shorter and straighter compared with the left one
before joining the inferior vena cava, suggesting that the lesion
was prone to invade the inferior vena cava from the right iliac
vein (30). The second access is
via the left ovarian vein to the left renal vein to the inferior
vena cava. Compared with the ovarian veins, the uterine vein is
more commonly involved. The different routes of venous channels
have an impact on the difficulty of surgical resection (31).
All these symptoms can be divided into three
categories: i) Symptoms associated with a pelvic mass, such as
irregular vaginal bleeding, pain, vaginal discomfort or pelvic
pressure; ii) symptoms associated with venous embolisms, such as
lower limb edema; and iii) cardiac symptoms, such as congestive
right-sided heart failure, intermittent syncope and dyspnea
(Figure 1).
 | Figure 1.Clinical manifestations of IVL. In
most cases, IVL is confined within the pelvis. Patients in this
stage are usually asymptomatic or demonstrate some symptoms caused
by pelvic masses, such as abdominal distention, unexplained pelvic
pain and abnormal vaginal bleeding. As the tumor grows out of the
pelvic cavity, reaching the renal vein and inferior vena cava,
patients experience edema and heaviness of the lower extremities.
When the tumor invades the right atrium or the pulmonary artery,
patients may suffer from chest discomfort, palpitations, dyspnea,
syncope, and in severe cases, develop congestive heart failure or
pulmonary embolism, or even experience sudden death. IVL,
intravenous leiomyomatosis. |
Imaging manifestations
Ultrasonography is the most convenient and simple
diagnostic method for IVL. Tubular or slit-like anechoic lesions
can be observed in IVL masses (32). Color Doppler flow imaging can detect
venous blood flow signals in slit-like anechoic lesions so that
they can be distinguished from common uterine fibroids. Cardiac
ultrasound can be used to evaluate intravascular and intracardiac
lesions when performed by experienced operators, which requires
specialized training (33). In
particular, transthoracic echocardiography is indispensable for
revealing the intracardiac lesion burden as well as the associated
compromise (30,33). Contrast-enhanced ultrasonography
(CEUS) can allow tracking of the origin and expansion of the lesion
(30). The echocardiography of IVL
cardiac extension manifests as solid or tubular masses in the right
atrium of the heart, and is soft and highly mobile, with no stalk
adhering to the wall of the heart (33). Different perfusion modes of CEUS are
shown in different types of IVL. Solid-mass IVL exhibits earlier
perfusion, and the intensity is the same as that of the myocardium.
Cystic conduit IVL has delayed perfusion, with an intensity similar
to that of the cardiac chambers. It is proposed that cystic tumors
are venous leiomyomas, with no vascular function, which is
responsible for the delay in blood supply in cystic tumors compared
with that in solid tumors. The ‘sieve hole sign’ and the
‘multi-track sign’ are reported to be the specific signs of IVL in
CEUS diagnosis (34).
Computed tomography (CT) imaging is an important
examination technique to display the distinct features of IVL, and
3D post-processing, including multiplanar reconstruction, maximum
intensity projection and CT angiography (CTA), is beneficial to
directly demonstrate the full-scale path of the tumor extension.
The chest/abdomen/pelvis combined scans are able to show the IVL
extension pathway clearly (35).
Usually, a tumor mass exhibits heterogeneous enhancement, and
typical CT findings include a low-density intravascular mass with
deficient filling (36). Tumors in
the inferior vena cava are continuous, with a stadium shape
(35). When tumors invade the right
atrium of the heart, a snake's head- or walking stick head-shaped
mass can be found (12,37). Moreover, CTA can also reveal the
condition of other organs, including hydronephrosis, abdominal
ascites, pulmonary or hepatic metastasis, collateral circulation
and pericardial effusion. This increased imaging information
facilitates the diagnosis of IVL (12). Due to the detailed information
regarding the tumor composition given by CT imaging, it serves as
the primary imaging technique in preoperative assessment. A recent
study reported that a contrast-enhanced CT-based radiomic nomogram
could provide evidence for the differential diagnosis of IVL and
uterine leiomyoma (38).
The excellent soft-tissue resolution of MRI makes
this non-invasive diagnostic technique desirable in spite of
shortcomings, such as its time-consuming nature, poor spatial
resolution and lack of suitability for patients with cardiac
pacemakers or intrauterine metal devices (12). It has been reported that the number
of smooth muscle cells and vessels containing hyalinized fibrous
tissue determines the signal intensity of the lesions on MRI.
Multiple signals (low, equal or high), depending on the ratio of
smooth muscle to fibrous tissue, and unevenly high signals may
appear on T2 and T1 weighted images (37). However, these signal features are
not specific for IVL. A luffa vegetable sponge and sieve pore-like
appearance on MRI are demonstrated to be important features for
differential diagnosis (6).
Pathological characteristics
On first examination, the morphology of IVL overlaps
with that of other uterine mesenchymal tumors. Cord-like, worm-like
or lobulated structures are characteristically visible
macroscopically in the myometrial or parametrial layers and extend
along the uterine and ovarian veins (39). These worm-like plugs are usually
white to gray in color, with a convoluted irregular border
(40).
On microscopic examination, IVL consists of smooth
muscle cells, which grow in the vascular cavity. The spindle-shaped
smooth muscle cells of the same size and shape are arranged in a
whirlpool, scattered in the thick-walled small blood vessels,
surrounded by banded glassy tissue. Moreover, the focal
distribution of edema degeneration and glassy areas is easily
found. Only a small number of nuclear division images, generally
<2/10 high-power field and nuclear atypia are found in IVL
(25).
Typically, positive expression of ER and PR is
reported in most cases of IVL. There is also positive expression
for smooth muscle actin, the peritumor vascular endothelial markers
(CD31 and CD34) and factor VIII in the intravascular parts of the
tumor (41), which could serve as
biomarkers to identify IVL. One molecular pathological study
identified a population of tumor stem-like cells in IVL, similar to
uterine leiomyosarcoma. The CD44-positive mesenchymal tumor cells
were hypothesized to be able to infiltrate into the vasculature,
participating in tumor metastasis (8). CD44 has also been identified as the
first integral receptor of hyaluronan. Hyaluronan is involved in
tumor invasion/metastases and can enhance tumor growth by
stimulating angiogenesis and malignant neovascularization (42). Elevated expression levels of
hyaluronan and CD44 are demonstrated in IVL, while in uterine
leiomyomas, hyaluronan is reported to be notably lower, suggesting
a difference in the pathogenesis. The grape-like appearance with a
large amount of fluid accumulation in the IVL tumor could be
associated with hyaluronan secretion, producing tissue hydration
(43). High positive expression
levels of cyclin E and Ki-67 are factors associated with a poor
prognosis in uterine leiomyosarcoma. However, in contrast to the
high expression levels of cyclin E and Ki-67 frequently observed in
uterine leiomyosarcoma, cyclin E and Ki-67-expressing cells are
rarely found in IVL, which is in line with the low malignancy of
IVL (44). The retinoblastoma (Rb)
pathway is known to serve a regulatory role in multiple steps of
cancer progression, including angiogenesis, the
epithelial-mesenchymal transition, invasion and migration (45). The findings from
immunohistochemistry (IHC) experiments suggest cytoplasmic
phosphorylated-Rb localization in IVL. The nuclear export of Rb may
be the mechanism for Rb inactivation, signifying the role of the Rb
pathway in IVL pathogenesis (19).
The p16/cyclin D1/Rb pathway serves a critical role in controlling
the transition from the G1 to the S phase, which is
routinely used to diagnose various tumor types (46). Increased expression of p16 and
Cyclin D1 can be detected in IVL using IHC (8,19).
Differential diagnosis
Leiomyosarcoma, intravenous thrombus, right atrial
myxoma and malignant thrombosis with carcinoma (such as renal cell
carcinoma, hepatocellular carcinoma and adrenocortical carcinoma)
have similar clinical manifestations or similar characteristics on
CT or MRI with IVL, and they need to be differentially diagnosed
from IVL (34,47).
Firstly, these diseases are not usually accompanied
by a history of uterine leiomyoma. No vessels grow inside the
intravenous thrombus and thus no enhancement arises inside the
intravenous thrombosis on contrast CT (35). It can be challenging to
differentiate IVL from primary leiomyosarcoma at an early stage due
to similar clinical and radiological manifestations. Leiomyosarcoma
is known to grow from the wall of the inferior vena cava, which
tends to involve the vascular walls and adjacent tissues (48). IVL, by contrast, has a clear
boundary with no adhesion to the vascular walls. Men account for a
large proportion of the patients with leiomyosarcoma, especially
primary vascular leiomyosarcoma in the extremities (34,48).
Right atrial myxoma only occurs in the heart chamber with a stalk
and may attach to the walls of the cardiac chambers and not affect
the inferior vena cava, while IVL, typically originating from the
uterus, has a serpentine appearance, with iliac or ovarian vein
extension into the inferior vena cava and right atrium. Renal cell
carcinoma is the most common tumor that can invade the inferior
vena cava and right atrium of the heart, and should be
distinguished from IVL by postoperative pathological examination
(49).
Treatment and prognosis
The age of the patient, fertility status and extent
of the lesion involvement must be considered in the treatment of
IVL. Tumor removal and prevention of recurrence are the main aims
of IVL treatment. Surgical treatment and antiestrogen therapy are
the most common treatment choices for patients with IVL (50,51).
Surgery
Although there is no current consensus on clinical
guidelines established for the treatment of IVL, a complete tumor
resection is the recommended course for most patients with IVL, and
resection of IVL tumors has been performed in almost all types of
organ, including intra-pelvic and extra-pelvic organs (52).
A preoperative staging system has been proposed by
Ma et al (53), which
reflects intravascular tumor progression before surgery and
facilitates the option of different surgical strategies in patients
with IVL. Stage I is defined by tumors that penetrate the uterine
venous wall, but are confined to the pelvic cavity; stage II
reflects tumors that extend into the abdominal cavity, but have not
grown into the renal vein; stage III refers to tumors that reach
the renal vein, inferior vena cava and even further into the right
atrium, but have not yet extended into the pulmonary arteries; and
stage IV refers to tumors that grow into the pulmonary arteries
and/or those with lung metastases. Based on these four stages,
different surgical strategies have been provided. Total tumor
resection, hysterectomy and bilateral salpingo-oophorectomy (BSO)
are suggested for patients in stage I. Complete tumor resection,
abdominal hysterectomy and BSO are also recommended for patients
graded as stage II or above. These surgeries require the
cooperation of gynecologists and vascular surgeons. For patients in
stages III or IV, cardiopulmonary bypass (CPB) is necessary to
prevent massive hemorrhage during tumor resection. Two surgical
procedures are recommended for patients with lesions involving the
cardiovascular system, especially those with severe hemodynamic
dysfunction or with large tumors strongly adhered to surrounding
tissue (54,55); in general, patients first receive
the resection of the intracardiac component, followed by resection
of the remaining intra-abdominal tumor. The interval between the
first and second operations ranges from 7 days to 2 years (51,56).
Due to the current understanding of IVL and improved technical
abilities, a one-stage resection of intracardiac leiomyomatosis
using a single laparotomy is also feasible; this is cost-effective
and could avoid tumor embolism and progression during the interval
between the two surgical stages (24,57,58).
Further classification of IVCL has been proposed for
individualized surgical treatment of IVCL cases (59). Types A, B, C, D or E were classified
depending on the tumor size and extension of IVCL. Type A IVCL
refers to tumors in which the maximal diameters of both the
intracardiac and intracaval sections of the tumor are smaller than
the minimum diameter of the inferior vena cava. A single laparotomy
is suggested for patients with type A IVCL. Type B refers to tumors
in which the maximal diameter of the intracardiac section of the
IVCL is greater than the minimum diameter of the inferior vena
cava. For patients with this type of IVCL, right atriotomy with CPB
and deep hypothermic arrest to remove the tumor, as well as an
abdominal procedure that includes removal of the pedicle of the
tumor in the internal iliac or ovarian vein, adnexectomy or
hysterectomy is suggested. Type C refers to tumors in which the
maximal diameter of the intracaval section of the IVCL is greater
than the minimum diameter of the inferior vena cava, and is the
most common type of IVCL. Sternolaparotomy and CPB are required for
these patients. Type D refers to tumors that are in two separate
segments. One originates from the ovarian or iliac vein, and
another from the retrohepatic inferior vena cava intima. Type E
refers to tumors that belong to any of the aforementioned types
accompanied by pulmonary embolism. Due to the rareness of types D
and E, individualized treatment approaches are recommended
(59).
Overall, complete resection of the tumor plus BSO is
considered the optimal and most efficient treatment option, which
is a crucial part of preventing recurrences. Strategies and
approaches to surgery should be individualized according to the
tumor diameter, degree of abdominal and pelvic adhesion, and degree
of obstruction caused by the IVCL. When IVL involves the heart
cavity, the surgical options include one-stage and two-stage
surgery, both of which have advantages and disadvantages, and the
best surgical method is still controversial.
Anti-estrogen hormone therapies
IHC results have identified positive expression of
ER and PR in IVL (60,61). Due to this estrogen-dependent
characteristic of IVL, estrogen deprivation therapy is suggested to
be another choice for those patients who cannot undergo complete
primary removal (62).
Gonadotrophin-releasing hormone agonist (GnRHa) administration
before surgery in a premenopausal case was reported to shrink the
volume of myoma, raising the possibility of positive surgical
intervention (63). The
perioperative complications could also be efficiently reduced by
applying estrogen deprivation therapies preoperatively, considering
that the intraoperative blood loss is 7.5 times higher in patients
with IVCL compared with that in patients with IVL and tumors are
confined to the abdominal cavity (53). In addition, GnRHa has also been
administered postoperatively to prevent recurrence after incomplete
resections (5,62,64).
In one case, a 30-year-old woman developed a pulmonary embolism and
multiple nodules of the lungs after postoperatively receiving GnRHa
for 6 months. No progression of the pulmonary nodules was found
(65). However, the duration and
benefits of this approach are still controversial (50). In a number of premenopausal women
who received complete tumor resection, the administration of GnRHa
postoperatively was found to have limited effects and even lead to
a small amount of growth in the residual tumors of the patients
(66). The side effects of
long-term therapy with GnRHa, including reduced bone mineral
density, vasomotor symptoms and altered lipid profile, deserve
serious consideration (67). Other
hormonal therapies include selective ER modulators (SERMs) and
aromatase inhibitors. Tamoxifen and raloxifene, as typical SERMs,
are used as both monotherapy and adjuvant treatments for IVL
(68). However, an analysis of 194
cases of IVL demonstrated that postoperative tamoxifen treatment
did not help to prevent recurrence (49). Therefore, the effectiveness of GnRHa
and other hormonal therapies in the adjuvant treatment of IVL still
requires investigation and large samples of clinical cases. Further
research is still required for using hormone therapy in patients
with IVL.
Follow-up and prognosis
The recurrence rate of the IVL varies among
different studies. Yu et al (69) reported a recurrence rate of 31.0% in
58 patients. In a comprehensive analysis of 194 cases of IVL, no
recurrence or postoperative mortality was reported in patients who
undewent complete tumor removal. However, for those who underwent
an incomplete removal, the recurrence rate was 33.3%, and 4
patients died during the follow-up period (49). Incomplete surgical tumor resection
and large vein involvement are the critical risk factors for
recurrence. In a recent retrospective single-center study, compared
with patients diagnosed non-incidentally and undergoing a complete
tumor resection, those patients diagnosed incidentally were
reported to have a higher risk of recurrence. A total of 5 (12.8%)
cases in the incidental group were reported to have recurrence,
while no recurrence was found among the 24 patients who are
non-incidentally diagnosed (7).
There are several reasons that may explain this result. Firstly,
the incidentally diagnosed patients may have an inadequate
evaluation without further imaging examination, such as
echocardiography. Secondly, the surgery of these patients could
also be insufficient, which would result in residual tumors and
recurrence. For patients diagnosed non-incidentally, further
evaluation could be conducted to identify the details of the IVL
before the operation, including use of CT, MRI and transesophageal
echocardiography (7,53). Furthermore, a multidisciplinary team
usually participates in the surgery of patients diagnosed
non-incidentally but not in the surgery being performed by
gynecologists only (39). Overall,
this medical evidence highlights the importance of extra-pelvic
imaging and multidisciplinary surgical treatment.
Long-term follow-up is necessary for patients with
IVL, especially those who have an incomplete tumor resection and
preservation of the uterus and ovaries. Although there is no
standard recommendation for the time and imaging modalities of
follow-up, indications are for a follow-up every 3–6 months for the
first 2 years after surgery and then every 12 months after that
(32). A CT scan of the chest,
abdomen and pelvis is necessary. When the CT scan is not available,
an ultrasound of the peritoneal cavity, retroperitoneal space and
pelvic cavity, including transvaginal and transanal examinations,
is recommended (3). Furthermore, if
any symptoms associated with thromboembolic events occur, a Doppler
ultrasound assessment of the corresponding vein, such as the
inferior vena cava, iliac vessels and veins of the lower limbs, is
vital to identify the progress of the disease (52).
Conclusions
In summary, IVL is a rare and unique benign smooth
muscle tumor with the potential to be malignant. Due to the
non-specific clinical manifestations, it is challenging for
clinicians to diagnose IVL before surgery. However, intracardiac
IVL must be considered in fertile women with a history of uterine
leiomyomatosis when the patient has a mobile mass in the right
atrium and inferior vena cava without attachment to the endothelium
or the endocardium. Imaging modalities, such as ultrasonography, CT
and MRI, are useful in displaying the precise location and
full-scale extension path of the tumor before surgery. The primary
treatment approach remains as surgery with a complete tumor
resection, which is the key to avoiding recurrence. The final
diagnosis also depends on the histopathological analysis after
surgery. The surgical procedure should be considered carefully
depending on the general condition of the patient, the tumor
diameter, the degree of abdominal and pelvic adhesion, and the
degree of obstruction. A thorough preoperative assessment and
intraoperative collaboration in a multi-disciplinary setting are
important for good patient outcomes. The application of hormone
therapies is still controversial and needs the support of further
large-scale clinical data. The unique molecular profile of IVL has
been investigated at different levels and further analysis is
required for an improved understanding of the pathogenesis of IVL
(Figure 2). The DEGs and tumor
stem-like cells in IVL could provide insights for the development
of new therapeutic and diagnostic approaches. Furthermore,
long-term follow-up is necessary for patients with IVL.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XTZ and FY designed and organized this manuscript.
XTZ contributed to the first draft of the manuscript, tables and
figures. XRQ and XZ revised the manuscript. All authors have read
and approved the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed M, Zangos S, Bechstein WO and Vogl
TJ: Intravenous leiomyomatosis. Eur Radiol. 14:1316–1317. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birch-Hirschfeld FV: Textbook of
pathological anatomy. FCW Vogel; Leipzig: pp. p2261896, (In
German).
|
|
3
|
Lim WH, Lamaro VP and Sivagnanam V:
Manifestation and management of intravenous leiomyomatosis: A
systematic review of the literature. Surg Oncol. 45:1018792022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Findakly D and Wang J: Molecular profiling
of benign metastasizing leiomyoma of the uterus revealing unique
novel therapeutic targets. Cureus. 12:e77012020.PubMed/NCBI
|
|
5
|
Low HY, Zhao Y, Huang KS, Shen HP, Wu PJ
and Tseng CJ: Intravenous leiomyomatosis of the uterus: A
clinicopathological analysis of nine cases and literature review.
Taiwan J Obstet Gynecol. 56:362–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang LQ, Zhang B, Liu BG and Liu FH:
Diagnosis of intravenous leiomyomatosis extending to heart with
emphasis on magnetic resonance imaging. Chin Med J (Engl).
125:33–37. 2012.PubMed/NCBI
|
|
7
|
Shi P, Xiao H, Li H, Tang W, Ren A, Ma L,
Tu R, Yin S and Zhang J: Management and prognosis comparison
between incidental and nonincidental intravenous leiomyomatosis: A
retrospective single-center real-life experience. Ann Transl Med.
10:5032022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashi T, Yaegashi N and Konishi I:
Molecular pathological approach of uterine intravenous
leiomyomatosis. Ann Transl Med. 10:7242022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grimer RJ and Armstrong GR: Intra-vascular
leiomyoma of the popliteal vein. Postgrad Med J. 64:247–248. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansson B, Bogers J, Colpaert C, De Roeck
J, De Backer A, Ceulemans P, De Maeseneer M and Hubens G: Leiomyoma
of the right common iliac vein presenting as a duodenal tumour. Eur
J Surg Oncol. 26:717–719. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang H, You Y, Cai F, Yang Y, Yang C and
Lv P: Intravenous leiomyomatosis of the subclavian vein. J Vasc
Surg Venous Lymphat Disord. 5:254–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gui T, Qian Q, Cao D, Yang J, Peng P and
Shen K: Computerized tomography angiography in preoperative
assessment of intravenous leiomyomatosis extending to inferior vena
cava and heart. BMC Cancer. 16:732016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miro A, Bottazzi EC, Vanella S, Palma T,
Noviello A, Apicella I, Lombardi G, Fiorani B and Crafa F:
Intravascular leiomyomatosis with intracardiac extension: A
toraco-abdominal approach. J Surg Case Rep. 202:rjab2492021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ordulu Z, Nucci MR, Cin PD, Hollowell ML,
Otis CN, Hornick JL, Park PJ, Kim TM, Quade BJ and Morton CC:
Intravenous leiomyomatosis: An unusual intermediate between benign
and malignant uterine smooth muscle tumors. Mod Pathol. 29:500–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Wu L, Xu R, Zhu C, Ma G, Zhang C,
Liu X, Zhao H and Miao Q: Identification of the molecular
relationship between intravenous leiomyomatosis and uterine myoma
using RNA sequencing. Sci Rep. 9:14422019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang W, Wang Y, Chen F, Zhang M, Jia R,
Liu X, Zhang C, Shao J, Cheng N, Ma G, et al: Intravenous
leiomyomatosis is inclined to a solid entity different from uterine
leiomyoma based on RNA-seq analysis with RT-qPCR validation. Cancer
Med. 9:4581–4592. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rein MS, Friedman AJ, Barbieri RL, Pavelka
K, Fletcher JA and Morton CC: Cytogenetic abnormalities in uterine
leiomyomata. Obstet Gynecol. 77:923–926. 1991.PubMed/NCBI
|
|
18
|
Schoenmakers EF, Wanschura S, Mols R,
Bullerdiek J, Van den Berghe H and Van de Ven WJ: Recurrent
rearrangements in the high mobility group protein gene, HMGI-C, in
benign mesenchymal tumours. Nat Genet. 10:436–444. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ordulu Z, Chai H, Peng G, McDonald AG, De
Nictolis M, Garcia-Fernandez E, Hardisson D, Prat J, Li P, Hui P,
et al: Molecular and clinicopathologic characterization of
intravenous leiomyomatosis. Mod Pathol. 33:1844–1860. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buza N, Xu F, Wu W, Carr RJ, Li P and Hui
P: Recurrent chromosomal aberrations in intravenous leiomyomatosis
of the uterus: High-resolution array comparative genomic
hybridization study. Hum Pathol. 45:1885–1892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsubara A, Sekine S, Yoshida M, Yoshida
A, Taniguchi H, Kushima R, Tsuda H and Kanai Y: Prevalence of MED12
mutations in uterine and extrauterine smooth muscle tumours.
Histopathology. 62:657–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Hu S, Xin F, Zhao H, Li G, Ran W,
Xing X and Wang J: MED12 exon 2 mutation is uncommon in intravenous
leiomyomatosis: Clinicopathologic features and molecular study. Hum
Pathol. 99:36–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krasny L, Wilding CP, Perkins E, Arthur A,
Guljar N, Jenks AD, Fisher C, Judson I, Thway K, Jones RL and Huang
PH: Proteomic profiling identifies co-regulated expression of
splicing factors as a characteristic feature of intravenous
leiomyomatosis. Cancers (Basel). 14:29072022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cassol DF, Junior FJRT, Dias do Couto
Netto S, Rengel LC, Ragazzo L, Gaiotto FA and Utiyama EM:
Symptomatic uterine leiomyomatosis with intracaval and intracardiac
invasion: Video case report. Gynecol Oncol Rep. 45:1011272023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan S, Wang X, Li Y and Zhai M:
Intravenous leiomyomatosis: A case study and literature review.
Radiol Case Rep. 17:4203–4208. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng J, Zhong F, Zhu Y, Zhang M, Zhang M,
Lu C, Wang Y, Qi X, Wang C and Li G: Clinical analysis of uterine
intravenous leiomyomatosis: A retrospective study of 260 cases. J
Obstet Gynaecol Res. 47:4357–4364. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin X, Li F, Lu Z and Cheng W: IV
Leiomyomatosis on FDG PET/CT. Clin Nucl Med. 41:580–582. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barksdale J, Abolhoda A and Saremi F:
Intravenous leiomyomatosis presenting as acute Budd-Chiari
syndrome. J Vasc Surg. 54:860–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Y and Song B: Three case reports of
intravenous leiomyomatosis with intracardiac extensions. Thorac
Cardiovasc Surg Rep. 9:e40–e43. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo X, Li R and Li Z: Combined
transthoracic echocardiography and contrast-enhanced
ultrasonography to trace intravenous leiomyomatosis with
intracardiac extension. Echocardiography. 36:1573–1576. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lam PM, Lo KW, Yu MY, Wong WS, Lau JY,
Arifi AA and Cheung TH: Intravenous leiomyomatosis: Two cases with
different routes of tumor extension. J Vasc Surg. 39:465–469. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, Yu X, Shi H, Fan Q, Lang J and
Liu B: Clinical characteristics and prognostic features of
intravenous leiomyomatosis with inferior vena cava or intracardiac
extension. J Vasc Surg Venous Lymphat Disord. 5:485–492. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma H, Niu Y, Yang Z and Zheng M:
Echocardiographic characteristics and contrast-enhanced imaging of
intravenous leiomyomatosis with intracardiac extension. J
Ultrasound Med. 41:1101–1108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge Z, Wang Y, Wang Y, Fang S, Wang H and
Li J: Diagnostic value of contrast-enhanced ultrasound in
intravenous leiomyomatosis: A single-center experiences. Front
Oncol. 12:9636752022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Nie P, Chen B, Hou F, Dong C, He F
and Xu W: Contrast-enhanced CT findings of intravenous
leiomyomatosis. Clin Radiol. 73:503e1–503e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng HJ, Zhao B, Yao QW, Qi HT, Xu ZD and
Liu C: Intravenous leiomyomatosis: CT findings. Abdom Imaging.
37:628–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng H, Xu Z, Zhang L, Luo YI, Chen H, Zhu
H, Peng L and Yu J: Intravenous leiomyomatosis with intracardiac
extension depicted on computed tomography and magnetic resonance
imaging scans: A report of two cases and a review of the
literature. Oncol Lett. 11:4255–4263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shao J, Wang C, Shu K, Zhou Y, Cheng N,
Lai Z, Li K, Xu L, Chen J, Du F, et al: A contrast-enhanced
CT-based radiomic nomogram for the differential diagnosis of
intravenous leiomyomatosis and uterine leiomyoma. Front Oncol.
13:12391242023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu X, Fu J, Cao T, Huang L, Qie M and
Ouyang Y: Clinicopathologic features and clinical outcomes of
intravenous leiomyomatosis of the uterus: A case series. Medicine
(Baltimore). 100:e242282021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei JL, Ji X, Zhang P, Chen WJ, Zhao YN
and Liu M: Complete intravenous leiomyomatosis: A case report and
literature review. Ann Palliat Med. 10:12039–12045. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du J, Zhao X, Guo D, Li H and Sun B:
Intravenous leiomyomatosis of the uterus: A clinicopathologic study
of 18 cases, with emphasis on early diagnosis and appropriate
treatment strategies. Hum Pathol. 42:1240–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartolazzi A, Peach R, Aruffo A and
Stamenkovic I: Interaction between CD44 and hyaluronate is directly
implicated in the regulation of tumor development. J Exp Med.
180:53–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen MJ, Peng Y, Yang YS, Huang SC, Chow
SN and Torng PL: Increased hyaluronan and CD44 expressions in
intravenous leiomyomatosis. Acta Obstet Gynecol Scand. 84:322–328.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tamura S, Hayashi T, Tokunaga H, Yaegashi
N, Abiko K and Konishi I: Oncological properties of intravenous
leiomyomatosis: Involvement of mesenchymal tumor stem-like cells.
Curr Issues Mol Biol. 43:1188–1202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peurala E, Koivunen P, Haapasaari KM,
Bloigu R and Jukkola-Vuorinen A: The prognostic significance and
value of cyclin D1, CDK4 and p16 in human breast cancer. Breast
Cancer Res. 15:R52013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sungur EC, Selçuk İ, Özbek HM, Aytekin O,
Köklü NO, Turhan N, Talay S, Sarıtaş A and Yalçın HR: Extrapelvic
intravenous uterine leiomyomatosis mimicing cardiac myxoma and deep
vein thrombosis. Turk Gogus Kalp Damar Cerrahisi Derg. 30:622–626.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yokoyama Y, Goda T, Sato K, Suzuki M,
Kanda T and Sato Y: Leiomyosarcoma arising from the ovarian vein as
a gynecologic malignancy: Two case reports and a review of the
literature. J Obstet Gynaecol Res. 48:2224–2230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li B, Chen X, Chu YD, Li RY, Li WD and Ni
YM: Intracardiac leiomyomatosis: A comprehensive analysis of 194
cases. Interact Cardiovasc Thorac Surg. 17:132–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liang J, Lei R, Xie M, Lin S, Xu J, Ling X
and Xie Q: The role of estrogen deprivation therapy in
premenopausal women with primary unresectable intracardiac
leiomyomatosis: A systematic review and meta-analysis. Orphanet J
Rare Dis. 16:4532021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li H, Xu J, Lin Q, Zhang Y, Zhao Y, Tong
H, Tu R, Xu D, Wang C and Lu W: Surgical treatment strategies for
extra-pelvic intravenous leiomyomatosis. Orphanet J Rare Dis.
15:1532020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stilidi I, Paianidi J, Bokhian V, Andreeva
J, Shevchuk A and Ramirez PT: Intracardiac intravenous
leiomyomatosis: Diagnosis and management. Int J Gynecol Cancer.
30:1243–1247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma G, Miao Q, Liu X, Zhang C, Liu J, Zheng
Y, Shao J, Cheng N, Du S, Hu Z, et al: Different surgical
strategies of patients with intravenous leiomyomatosis. Medicine
(Baltimore). 95:e49022016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang C, Fang H, Yang Y, Cai F, Zheng H,
Jin B, Li Y, Liu Z and Zayed MA: Diagnosis and surgical management
of inferior vena cava leiomyomatosis. J Vasc Surg Venous Lymphat
Disord. 6:636–645. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gissey LC, Mariano G, Musleh L, Lepiane P,
Colasanti M, Meniconi RL, Ranocchi F, Musumeci F, Antonini M and
Ettorre GM: Massive pelvic recurrence of uterine leiomyomatosis
with intracaval-intracardiac extension: Video case report and
literature review. BMC Surg. 17:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luo G, Pan H, Bi J, Luo Y, Zhu J, Feng Z,
Fan H, Zhang Y and Dai X: Surgical treatment of intravenous
leiomyomatosis involving the right heart: A case series. J Int Med
Res. 47:3465–3474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Price JD, Anagnostopoulos C, Benvenisty A,
Kothuru RK and Balaram SK: Intracardiac extension of intravenous
leiomyomatosis. Ann Thorac Surg. 103:e145–e147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang C, Shao J, Ma X, Zhou Y, Ma G, Cheng
N, Cao D, Lai Z, Song X, Li K and Liu B: One-stage resection of
intravascular leiomyomatosis involving the right heart chamber
through a single laparotomy. Front Cardiovasc Med. 9:9764782022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu J, Liang M, Ma G, Liu X, Cheng N, Cao
D, Yu C, Du S, Miao Q and Zhang C: Surgical treatment for
intravenous-cardiac leiomyomatosis. Eur J Cardiothorac Surg.
54:483–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kokawa K, Yamoto M, Yata C, Mabuchi Y and
Umesaki N: Postmenopausal intravenous leiomyomatosis with high
levels of estradiol and estrogen receptor. Obstet Gynecol.
100:1124–1126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Akinseye OA, Nayyar M and Das P: Uterine
intravenous leiomyomatosis with femoral vein, intracaval,
intracardiac and pulmonary artery extension. Future Cardiol.
16:27–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mitsuhashi A, Nagai Y, Sugita M, Nakajima
N and Sekiya S: GnRH agonist for intravenous leiomyomatosis with
cardiac extension. A case report. J Reprod Med. 44:883–886.
1999.PubMed/NCBI
|
|
63
|
Khayata GM, Thwaini S and Aswad SG:
Intravenous leiomyomatosis extending to the heart. Int J Gynaecol
Obstet. 80:59–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mizoguchi C, Matsumoto H, Nasu K, Arakane
M, Kai K and Narahara H: Intravenous leiomyomatosis treated with
radical hysterectomy and adjuvant aromatase inhibitor therapy. J
Obstet Gynaecol Res. 42:1405–1408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bodner-Adler B, Bartl M and Wagner G:
Intravenous leiomyomatosis of the uterus with pulmonary metastases
or a case with benign metastasizing leiomyoma? Anticancer Res.
29:495–496. 2009.PubMed/NCBI
|
|
66
|
Hameleers JA, Zeebregts CJ, Hamerlijnck
RP, Elbers JR and Hameeteman TM: Combined surgical and medical
approach to intravenous leiomyomatosis with cardiac extension. Acta
Chir Belg. 99:92–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Doyle MP, Li A, Villanueva CI, Peeceeyen
SC, Cooper MG, Hanel KC, Fermanis GG and Robertson G: Treatment of
intravenous leiomyomatosis with cardiac extension following
incomplete resection. Int J Vasc Med. 2015:7561412015.PubMed/NCBI
|
|
68
|
Lo KW and Lau TK: Intracardiac
leiomyomatosis. Case report and literature review. Arch Gynecol
Obstet. 264:209–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yu X, Zhang G, Lang J, Liu B and Zhao D:
Factors associated with recurrence after surgical resection in
women with intravenous leiomyomatosis. Obstet Gynecol.
128:1018–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|