Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and fourth leading cause of cancer-related
death worldwide (1). HCC often
presents as a hyperenhancement in the arterial phase of dynamic
computed tomography (CT) or dynamic magnetic resonance imaging
(MRI) and washout in the portal vein phase or equilibrium phase.
The latter test has a sensitivity of 66–82% and a specificity of
91–92% (2). HCC cases with typical
patterns can be diagnosed by contrast-enhanced ultrasound, CT or
MRI, and pathological diagnosis is not required, which avoids the
risk of tumor seeding via biopsy. Sarcomatoid HCC (SHCC) is a rare
and highly lethal subtype of HCC that is characterized by the
presence of spindle-shaped, pleomorphic or bizarre giant cells
(3,4), with an incidence rate of 1.7–1.9% in
postoperative HCC cases (5). It has
been reported that SHCC is associated with a 3-year overall
survival rate of <20% (6–8), and
in some cases, repeated non-surgical therapies of HCC can lead to
necrosis and degeneration of hepatocytes, resulting in SHCC
(9). Compared with conventional
HCC, SHCC has been reported to have larger tumor sizes, a higher
incidence of lymph node metastasis, a higher proportion of advanced
lesions and a significantly poorer overall and disease-free
survival (5). Thus, SHCC is
characterized by an aggressive clinical course and a high incidence
of early recurrence (10).
At present, no standardized therapy has been
established for this rare type of cancer, and its pathogenesis
remains largely unclear. Previous integrated genomic analyses of
HCC samples demonstrated that catenin b1 (40%) and TP53
(21%) mutations are mutually exclusive and define two notable
groups of HCC characterized by distinct phenotypes, but few studies
have investigated the molecular features of SHCC (11). Sarcomatoid carcinoma of various
tissues, including SHCC, is characterized pathologically by the
presence of both carcinomatoid and sarcomatoid components, with
intratumor heterogeneity and a propensity for intratumor
transformation from carcinomatoid to sarcomatoid type (12,13).
The distinction between sarcomatoid and carcinomatoid components in
SHCC is clinically important because of potential impact on
treatment strategies and patient outcomes. Understanding the
molecular characteristics of these components through whole
transcriptome analysis may identify potential biomarkers for more
accurate SHCC diagnosis, leading to improved patient care and
tailored therapeutic interventions. Therefore, the aim of the
present study was to clarify the molecular characteristics of the
sarcomatoid component compared with the carcinomatoid component,
based on the whole transcriptome, and to identify a new biomarker
for the diagnosis of SHCC.
Materials and methods
Patients and tissue samples
A search for patients diagnosed with SHCC at three
institutions [Kansai Medical University Hospital (Hirakata, Japan),
Osaka Metropolitan University Hospital (Osaka, Japan) and Kindai
University Hospital (Osakasayama, Japan)] over ~25 years (dating
back from 2019) was conducted at the time of ethical approval.
Subsequently, patients diagnosed with SHCC at the three
institutions between 2006 and 2015 were retrospectively selected
based on their medical records. This was due to specimens collected
before 2006 not being preserved at the hospitals or being too old
for use. In addition, no SHCC cases were identified between 2016
and 2019. From this pool, formalin-fixed, paraffin-embedded (FFPE)
tumor tissues that had been collected and stored for diagnostic
purposes at the time of surgery were obtained from 7 patients with
SHCC who underwent surgical resection at Kansai Medical University
Hospital (n=4), Osaka Metropolitan University Hospital (n=1) and
Kindai University Hospital (n=2) between March, 2006 and December,
2015. The inclusion criteria for patient selection in the present
study were as follows: Patients with HCC who underwent surgical
resection and had carcinomatoid and sarcomatoid tumor components as
confirmed by pathological diagnosis following tumor resection. No
specific exclusion criteria were applied in the present study,
except for the absence of an adequate amount of FFPE specimen for
analysis. All patients provided written informed consent to
participate in the present study and for tumor tissue sample
collection for analysis. SHCC was diagnosed during the
postoperative pathological examination by pathologists at each
institution. SHCC specimens were further analyzed by the same
pathologist during the present study. The present study was
conducted in accordance with the Declaration of Helsinki and the
Ethical Guidelines for Medical and Health Research Involving Human
Subjects in Japan (14). The
Institutional Ethics Review Boards of Kansai Medical University
Hospital (approval no. 2019074), Osaka Metropolitan University
Hospital (approval no. 2020-185) and Kindai University Faculty of
Medicine (approval no. 31-201) approved the present study.
Whole transcriptome analysis
The FFPE samples were subjected to RNA extraction
using the Allprep DNA/RNA FFPE Kit (cat. no. 80234; Qiagen, Inc.)
according to the manufacturer's instructions. The quality and
quantity of the RNA were determined with NanoDrop 2000 device
(Thermo Fisher Scientific, Waltham, MA) and RiboGreen dsDNA Assay
Kit (cat. no. R11490; Thermo Fisher Scientific). The AmpliSeq
Transcriptome Human Gene Expression Kit (cat. no. A26325; Thermo
Fisher Scientific, Inc.) was subsequently used for whole
transcriptome analysis according to the manufacturer's
instructions. For library preparation, barcoded cDNA libraries were
generated from 10 ng total RNA using the SuperScript VILO cDNA
Synthesis Kit (cat. no. 11754050; Thermo Fisher Scientific, Inc.).
The cDNA was then amplified for 16 cycles following addition of the
PCR master mix and AmpliSeq human transcriptome gene expression
primer pool (Thermo Fisher Scientific, Inc.). After multiplex PCR,
IonXpress Barcode Adapters (cat. no. 4474517; Thermo Fisher
Scientific, Inc.) were ligated to the PCR products and purified
using Agencourt AMPure XP beads (cat. no. A63881; Beckman Coulter,
Inc.). The purified libraries were quantified using the Ion Library
TaqMan Quantitation Kit (cat. no. 4468802; Thermo Fisher
Scientific, Inc.) and the adjusted to 50 pM with low TE buffer (10
mM Tris-HCl, pH 8.0, 0.1 mM EDTA), and then pooled and sequenced
using an Ion Torrent S5 system (Thermo Fisher Scientific, Inc.) and
an Ion 550 Chip Kit (cat. no. A34538; Thermo Fisher Scientific,
Inc.). The Ion Torrent S5 system produces single-end short-reads
with 120-bp read length. Base calling, alignment to the human
reference genome (hg19) and quality control were performed using
Ion Torrent Suite v5.12 software (Thermo Fisher Scientific, Inc.).
The raw reads were analyzed using the AmpliSeqRNA plugin to
generate gene-level expression values for all 20,802 human
reference sequence (Seq) genes.
Gene selection and pathway
analysis
Data were processed with Transcriptome Analysis
Console (ver. 4.0.3; Thermo Fisher Scientific, Inc.) to determine
the differentially expressed genes (DEGs). DEGs were selected using
|fold change| >2 and P<0.05 as the cut-off. Hierarchical
clustering of these genes was performed using the mean linkage
method with 1-Pearson correlation coefficient as the distance
measure using Morpheus (software.broadinstitute.org/morpheus/).
Functional and pathway enrichment analysis was performed using
Metascape (ver. 3.5; accessed on 21 Feb 2023) (15).
Immunohistochemistry (IHC)
FFPE sections of 4-µm thickness were stained for
polybromo 1 (PBRM1) using a validated and published IHC method
(16,17). Briefly, FFPE tissue samples were
sectioned and placed on positively charged slides. Sections were
deparaffinized, hydrated and pretreated in DAKO target retrieval
solution (cat. no. S1699; Agilent Technologies, Inc.) for 20 min in
a steamer. The VECTASTAIN ABC-HRP Kit, Peroxidase (Rabbit IgG)
(cat. no. PK-4001; Vector Laboratories, Inc.) was used and includes
the blocking serum and the 2nd HP antibody. The procedure was
carried out according to the manufacturer's instructions. The
slides were blocked in goat blocking serum for 30 min at room
temperature, washed in phosphate buffered saline (PBS) and then
incubated with rabbit anti-PBRM1 monoclonal antibody (1:50; cat.
no. 38439; Cell Signaling Technology, Inc.) overnight at 4°C. The
slides were then washed in PBS and incubated with the supplied
biotinylated anti-rabbit secondary antibody for 30 min at room
temperature. The slides were washed in PBS and incubated with the
supplied VECTASTAIN Elite ABC Reagent for 30 min at room
temperature. The slides were washed in PBS and developed in DAB
(cat. no. 8801-4965-72, Invitrogen; Thermo Fisher Scientific, Inc.)
and counterstained with hematoxylin for 2 min at room temperature.
Assessment of immunohistochemical staining was performed on scanned
sections captured at magnification, ×10 using the Keyence BZ-X810
All-in-One light microscope. H scores were calculated for PBRM1
positivity in the sarcomatoid and carcinomatoid regions using
QuPath v0.2.0-m4 image analysis software (https://qupath.github.io/) (18,19).
Statistical analysis
PBRM1 gene expression was compared by
unpaired t test. Protein expression was compared using the Wilcoxon
signed-rank test. All statistical analyses were performed using
GraphPad Prism software (version 8.4; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of differentially
expressed genes in the sarcoma tissue components
A total of 7 tumor specimens, of which 5 contained
sarcomatoid components and 7 contained carcinoma components, were
obtained from 7 patients (Table I).
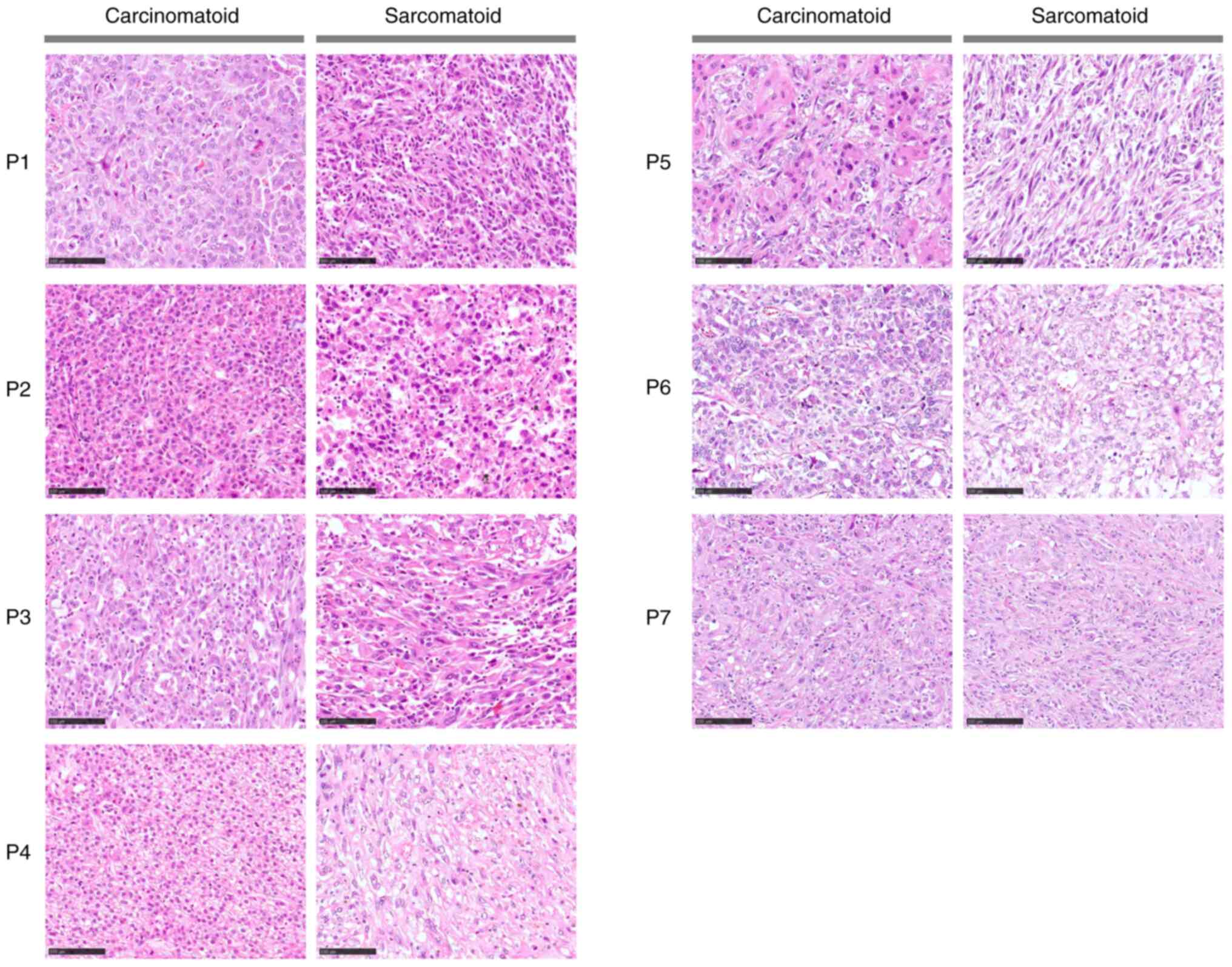
Representative images of hematoxylin and eosin-stained sections
showing histological differences between the sarcomatoid and
carcinomatoid components in SHCC are presented in Fig. 1. Following whole transcriptome
analysis of the samples, 336 genes were found to be differentially
expressed between the sarcomatoid and carcinomatoid components. The
top 10 downregulated and top 10 upregulated differentially
expressed genes are shown in Table
II. The sarcomatoid components had increased ZRANB1
(zinc finger RANBP2-type containing 1), IGF2BP1
(Insulin-like growth factor 2 mRNA binding protein 1), DHRSX
(dehydrogenase/reductase X-linked), TMEM19 (Transmembrane
protein 19), DNAJC9 (DnaJ homolog subfamily C member 9),
UBA7 (Ubiquitin-activating enzyme 7), OR10K2
(Olfactory receptor family 10 subfamily K member 2), DEPDC1B
(DEP domain containing 1B), ATP10D (ATPase phospholipid
transporting 10D (putative)) and HSPA7 (Heat shock protein
family A (Hsp70) member 7 (pseudogene)) expression levels, and
decreased ADRA2B (Adrenoceptor alpha 2B), NHLRC1 (NHL
repeat containing E3 ubiquitin protein ligase 1), PBRM1,
DHCR7 (7-dehydrocholesterol reductase), SPATA7
(Spermatogenesis associated 7), WRB (Tryptophan-rich basic
protein), PAFAH1B1 (Platelet activating factor
acetylhydrolase 1B regulatory subunit 1), PET112,
(Glutamyl-tRNA amidotransferase subunit B), GDA (Guanine
deaminase) and MRPS5 (Mitochondrial ribosomal protein S5)
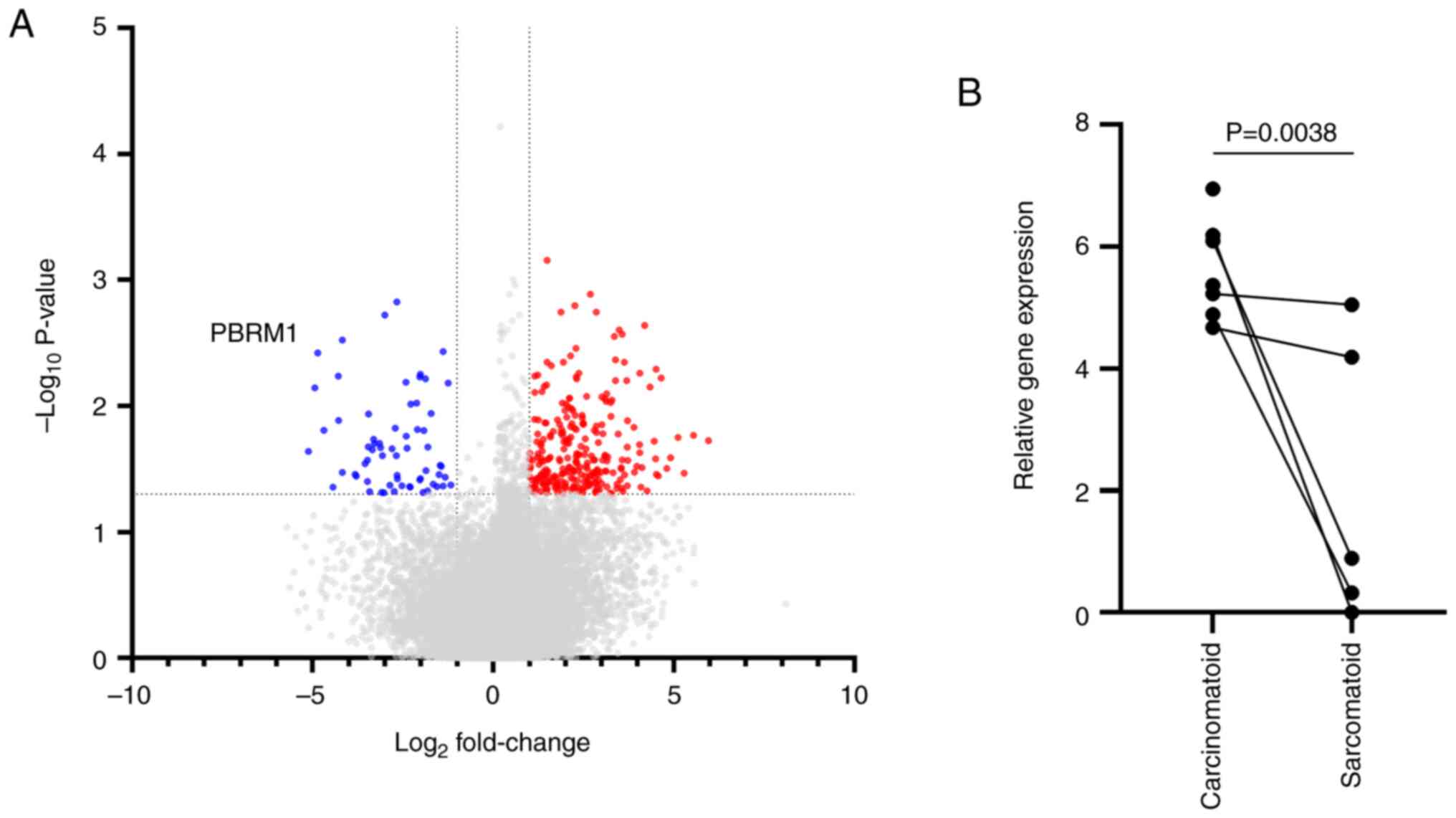
expression levels. Moreover, visualization of the differentially
expressed genes using a volcano plot indicated that decreased
expression of PBRM1 in the sarcoma component of SHCC tissues
may serve as a biologically relevant marker of the sarcoma subtype
(Fig. 2).
 | Table I.Patients and sample information. |
Table I.
Patients and sample information.
|
|
|
|
|
|
|
| Tissue
availability |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
| Gene
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient ID | Age, years | Sex | AFP, ng/ml | PIVKA-II,
mAU/ml | Hepatitis
status | Tumor size, mm | Carcinomatoid
component | Sarcomatoid
component | Protein
expression |
|---|
| P1 | 63 | Female | 4.3 | 30.0 | - | 55×45×50 | Yes | Yes | Yes |
| P2 | 70 | Male | 3959.0 | 61.0 | - | 105c | Yes | Yes | Yes |
| P3 | 69 | Male | 2.7 | 22.0 | Ca | 80×100×60 | Yes | No | Yes |
| P4 | 72 | Male | 2.0 | 1425.0 | - | 65×60×60 | Yes | Yes | Yes |
| P5 | 69 | Female | 7.8 | 61.0 | Ca | 50c | Yes | No | Yes |
| P6 | 67 | Male | 3.0 | 64107.0 | - | 109×118×113 | Yes | Yes | Yes |
| P7 | 65 | Male | 11.0 | 3.0 | Bb | 32×29×38 | Yes | Yes | Yes |
 | Table II.Top 10 differentially expressed genes
between sarcoma and carcinoma components, listed based on the fold
change. |
Table II.
Top 10 differentially expressed genes
between sarcoma and carcinoma components, listed based on the fold
change.
| A, Downregulated in
the sarcoma component |
|---|
|
|---|
| Gene | Fold change | P-value |
|---|
| ADRA2B | −34.80 | 0.0229 |
| NHLRC1 | −30.70 | 0.0072 |
| PBRM1 | −29.09 | 0.0038 |
| DHCR7 | −25.82 | 0.0156 |
| SPATA7 | −21.67 | 0.0440 |
| WRB | −19.56 | 0.0058 |
|
PAFAH1B1 | −19.35 | 0.0130 |
| PET112 | −18.19 | 0.0030 |
| GDA | −18.14 | 0.0337 |
| MRPS5 | −14.04 | 0.0349 |
|
| B, Upregulated
in the sarcoma component |
|
| Gene | Fold
change | P-value |
|
| ZRANB1 | 62.08 | 0.0189 |
| IGF2BP1 | 46.88 | 0.0171 |
| DHRSX | 39.15 | 0.0342 |
| TMEM19 | 34.95 | 0.0178 |
| DNAJC9 | 30.09 | 0.0258 |
| UBA7 | 27.91 | 0.0313 |
| OR10K2 | 24.94 | 0.0060 |
| DEPDC1B | 23.72 | 0.0360 |
| ATP10D | 22.88 | 0.0348 |
| HSPA7 | 22.71 | 0.0263 |
Gene enrichment analysis of the
differentially expressed genes
Next, the biological relevance of genes
differentially expressed between the sarcomatoid and carcinomatoid
components of SHCC was investigated. Subsequently, 336 genes were
identified as differentially expressed genes between the
sarcomatoid and carcinomatoid components of the tumors.
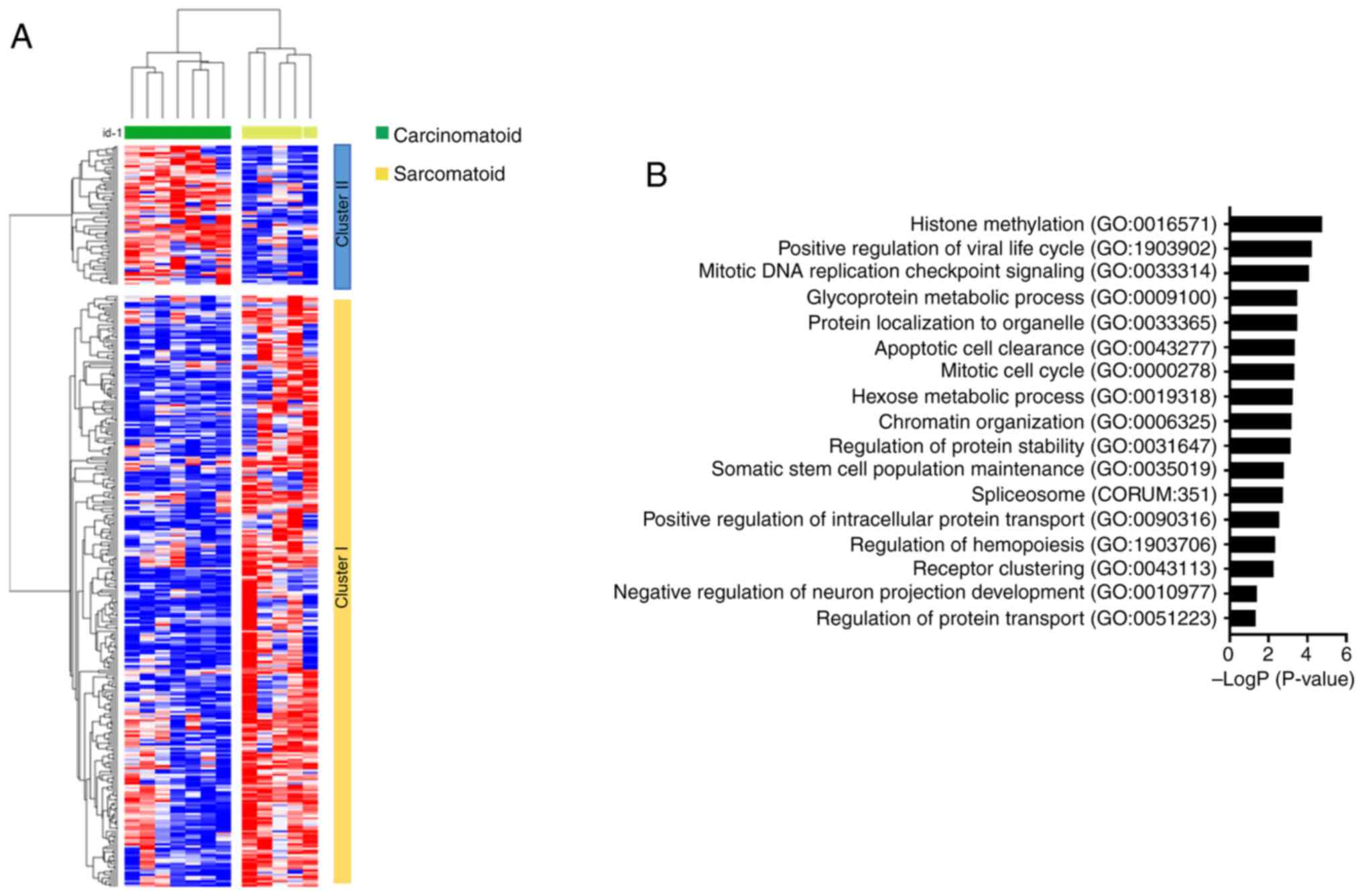
Unsupervised hierarchical clustering analysis of the 336
differentially expressed genes revealed two distinct clusters,
clusters I and II, which showed a strong association to the
sarcomatoid and carcinomatoid components, respectively (Fig. 3A). To determine the biological
relevance of the genes downregulated in the sarcomatoid components,
a gene ontology analysis was performed using Metascape, and
biological pathways enriched in genes from cluster II were
identified. The pathways enriched in this gene cluster included
‘histone methylation’ and ‘mitotic DNA replication checkpoint
signaling’, which indicated that these processes were disrupted in
the sarcomatoid components (Fig.
3B). These results supported our hypothesis that impaired
function of PBRM1 may be associated with changes resulting
in sarcomatoid differentiation.
Quantitative analysis of PBRM1 protein
expression in SHCC
The aforementioned findings of the present study
indicated the presence of altered PBRM1 expression in SHCC,
which indicated that PBRM1 may serve as a marker of
sarcomatoid differentiation. To evaluate the potential of
PBRM1 as a marker, PBRM1 protein expression levels in
sarcomatoid and carcinomatoid components of SHCC tissues were
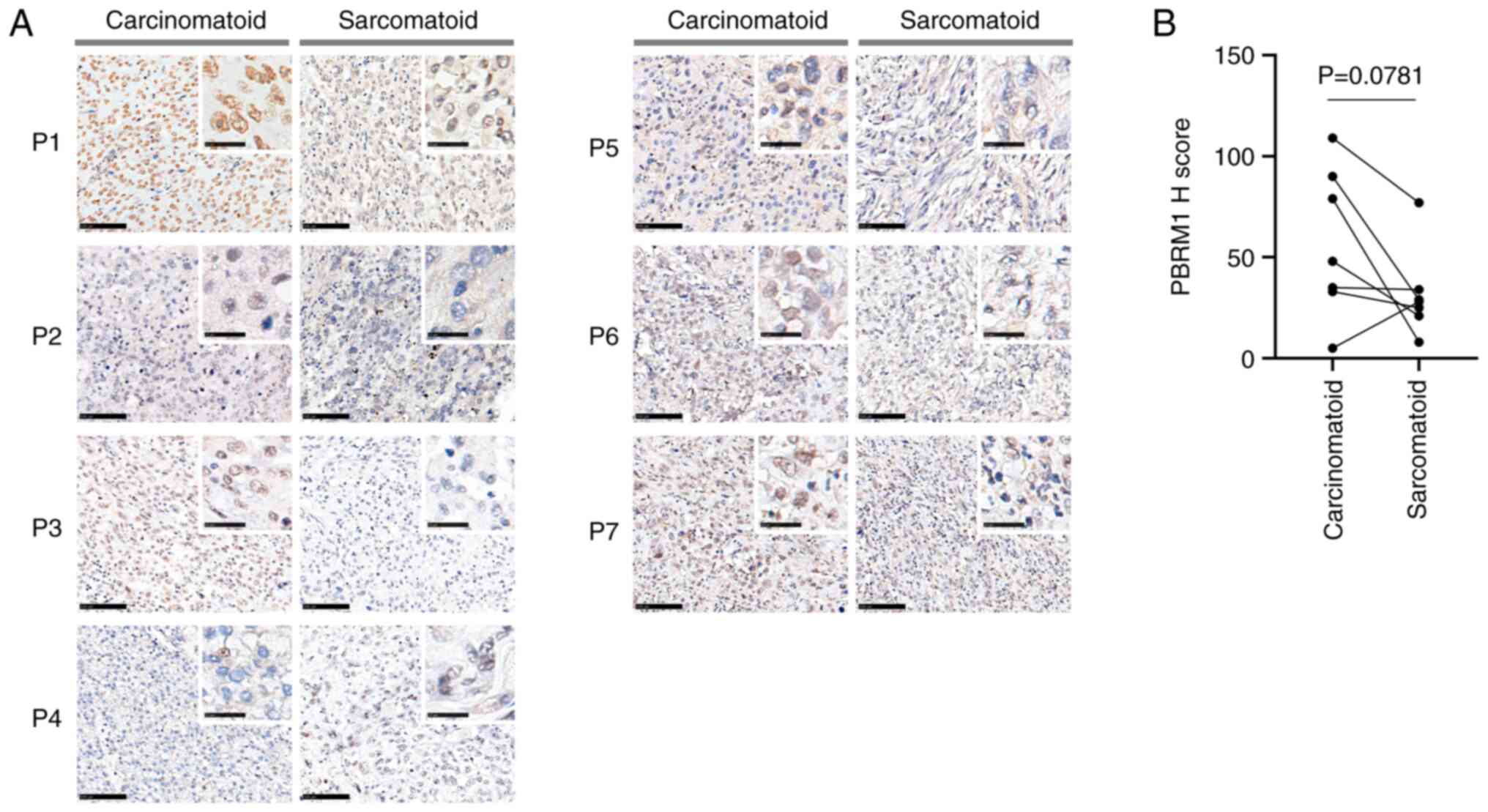
examined by quantitative IHC. The tumors were categorized as
PBRM1+ when the cells had strong diffuse nuclear
staining, and as PBRM1− when the cells had an absence of
diffuse nuclear staining (Fig. 4A)
(19). Quantitative analysis
demonstrated that the PBRM1 protein expression level was markedly
lower was lower in the sarcomatoid than the carcinomatoid
component, although the difference was not statistically
significant (P=0.0781; Fig. 4B).
Quantitative analysis revealed a noticeable reduction in PBRM1
protein expression levels within the SHCC, although statistical
significance was not achieved (P=0.0781; Fig. 4B). This discovery implies a
potential link between diminished PBRM1 protein expression and
sarcomatoid tumors, offering preliminary insights into the
biological role of PBRM1 in these tumors. Nonetheless, further
investigations employing larger datasets are imperative to
ascertain the utility of PBRM1 in IHC as a biomarker for
distinguishing sarcomatoid components.
Discussion
The present study revealed that expression of the
PBRM1 gene was reduced in the sarcomatoid differentiated
tumor components of SHCC tissues, compared with the matched
carcinomatoid components. PBRM1 is the second most highly
expressed gene in clear cell renal cell carcinoma (ccRCC) after the
von Hippel-Lindau tumor suppressor gene, and it is located
on the short arm of chromosome 3 (3p). Truncating mutations in
PBRM1 are found in 41% of ccRCC cases, and genetic
alterations in PBRM1 have been detected in 4% of HCC cases
included in The Cancer Genome Atlas database (20). Several previous studies have
suggested that a loss of PBRM1 protein may be a potential biomarker
of RCC and associated with adverse pathological factors and poor
patient prognosis in this disease (19,21,22).
However, to the best of our knowledge, the expression status of
PBRM1 in SHCC has not been previously reported. In the
present study, decreased PBRM1 expression in the sarcomatoid
components of SHCC was demonstrated, although DNA sequencing of the
PBRM1 gene in the sample cohort was unsuccessful (data not
shown). Gene expression analysis using FFPE specimens failed in two
components of the sarcoma-like component. of samples, suggesting
the importance of high-quality specimens, such as frozen specimens.
Frozen specimens are superior to FFPE samples for sequencing due to
the absence of artificial alterations caused by formalin fixation.
Gene mutation analysis using DNA is greatly affected by chemical
modifications of nucleobases (especially C>T changes) because it
analyzes base changes in sequences. On the other hand, the gene
expression analysis used in this study is based on a method that
amplifies a set region for each gene and estimates changes in gene
expression between samples based on the number of reads obtained,
and thus is considered to be less susceptible to the effects of
aging degradation and artificial changes in bases due to formalin
fixation than DNA analysis.
The biological significance of downregulated PBRM1
expression is largely unknown, but the gene ontology analyses of
the present study suggested that chromatin-related pathways are
altered in SHCC, suggesting that reduced PBRM1 expression
may affect chromatin function, given that PBRM1 is an
essential gene for chromatin remodeling (23). Other identified differentially
expressed genes may also serve a role in the alteration of
chromatin-related pathways in SHCC. While reduced PBRM1 expression
is suggested to affect chromatin function due to its role in
chromatin remodeling, it is common for multiple genes to
collaborate or interact in complex biological processes. Other
differentially expressed genes identified in the study could
potentially contribute to the alteration of chromatin-related
pathways through various mechanisms, such as transcriptional
regulation, protein-protein interactions, or downstream signaling
pathways. Moreover, this finding was consistent with the molecular
functions of PBRM1. PBRM1 is part of a protein associated
with cell proliferation, and its role is of particular interest in
cancer; PBRM1 is a component of the BAF complex (Brahma associated
factor complex), which is involved in chromatin remodeling and
regulation of gene expression (24). Mutations or deletions in
PBRM1 are associated with some types of cancer, particularly
renal cell carcinoma, and may affect the abnormal cell growth of
cancer cells (25). Therefore,
PBRM1 is considered one of the genes that play an important
role in cancer research. It has also been reported to be involved
in cell proliferation, such as being a regulator of TP53,
which plays a central role in the cell cycle check mechanism
(26), or PBRM1 being
regulated by TP53 to become a critical regulator of p21
(27,28). Further research and functional
studies would be needed to determine the specific roles of these
genes and how they collectively impact chromatin function in SHCC.
Functional deletion of PBRM1 has been previously reported to
be associated with an upregulation of IL-6 and its downstream
molecules, such as TNFα, which act on surrounding T cells to
stimulate cancer immunity (29). In
particular, JAK-STAT3 is also known to be involved in interferon
signaling (30,31), which appears to be relevant to
kidney cancer treatments because interferon therapy, which exhibits
immunomodulatory, anti-angiogenic, and direct antitumor activity,
has been used as a treatment for HCC (32). Loss of PBRM1 function suppresses the
expression of β2-microglobulin (33), which is required for cancer antigen
presentation, thereby reducing the efficacy of immune checkpoint
inhibitor therapy. However, additional studies involving more
samples are needed to validate these findings.
The present study has limitations, including its
retrospective design, the absence of blood sample analysis, and a
small sample size. Blood samples play a crucial role in diagnosis,
and the absence of their analysis in this study hinders clinical
application. To confirm the present findings and advance the
development of a simplified diagnostic method for SHCC, future
studies should involve larger sample sizes and incorporate blood
sample analysis. Another limitation of the present study is that
the RNA-seq results could not be confirmed by reverse
transcription-quantitative PCR. This is, due to the retrospective
nature of the present study, because frozen or freshly isolated
tissues were not available. A further limitation of the present
study is an absence of normal tissue samples, again due to its
retrospective nature. In 4 selected cases, whole transcriptome
analysis was performed using RNA extracted from a small region of
normal cells in the same tissue section that the pathologist
determined to be normal hepatocytes. However, evaluable data was
obtained from only 1 case, and therefore, the results of the normal
tissue analysis were not included in the present study.
The present study demonstrated that PBRM1
expression status may serve as a diagnostic marker for SHCC when
assessed through gene expression analyses or IHC. However, it's
important to note that these findings should be interpreted with
caution, as the study did not compare the results with normal
tissues, and the IHC results comparing the sarcomatoid and
carcinomatoid components were not statistically significant.
Further validation and comparison with normal tissues would be
necessary to confirm the utility of PBRM1 as a diagnostic marker
for SHCC. The expression of PBRM1 in SHCC was associated
with the protein expression level determined by IHC (34), and this could be used to develop
tools for routine diagnosis and potential treatment of SHCC. A
histological diagnosis of HCC is not mandatory when the imaging
diagnosis of HCC is clear. However, the presence of a sarcomatoid
component in the tumor tissue is unclear in most patients with HCC.
PBRM1 may aid in the diagnosis of SHCC and enable a uniform
diagnosis. SHCC has a poorer prognosis than typical hepatocellular
carcinoma and may require different therapeutic approaches. Uniform
diagnosis of the presence of sarcomatoid features by PBRM1 (e.g.,
immunostaining) will lead to analysis of the mechanisms underlying
SHCC, help provide a more accurate prognosis for all patients with
sarcomatoid HCC, and allow better-informed decisions regarding
patient treatment. Future clinical performance studies using
receiver operating characteristic curve analyses are necessary to
further establish the predictive value of PBRM1 expression for the
diagnosis of sarcomatoid components.
Acknowledgements
The authors would like to thank Mr. Yoshihiro Mine
(Center for Instrumental Analyses Central Research Facilities,
Kindai University Faculty of Medicine, Osakasayama, Japan) and Ms.
Ayaka Kitano (Department of Genome Biology, Kindai University
Faculty of Medicine, Osakasayama, Japan) for their technical
assistance with sample preparation during the study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to privacy considerations. Genomic data
consisting of DNA or RNA sequences obtained from tumor cells from
individual patients that contain >40 loci are considered an
‘individual identification code’ under the Guideline for Personal
Information Protection Act in Japan (14). The RNA-seq data in the present study
are considered as personal information as they contain >40 loci.
Consent for public release of sequence data as an individual
identification code has not been obtained from the 7 patients of
the present study. Therefore, the public release of these data were
not allowed. However, the datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TY, MK and KN designed and supervised the research.
MI, ST, SK, TN, HM, KT and MS collected the samples and clinical
information. KS and KN analyzed the data. KN and MADV performed the
statistical analysis. TY, KS and KN prepared the figures and
tables. KS and KN wrote the manuscript. TY and KS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Ethics Review Boards of Kansai
Medical University Hospital (Hirakata, Japan; approval no.
2019074), Osaka Metropolitan University Hospital (Osaka, Japan;
approval no. 2020-185) and Kindai University Faculty of Medicine
(Osaka-Sayama, Japan; approval no. 31-201) approved the present
study. All patients provided written informed consent for
participating in the study and allowed the collection of tumor
tissue specimens for analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts LR, Sirlin CB, Zaiem F, Almasri J,
Prokop LJ, Heimbach JK, Murad MH and Mohammed K: Imaging for the
diagnosis of hepatocellular carcinoma: A systematic review and
meta-analysis. Hepatology. 67:401–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torbenson MS: Morphologic subtypes of
hepatocellular carcinoma. Gastroenterol Clin North Am. 46:365–391.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe Y, Matsumoto N, Ogawa M, Moriyama
M and Sugitani M: Sarcomatoid hepatocellular carcinoma with
spontaneous intraperitoneal bleeding. Intern Med. 54:1613–1617.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lv TR, Hu HJ, Regmi P, Liu F and Li FY:
Sarcomatoid hepatocellular carcinoma versus conventional
hepatocellular carcinoma: A systematic review and meta-analysis. J
Cancer Res Clin Oncol. 148:1685–1696. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benusiglio PR, Couve S, Gilbert-Dussardier
B, Deveaux S, Le Jeune H, Da Costa M, Fromont G, Memeteau F, Yacoub
M, Coupier I, et al: A germline mutation in PBRM1 predisposes to
renal cell carcinoma. J Med Genet. 52:426–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwang S, Lee SG, Lee YJ, Ahn CS, Kim KH,
Park KM, Moon KM, Moon DB, Ha TY, Yu ES and Choi GW: Prognostic
impact of sarcomatous change of hepatocellular carcinoma in
patients undergoing liver resection and liver transplantation. J
Gastrointest Surg. 12:718–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao SH, Su TH, Jeng YM, Liang PC, Chen
DS, Chen CH and Kao JH: Clinical manifestations and outcomes of
patients with sarcomatoid hepatocellular carcinoma. Hepatology.
69:209–221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kojiro M, Sugihara S, Kakizoe S, Nakashima
O and Kiyomatsu K: Hepatocellular carcinoma with sarcomatous
change: A special reference to the relationship with anticancer
therapy. Cancer Chemother Pharmacol. 23 (Suppl):S4–S8. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Xiong XZ, Li FY, Ye H, Lin YX, Zhou
RX, Cai YL, Jin YW and Cheng NS: Prognostic significance of
sarcomatous change in patients with hepatocellular carcinoma after
surgical resection. Ann Surg Oncol. 22 (Suppl 3):S1048–S1056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morisue R, Kojima M, Suzuki T, Nakatsura
T, Ojima H, Watanabe R, Sugimoto M, Kobayashi S, Takahashi S,
Konishi M, et al: Sarcomatoid hepatocellular carcinoma is distinct
from ordinary hepatocellular carcinoma: Clinicopathologic,
transcriptomic and immunologic analyses. Int J Cancer. 149:546–560.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Feng S, Tu Z, Sun J, Rui T, Zhang
X, Huang H, Ling Q and Zheng S: Sarcomatoid hepatocellular
carcinoma: From clinical features to cancer genome. Cancer Med.
10:6227–6238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ministry of Education Culture, Sports,
Science and Technology, Ministry of Health, Labour and Welfare, and
Ministry of Economy, Trade and Industry, . Ethical Guidelines for
Medical and Biological Research Involving Human Subjects.
https://www.mhlw.go.jp/content/001077424.pdfJanuary
19–2024(In Japanese).
|
|
15
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Velasco MA, Kura Y, Ando N, Sako N,
Banno E, Fujita K, Nozawa M, Yoshimura K, Sakai K, Yoshikawa K, et
al: Context-Specific efficacy of apalutamide therapy in preclinical
models of pten-deficient prostate cancer. Cancers (Basel).
13:39752021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pena-Llopis S, Vega-Rubin-de-Celis S, Liao
A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L,
Sivanand S, Spence P, et al: BAP1 loss defines a new class of renal
cell carcinoma. Nat Genet. 44:751–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bankhead P, Loughrey MB, Fernandez JA,
Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ,
Coleman HG, et al: QuPath: Open source software for digital
pathology image analysis. Sci Rep. 7:168782017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eckel-Passow JE, Serie DJ, Cheville JC, Ho
TH, Kapur P, Brugarolas J, Thompson RH, Leibovich BC, Kwon ED,
Joseph RW and Parker AS: BAP1 and PBRM1 in metastatic clear cell
renal cell carcinoma: Tumor heterogeneity and concordance with
paired primary tumor. BMC Urol. 17:192017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carril-Ajuria L, Santos M, Roldan-Romero
JM, Rodriguez-Antona C and de Velasco G: Prognostic and predictive
value of PBRM1 in clear cell renal cell carcinoma. Cancers (Basel).
12:162019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brugarolas J: PBRM1 and BAP1 as novel
targets for renal cell carcinoma. Cancer J. 19:324–332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Costa WH, Rezende M, Carneiro FC, Rocha
RM, da Cunha IW, Carraro DM, Guimaraes GC and de Cassio Zequi S:
Polybromo-1 (PBRM1), a SWI/SNF complex subunit is a prognostic
marker in clear cell renal cell carcinoma. BJU Int. 113:E157–E163.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porter EG and Dykhuizen EC: Individual
bromodomains of polybromo-1 contribute to chromatin association and
tumor suppression in clear cell renal carcinoma. J Biol Chem.
292:2601–2610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pawlowski R, Muhl SM, Sulser T, Krek W,
Moch H and Schraml P: Loss of PBRM1 expression is associated with
renal cell carcinoma progression. Int J Cancer. 132:E11–E17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burrows AE, Smogorzewska A and Elledge SJ:
Polybromo-associated BRG1-associated factor components BRD7 and
BAF180 are critical regulators of p53 required for induction of
replicative senescence. Proc Natl Acad Sci USA. 107:14280–14285.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Macher-Goeppinger S, Keith M, Tagscherer
KE, Singer S, Winkler J, Hofmann TG, Pahernik S, Duensing S,
Hohenfellner M, Kopitz J, et al: PBRM1 (BAF180) protein is
functionally regulated by p53-induced protein degradation in renal
cell carcinomas. J Pathol. 237:460–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia W, Nagase S, Montia AG, Kalachikov SM,
Keniry M, Su T, Memeo L, Hibshoosh H and Parsons R: BAF180 is a
critical regulator of p21 induction and a tumor suppressor mutated
in breast cancer. Cancer Res. 68:1667–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Xia Q, Zhang H, Wang Z, Yang W, Gu
X, Hou T, Chen Y, Pei X, Zhu G, et al: CCL5-dependent mast cell
infiltration into the tumor microenvironment in clear cell renal
cell carcinoma patients. Aging (Albany NY). 12:21809–21836. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo C, Yang G, Khun K, Kong X, Levy D, Lee
P and Melamed J: Activation of Stat3 in renal tumors. Am J Transl
Res. 1:283–290. 2009.PubMed/NCBI
|
|
31
|
Horiguchi A, Oya M, Shimada T, Uchida A,
Marumo K and Murai M: Activation of signal transducer and activator
of transcription 3 in renal cell carcinoma: A study of incidence
and its association with pathological features and clinical
outcome. J Urol. 168:762–765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu CS, Chao YC, Lin HH, Chen DS and Kao
JH: Systematic Review: Impact of interferon-based therapy on
HCV-related hepatocellular carcinoma. Sci Rep. 5:99542015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miao D, Margolis CA, Gao W, Voss MH, Li W,
Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, et al:
Genomic correlates of response to immune checkpoint therapies in
clear cell renal cell carcinoma. Science. 359:801–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
da Costa WH, da Cunha IW, Fares AF,
Bezerra SM, Shultz L, Clavijo DA, da Silva DV, Netto GJ, Guimaraes
GC and Cassio Zequi S: Prognostic impact of concomitant loss of
PBRM1 and BAP1 protein expression in early stages of clear cell
renal cell carcinoma. Urol Oncol. 36:243 e1–243 e8. 2018.
View Article : Google Scholar : PubMed/NCBI
|