Introduction
Based on the latest data on global cancer incidence
in 2020, breast cancer (BC) is ranked as the most prevalent type of
cancer in women (1). In 2020 alone,
>2.3 million women worldwide were newly diagnosed with BC
(2). At the time of diagnosis,
3-10% of newly diagnosed patients with BC had distant metastases,
and 30-40% of early-stage patients with BC progressed to an
advanced stage (3). BC metastasis
significantly reduces survival rates and only 29% of women with
metastatic BC experience a 5-year survival time (3). Therefore, understanding the processes
of BC metastasis may lead to the development of new therapeutic
approaches.
BC is subtyped using specific molecular biomarkers,
namely an estrogen receptor (ER), the progesterone receptor (PR)
and human epidermal growth factor 2 (HER2). The Ki67 index, a
proliferation indicator, is also considered during subtyping
(4). Based on the expression levels
of these specific biomarkers, there are five defined BC subtypes,
namely Luminal A (Lum A; ER+ and PR+, but
HER2− and Ki67 <14%), Luminal B (Lum
B)-HER2− (ER+ and PR+, but
HER2− and Ki67 >14%), Lum B-HER2+
(ER+ and PR+, but HER2+ and any
Ki67 level), HER2 overexpressed [ER− and PR−,
but high HER2+ (HER2 score of >3) and any Ki67 level]
and triple-negative BC (TNBC; ER−, PR−,
HER2− and any Ki67 level) (5,6) Each
subtype displays distinct behavior with regards to their metastatic
potential, expression of cell surface markers, response to
treatments, and their cellular and molecular characteristics
(7). Therefore, understanding the
metastatic differences of each subtype is critical for designing
effective, subtype-specific treatments.
In the present study, changes in the expression
levels of two enzymes, glycerol-3-phosphate dehydrogenase (GPD1)
and monoacylglycerol lipase (MAGL), in the axillary lymph nodes of
metastatic BC tissues were investigated across different subtypes.
Prior research, performed by both our team and other researchers,
has already reported altered levels of these enzymes in certain BC
subtypes, suggesting reduced expression in cancer cells compared
with controls (6,8–10). The
aim of the present study was to investigate the potential
significance of GPD1 and MAGL as candidate biomarkers for
diagnosing and subtyping BC.
Material and methods
Patient characteristics and tissue
collection
Tissues from 26 female patients diagnosed with
infiltrating ductal carcinoma BC were collected at the Department
of General Surgery of Kocaeli University Medical School (Kocaeli,
Turkey) from March 2019 to August 2021. Written informed consent
was obtained from each patient before participation in the present
study. The study specifically included patients who had not
undergone any form of cancer therapy. For comparison,
non-metastatic lymph nodes were taken from an area separate from
the metastatic lymph nodes and utilized as control samples. Control
breast tissues were collected from adjacent non-tumor tissue
regions. Table I presents a
comprehensive list of the clinical features of the patients
included in the present study.
 | Table I.Clinical features of patients with
breast cancer included in the present study. |
Table I.
Clinical features of patients with
breast cancer included in the present study.
| Clinical feature | Luminal A | Luminal
B/HER2− | Luminal
B/HER2+ | HER2-OE | TNBC |
|---|
| Patients, n | 7 | 5 | 4 | 4 | 6 |
| Mean (SD) age,
years | 41.25 (5.3) | 47.3 (5.6) | 59.25 (10.8) | 49 (5.2) | 62.75 (12.25) |
| Ki67 level, % | <14 | ≥14 | ≤14≥ | ≤14≥ | ≤14≥ |
Subtyping study groups
Molecular subtyping was performed based on ER, PR,
HER2 and Ki67 protein expression levels (data not shown) (11). Molecular subtyping of tumor samples
was performed by the Department of Pathology at Kocaeli University
Medical School by analyzing expression levels of ER, PR, HER2 and
Ki-67 proteins. The expression levels of these proteins were
evaluated using routine immunohistochemical methods, as previously
described (5). Based on the
expression patterns, patients were divided into the following five
subgroups: Lum A (ER+ and/or PR+,
HER2− and Ki67 <14%), Lum B/HER2−
(ER+ and/or PR+, HER2− and Ki67
>14%), Lum B/HER2+ (ER+ and/or
PR+, HER2+ and any Ki67 level), HER2-OE
[ER−, PR−, HER2 (score of >3) and any Ki67
level] and TNBC (ER−, PR−, HER2−
and any Ki67 level) (12). The Lum
A, Lum B/HER2+, Lum B/HER2−, HER2-OE and TNBC
groups included 7, 4, 5, 4 and 6 patients, respectively. Surrogate
definitions of intrinsic sub-types of breast cancer for HER2
expression were scored based on staining patterns and scored as 1,
2 and 3. Any score beyond 3 was considered as overexpressed.
Sample preparation
The collected tissues were first diced into small
pieces and then washed with buffer containing 10 mM Tris (pH 7.2)
and 250 mM sucrose. After centrifugation at 10,000 × g for 10 min,
supernatant containing trace amounts of blood (from the tissue
samples) was carefully removed. Subsequently, the tissue samples
were homogenized using a Scilogex homogenizer in buffer containing
10 mM Tris-HCl containing 7 M urea, 2 M thiourea, 5 mM magnesium
acetate, with 4% CHAPS (pH 8.0). To ensure thorough homogenization,
further treatment with 1.4 mm stainless steel beads using a
bead-beater (Bullet Blender; Next Advance, Inc.) was performed. The
resulting homogenates were then subjected to centrifugation for 15
min at 15,000 × g at 4°C to obtain cell-free extracts. Protein
concentrations in these extracts were determined using a modified
Bradford assay (Bio-Rad Laboratories, Inc.). Finally, the cell-free
extracts were stored at −80°C until further use.
Preparation of protein pools
Protein pools were created by mixing equal amounts
of protein from the cell-free extracts of samples from the
following sample types: Healthy breast (BH), tumor breast (BT),
healthy lymph node (LH) and metastatic lymph node (LM). Protein
concentrations in each pool were determined using the Qubit Protein
Assay (Thermo Fisher Scientific, Inc.). The protein integrity of
each pool was also assessed by visual examination of Coomassie
stained 12% SDS-PAGE gels (data not shown). Protein (30 µg per
lane) was used for qualitative and quantitative analyses. Quantity
One software version 4.6.7 was used to compare protein band
intensities among the pools (data not shown) (Bio-Rad Laboratories,
Inc.).
Western blotting analysis
Protein pools and individual cell-free extracts were
analyzed by SDS-PAGE using 12% acrylamide gels. Protein (20 µg per
lane) was used for qualitative and quantitative analyses.
Electrophoretic transfer of proteins onto positively charged
nitrocellulose membranes was performed in a semi-dry
electrophoretic transfer cell (Bio-Rad Laboratories, Inc.) for 20
min at 15 V, in a buffer containing 48 mM Tris (pH 9.2), 39 mM
glycine, 20% (v/v) methanol and 0.0375 g/l SDS. The membranes were
blocked in TBS-T buffer (Tris-HCl 25 mM pH 7.2, NaCl 150 mM and
0.1% Tween 20) containing 5% nonfat dry milk for 1 h at room
temperature, and washed with TBS-T three times before incubation
with primary antibodies diluted in TBS-T overnight at 4°C. The
membranes were then washed three times with TBS-T and incubated
with goat anti-mouse HRP-labelled secondary antibody (cat. no.
170-5047; Bio-Rad) for 1 h at room temperature. Monoclonal
anti-β-actin antibody (cat. no. sc81178; Santa Cruz Biotechnology,
Inc.), monoclonal anti-glycerol-3-phosphate dehydrogenase NAD+,
cytoplasmic antibody (anti-GPD1) (cat. no. sc-376219; Santa Cruz
Biotechnology, Inc.) and monoclonal anti-monoglyceride lipase
antibody (anti-MAGL) (cat. no. sc398942; Santa Cruz Biotechnology,
Inc.) were used at the respective dilutions of 1:1,000, 1:750 and
1:750. Following a subsequent three washes with TBST, protein bands
were visualized with an enhanced chemiluminescence detection system
(Thermo Fisher Scientific, Inc.). Protein band intensities were
analyzed using Image J (version 1.40 g; National Institutes of
Health). Β-actin was used as the internal normalization control for
the band intensities. Western blotting analyses of the protein
pools were performed three times.
Statistical analysis
Statistical analyses of the differences in MAGL and
GPD1 protein expression levels between the BH and BT groups, as
well as between the LH and LM groups were performed. All
statistical analyses were performed using GraphPad Prism software,
version 5.0 (Dotmatics). Datasets with two groups were analyzed
using paired Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Protein pools within each subtype were labelled as
follows: BH, BT, LH and LM. After determination and normalization
of protein concentrations, the protein expression levels of GPD1
and MAGL in each BC subtype were analyzed using western blotting.
Similarly to results previously observed (6,8,9), there
was a significant decrease in GPD1 and MAGL protein levels in the
BT pool compared with the BH pool, regardless of the BC subtype
(Fig. 1). However, when comparisons
were made between the LH and LM protein pools, statistically
significant decreases in GPD1 and MAGL protein levels were only
observed in the TNBC subtype (Fig.
1).
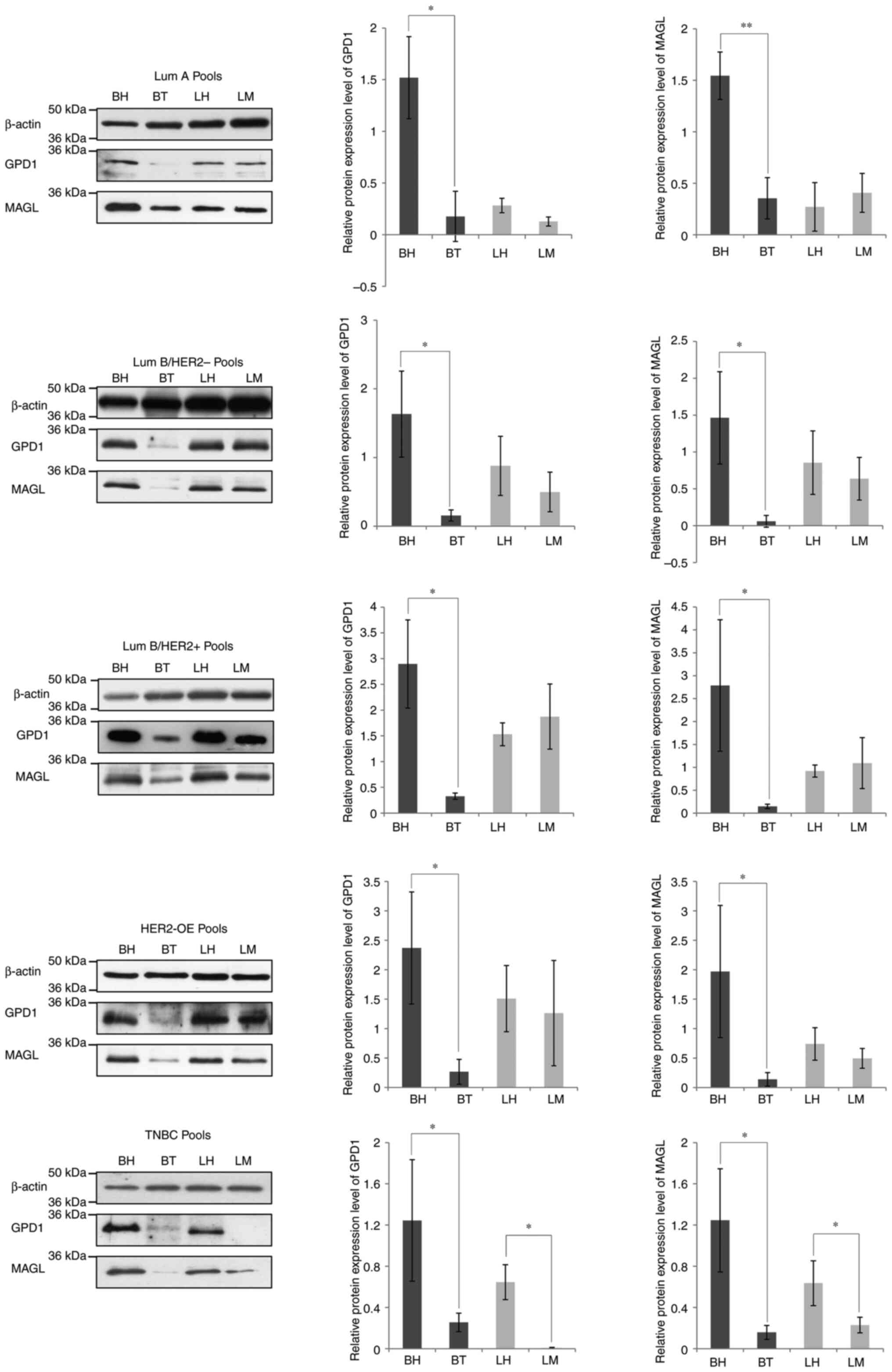 | Figure 1.Western blot analysis of protein pools
prepared from Lum A, Lum B/HER2−, Lum
B/HER2+, HER2-OE and TNBC tissues for the assessment of
GPD1 and MAGL levels. The western blot was re-probed with an
anti-β-actin antibody for the normalization of protein expression
levels. The band intensities were semi-quantified using ImageJ
software and statistical significance was calculated using GraphPad
Prism software. *P<0.05, **P<0.001. BH, healthy breast; BT,
breast tumor; GPD1, glycerol-3-phosphate dehydrogenase; HER2, human
epidermal growth factor 2; LH, healthy lymph node; LM, metastatic
lymph node; Lum, Luminal; MAGL, monoacylglycerol lipase; OE,
overexpressed; TNBC, triple-negative breast cancer. |
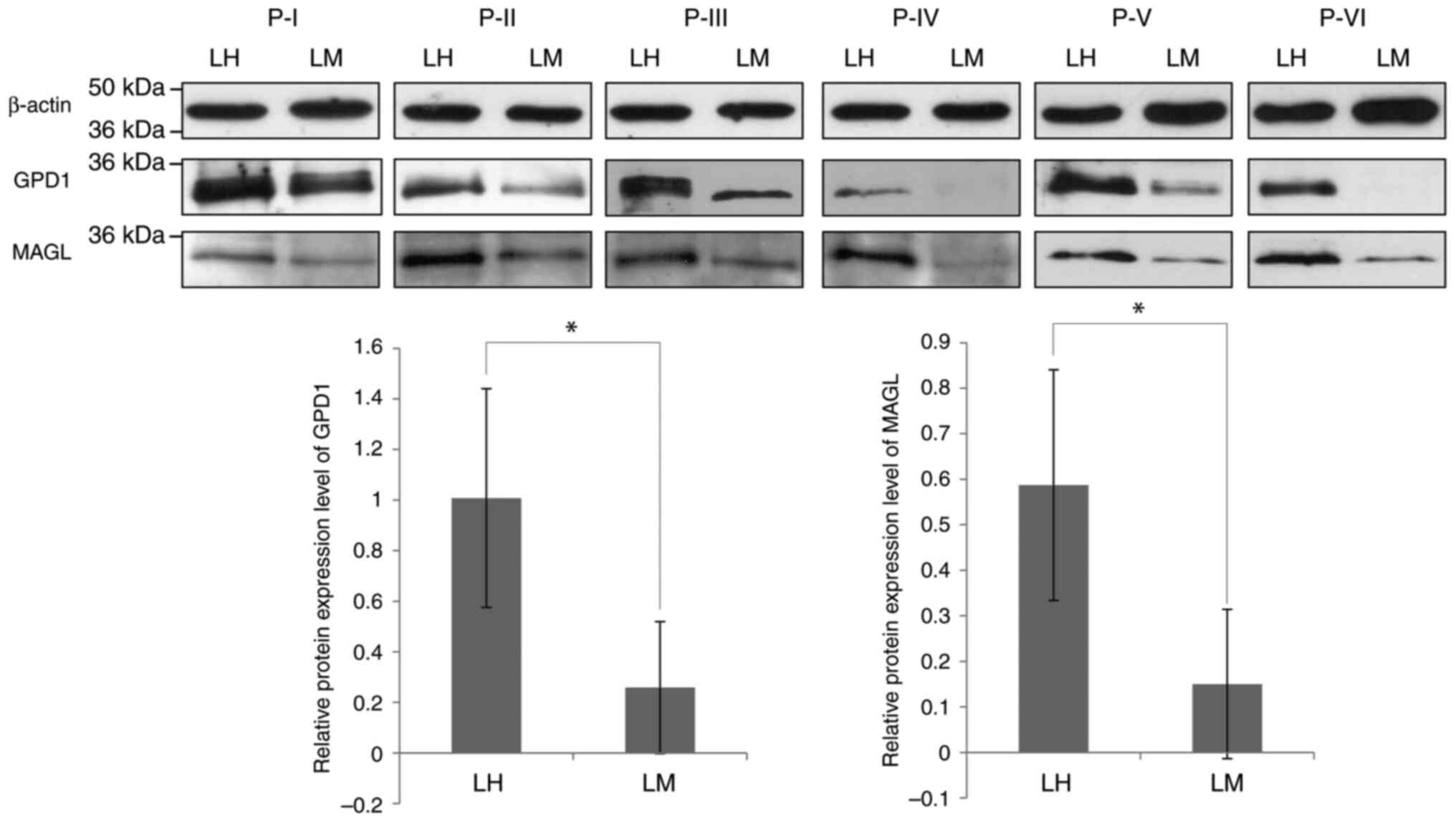
To assess whether the decrease in GPD1 and MAGL
protein levels observed in the LM protein pool, compared with the
LH protein pool, was detectable in each individual protein sample
prepared from tissues from patients with TNBC, western blotting of
the separate TNBC samples was performed (Fig. 2). Significantly lower mean protein
expression levels of GPD1 and MAGL were detected in the LM samples
compared with the LH samples, although this decrease in protein
expression appeared to vary from sample to sample. Notably, the
absence or low levels of GPD1 protein in LM samples suggests that
it might serve as a more effective discriminator between LM and LH
samples.
Discussion
BC is a metabolically complicated and heterogeneous
disease and requires greater understanding at the molecular level
(13). Onset, progression and
metastasis are the three main steps in BC, and each step is
markedly different in terms of epigenetic changes, mutations rates,
secretion of certain growth factors, changes in expression of
different receptor types and key cell-cell adhesion molecules
(14). Metastasis is the leading
cause of cancer mortality; therefore, the metastatic cascade is
potentially the most important step to target for reducing the
death rate. Metastasis itself is a multistep process involving
local tumor cell invasion, entry into the vasculature, exiting of
carcinoma cells from the circulation and colonization at distal
sites (15). The local axillary
lymph nodes are initially involved in metastatic BC (16). In patients with BC, a detailed and
comprehensive assessment of the condition of the axillary lymph
nodes is important for determining prognosis (16).
In our previous study, proteomic profiles of BC
subtypes were compared and the existence of differentially
regulated proteins was reported (6). Among the differentially regulated
proteins, GPD1 and MAGL showed significant downregulation in tumor
tissues compared with controls collected from the adjacent
non-tumor tissue, which suggested that these two proteins may
possess biomarker properties for the diagnosis of BC. Furthermore,
GPD1 and MAGL were more notably downregulated in the TNBC subtype
(an aggressive form of BC) compared with the controls, which
indicated their possible role in cancer metastasis (6). Further investigation by our group
indicated that these two proteins were also present at lower levels
in serum samples from patients with BC compared with the serum
samples from healthy controls (9).
Similar to these findings, Zhou et al (8) independently reported GPD1 as a
potential tumor suppressor protein upon investigating changes in
mRNA expression levels in BC tissues. The study demonstrated that
GPD1 was significantly downregulated in BC tissues compared with
controls. Additionally, low GPD1 expression levels were correlated
with lower survival rates.
The differential regulation of GPD1 is not unique to
BC as GPD1 is also downregulated in other cancer types, including
bladder cancer, ovarian cancer and renal clear cell carcinoma
(17–19). However, to the best of our
knowledge, there has not yet been a detailed study investigating
the involvement of GPD1 in cancer metastasis. Therefore, in the
present study, changes in GPD1 and MAGL expression levels in BC
metastasized to axillary lymph nodes were investigated. Both
proteins were significantly downregulated in the axillary lymph
nodes of patients from all BC subtypes compared with the controls,
which indicated the potential involvement of these two proteins in
metastasis. However, the cause of this downregulation in BC and how
much contribution GPD1 and MAGL make to the metastatic process is
currently unknown.
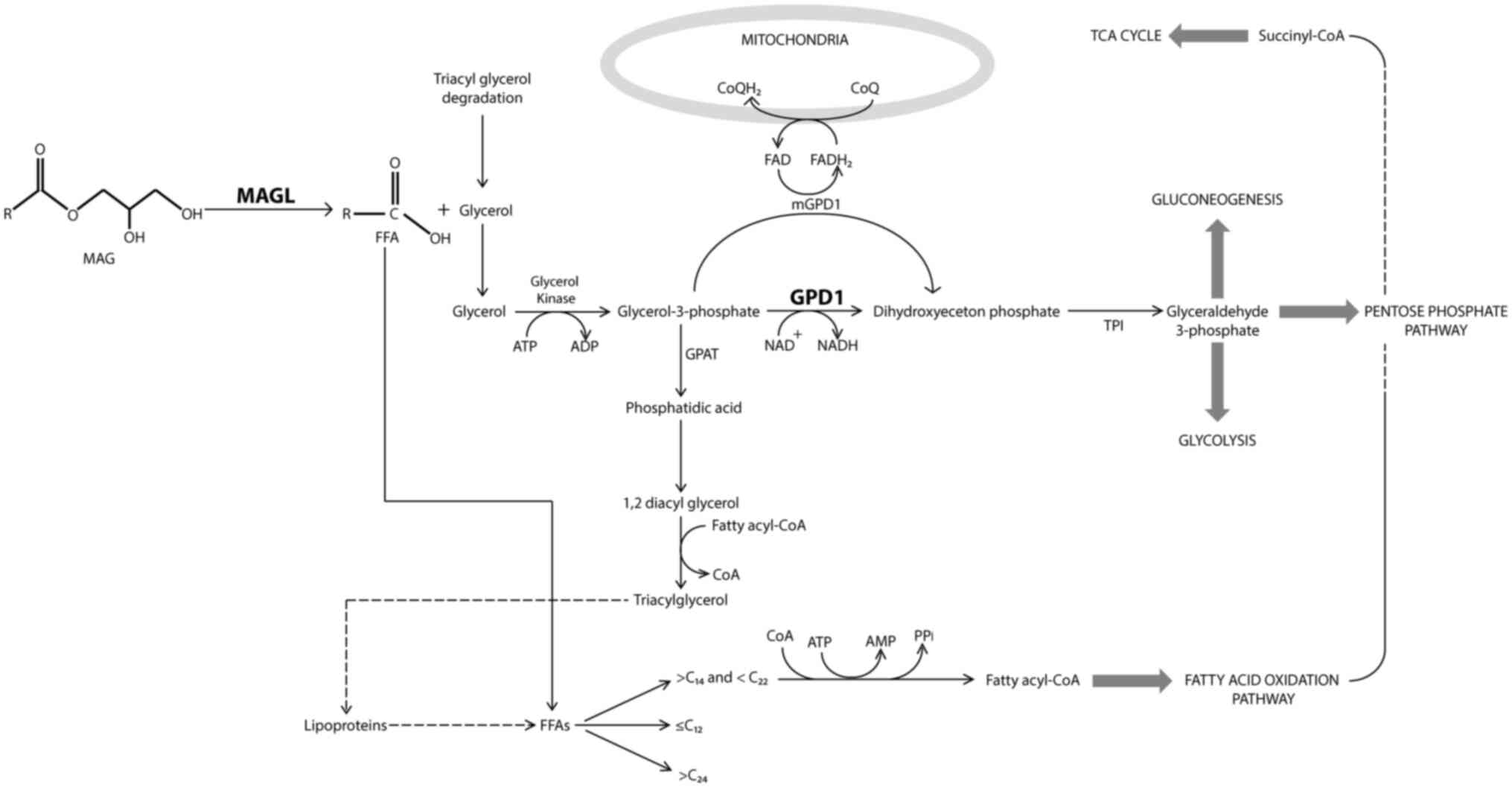
Glycerol is a key metabolite likely involved in the
cross talk between MAGL and GPD1 (Fig.
3). MAGL catalyzes the hydrolysis of monoacylglycerols in the
production of free fatty acids and glycerol (20). The free fatty acids are then used in
numerous metabolic processes, including the production of fatty
acyl-CoA, which then undergoes fatty acid oxidation for energy
release. The glycerol produced by MAGL can be converted to
glycerol-3-phosphate (G-3-P) by glycerol kinase, which then serves
as a substrate for certain enzymes such as glycerol-3-phosphate
dehydrogenase, glycerol-3-phosphate acyltransferase and
glycerol-3-phosphate deacylase (21). G-3-P can be converted to dihydroxy
acetone phosphate by GPD1, which is used as a source in
carbohydrate metabolism. Depending on the cellular energy
requirement, G-3-P can be used in glycolysis, gluconeogenesis or
the pentose phosphate pathway. G-3-P is also the substrate for the
enzyme, glycerol-3-phosphate acyl transferase (GPAT) (22). GPAT produces the simplest form of
phospholipids, phosphatidic acid, which is then used for the
production of triacylglycerols using fatty acyl-CoA. It is known
that triacylglycerols are good for storing free fatty acids for
later use (23). Looking at this
metabolic scheme, it is clear that there is a complex intertwined
chain of events occurring within cells. A decrease in MAGL levels
may result in a decrease in GPD1 levels since MAGL produces the
precursor molecule, glycerol, for the production of G-3-P, which in
turn is a substrate of GPD1. The decrease in GPD1 levels could
direct the synthesis of triacylglycerols via the GPAT-associated
pathway. However, whether GPAT levels in BC tissues are associated
with a downregulation in GPD1 remains to be determined. A
functional proteomics study comparing the activities of MAGL, GPD1
and other metabolically relevant enzymes would also indicate how
the activities of these enzymes change in parallel to the changes
observed at the protein expression levels.
A limitation of the present study was the low number
of enrolled cases. However, it was challenging to identify and
include suitable cases for inclusion in the study, as not all
patients wanted to participate and samples could only be used from
Kocaeli University Medical School according to Ethics Committee
requirements. Additionally, certain potential participants who met
the inclusion criteria declined to take part. As a result, the
process of collecting samples extended beyond 2 years. Despite
these challenges, the preliminary results of the present study may
pave the way for future studies with a larger number of enrolled
cases.
The present study was a follow-up study,
specifically aimed at addressing a particular question: The
association between GPD1 and MAGL protein levels and lymph node
metastasis. To further establish this association, western
blotting, ELISA, flow cytometry analysis and targeted mass
spectrometry analysis could be performed. In combination, these
approaches may provide a more comprehensive understanding of the
protein levels in lymph node metastasis. The western blotting
analysis performed in the present study indicated that GPD1 and
MAGL proteins were downregulated in lymph node metastasis,
particularly in the TNBC subtype when compared with the control
samples. Additional methods, such as ELISA, flow cytometry and
targeted mass spectrometry analysis, could be employed in future
studies to establish reference values for tumor diagnosis and
monitoring. Performing such studies would necessitate more
extensive sample collection and substantial financial resources,
and future work will need to meet the requirements in this
regard.
In conclusion, an accelerated lipid metabolism is
crucial to support cellular proliferation and biosynthetic
activities in cancer cells (24).
Evidence suggests that cell proliferation can be suppressed through
a reduction in the availability of fatty acids to the cell, making
free fatty acids play a central and important role in the process
of the suppression of cell proliferation (25). In addition, aggressive cancer cells
have been shown to have a higher lipid content and elevated
lipogenic and lipolytic switching. MAGL and GPD1 are important for
the modulation of lipid synthesis, along with GPAT, which has
previously been determined to be an effective target for cancer
treatment. Future research should therefore address the possible
utility of these three proteins as tools in diagnostic, prognostic
or therapeutic strategies.
Acknowledgements
The authors would like to thank Professor Dr Muge
Alvur (Department of Family Medicine, Kocaeli University Medical
School, Kocaeli, Turkey) for providing valuable comments during the
statistical analysis of the data.
Funding
This study was supported by Kocaeli University Scientific
Research Projects Coordination Unit (grant no. 2014/057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, MGBA, GA, ZC and MK confirm the authenticity of
all the raw data. Study conception and experimental design were
performed by MK and GA. Tissue collection during surgeries and
storage were performed by TS and NZC. Sample preparation and
protein isolations were performed by MGBA and TS. Western blotting
analyses were performed by TS, MGBA, MK, and GA. Data analyses were
performed by MGBA and GA. All authors read and approved the final
version of the manuscript.
Ethical approval and consent to
participate
This study was approved by The Ethics Committee of
Kocaeli University (Kocaeli, Turkey; approval no. KOU
GOKAEK-2019/16.04 2019/139). Written informed consent, approved by
the ethics committee, was obtained for each patient before
participation in the study. The Declaration of Helsinki was
complied with to safeguard human subjects and uphold the highest
ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of ıncidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giaquinto AN, Sung H, Miller KD, Kramer
JL, Newman LA, Minihan A, Jemal A and Siegel RL: Breast cancer
statistics, 2022. CA Cancer J Clin. 72:524–541. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eliyatkın N, Yalçın E, Zengel B, Aktaş S
and Vardar E: Molecular classification of breast carcinoma: From
traditional, old-fashioned way to a new age, and a new way. J
Breast Health. 11:59–66. 2013. View Article : Google Scholar
|
|
6
|
Yoneten KK, Kasap M, Akpınar G, Gunes A,
Gurel B and Utkan NZ: Comparative proteome analysis of breast
cancer tissues highlights the ımportance of glycerol-3-phosphate
dehydrogenase 1 and monoacylglycerol lipase in breast cancer
metabolism. Cancer Genomics Proteomics. 16:377–397. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q,
Wei W, Zhang Y and Sun S: Breast cancer subtypes predict the
preferential site of distant metastases: A SEER based study.
Oncotarget. 8:27990–27996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Yu J, Wang M, Yang J, Xiong H,
Huang H, Wu D, Hu S, Wang Y, Chen XZ and Tang J: Identification of
glycerol-3-phosphate dehydrogenase 1 as a tumour suppressor in
human breast cancer. Oncotarget. 8:101309–101324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoneten KK, Kasap M, Arga KY, Akpinar G
and Utkan NZ: Decreased serum levels of glycerol-3-phosphate
dehydrogenase 1 and monoacylglycerol lipase act as diagnostic
biomarkers for breast cancer. Cancer Biomark. 34:67–76. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Liu Z, Lian Z, Liao R, Chen Y,
Qin Y, Wang J, Jiang Q, Wang X and Gong J: Monoacylglycerol lipase:
A novel potential therapeutic target and prognostic ındicator for
hepatocellular carcinoma. Sci Rep. 6:357842016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams SL, Birdsong GG, Cohen C and
Siddiqui MT: Immunohistochemical detection of estrogen and
progesterone receptor and HER2 expression in breast carcinomas:
Comparison of cell block and tissue block preparations. Int J Clin
Exp Pathol. 2:476–480. 2009.PubMed/NCBI
|
|
12
|
Ye L, Zhang B, Seviour EG, Tao KX, Liu XH,
Ling Y, Chen JY and Wang GB: Monoacylglycerol lipase (MAGL)
knockdown inhibits tumor cells growth in colorectal cancer. Cancer
Lett. 307:6–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong JM, Li J, Kang AD, Huang SQ, Liu WB,
Zhang Y, Liu ZH and Zeng L: Protein S100-A8: A potential
metastasis-associated protein for breast cancer determined via
iTRAQ quantitative proteomic and clinicopathological analysis.
Oncol Lett. 15:5285–5293. 2018.PubMed/NCBI
|
|
14
|
Zhang X: Molecular classification of
breast cancer: Relevance and challenges. Arch Pathol Lab Med.
147:46–51. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen F, Li X, Lin X, Chen L, Lin Z, Wu H
and Chen J: Can axillary lymph node dissection be omitted in breast
cancer patients with metastatic sentinel lymph nodes undergoing
mastectomy? A systematic review and meta-analysis of real-world
evidence. World J Surg. 47:2446–2456. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, He X, Yin H, Cao W, Lin T, Chen
W, Diao W, Ding M, Hu H, Mo W, et al: Allosteric activation of the
metabolic enzyme GPD1 inhibits bladder cancer growth via the
lysoPC-PAFR-TRPV2 axis. J Hematol Oncol. 15:932022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen LY, Huang RL, Su PH, Chu LH, Weng YC,
Wang HC, Lai HC and Wen KC: Epigenomic profiling of epithelial
ovarian cancer stem-cell differentiation reveals GPD1 associated
ımmune suppressive microenvironment and poor prognosis. Int J Mol
Sci. 23:51202022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu R, Feng Y, Deng Y, Zou Z, Ye J, Cai Z,
Zhu X, Liang Y, Lu J, Zhang H, et al: A HIF1α-GPD1 feedforward loop
inhibits the progression of renal clear cell carcinoma via
mitochondrial function and lipid metabolism. J Exp Clin Cancer Res.
40:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nomura DK, Long JZ, Niessen S, Hoover HS,
Ng SW and Cravatt BF: Monoacylglycerol lipase regulates a fatty
acid network that promotes cancer pathogenesis. Cell. 140:49–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi K and Reue K: Biochemistry,
physiology, and genetics of GPAT, AGPAT, and lipin enzymes in
triglyceride synthesis. Am J Physiol Endocrinol Metab.
296:E1195–E1209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Loh K, Song ZY, Yang HQ, Zhang Y and
Lin S: Update on glycerol-3-phosphate acyltransferases: The roles
in the development of insulin resistance. Nutr Diabetes. 8:342018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmadian M, Duncan RE, Jaworski K,
Sarkadi-Nagy E and Sul HS: Triacylglycerol metabolism in adipose
tissue. Futur Lipidol. 2:229–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu Y, Zou T, Shen X, Nelson PJ, Li J, Wu
C, Yang J, Zheng Y, Bruns C, Zhao Y, et al: Lipid metabolism in
cancer progression and therapeutic strategies. MedComm (2020).
2:27–59. 2020.PubMed/NCBI
|
|
25
|
Wang S, Wang Y, Wang Y, Li Q, Zeng K, Li X
and Feng X: Myc derived circRNA promotes triple-negative breast
cancer progression via reprogramming fatty acid metabolism. Discov
Oncol. 14:672023. View Article : Google Scholar : PubMed/NCBI
|