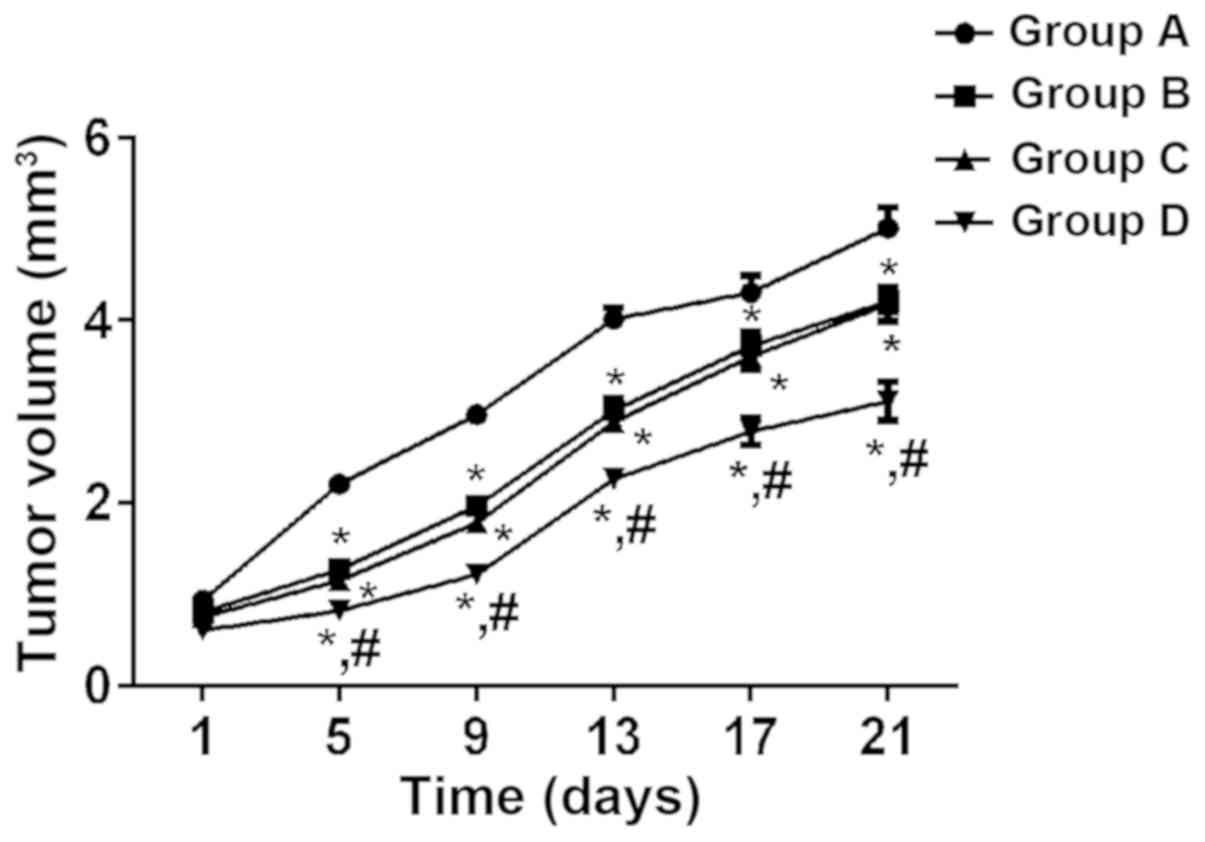
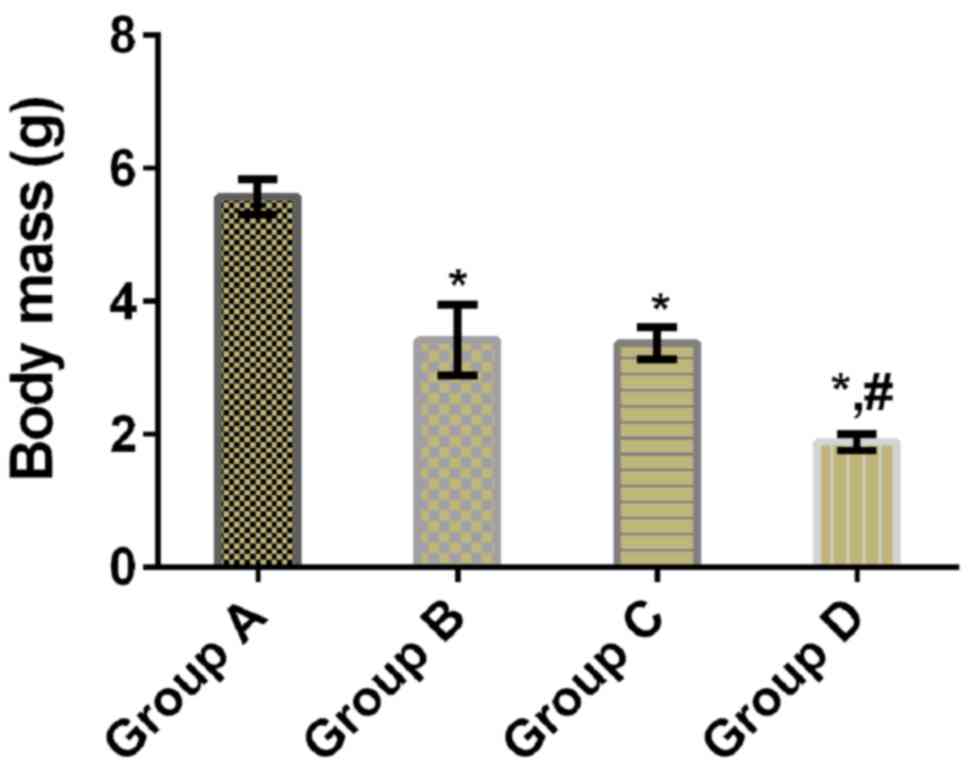
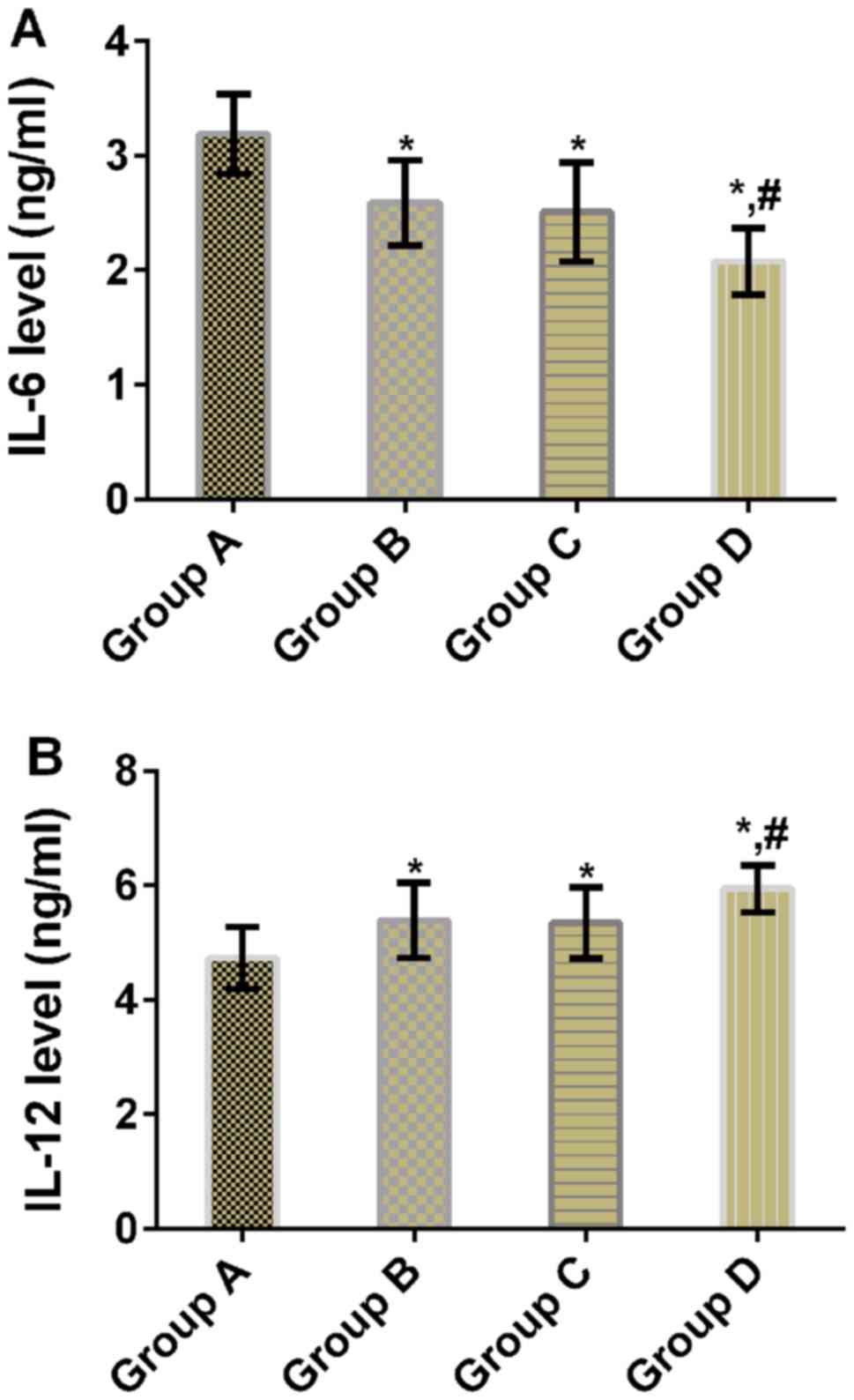
|
1
|
Cetta F, Renieri A and Frullanti E:
Germline mutations in lung cancer and personalized medicine. Fam
Cancer. 17:429–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung cancer -
Recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al OAK Study Group, : Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaplan JA, Liu R, Freedman JD, Padera R,
Schwartz J, Colson YL and Grinstaff MW: Prevention of lung cancer
recurrence using cisplatin-loaded superhydrophobic nanofiber
meshes. Biomaterials. 76:273–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al CheckMate 026 Investigators, : First-line nivolumab in stage
IV or recurrent non-small-cell lung cancer. N Engl J Med.
376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H and Song Y: MDT is still important
in the treatment of early stage lung cancer. J Thorac Dis. 10
(Suppl 33):S3984–S3985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu S, Yu Y and Yang Y: Retrospect and
prospect for lung cancer in China: Clinical advances ofimmune
checkpoint inhibitors. Oncologist. 24 (Suppl 1):S21–S30. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korytowsky B, Radtchenko J, Nwokeji ED,
Tuell KW, Kish JK and Feinberg BA: Understanding total cost of care
in advanced non-small cell lung cancer pre- and postapproval of
immuno-oncology therapies. Am J Manag Care. 24 (Suppl
20):S439–S447. 2018.PubMed/NCBI
|
|
11
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tugues S, Burkhard SH, Ohs I, Vrohlings M,
Nussbaum K, Vom Berg J, Kulig P and Becher B: New insights into
IL-12-mediated tumor suppression. Cell Death Differ. 22:237–246.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garon EB, Siegfried JM, Stabile LP, Young
PA, Marquez-Garban DC, Park DJ, Patel R, Hu EH, Sadeghi S, Parikh
RJ, et al: Randomized phase II study of fulvestrant and erlotinib
compared with erlotinib alone in patients with advanced or
metastatic non-small cell lung cancer. Lung Cancer. 123:91–98.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doki Y, Murakami K, Yamaura T, Sugiyama S,
Misaki T and Saiki I: Mediastinal lymph node metastasis model by
orthotopic intrapulmonary implantation of Lewis lung carcinoma
cells in mice. Br J Cancer. 79:1121–1126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogden BE, Pang William W, Agui T and Lee
BH: Laboratory animal laws, regulations, guidelines and standards
in China Mainland, Japan, and Korea. ILAR J. 57:301–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karachaliou N, Fernandez-Bruno M, Bracht
JWP and Rosell R: Challenges and unanswered questions for the next
decade of immune-oncology research in NSCLC. Transl Lung Cancer
Res. 7:691–702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berland L, Heeke S, Humbert O, Macocco A,
Long-Mira E, Lassalle S, Lespinet-Fabre V, Lalvée S, Bordone O,
Cohen C, et al: Current views on tumor mutational burden in
patients with non-small cell lung cancer treated by immune
checkpoint inhibitors. J Thorac Dis. 11 (Suppl 1):S71–S80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YH: Durvalumab after chemoradiotherapy
in Stage III non-small-cell lung cancer. N Engl J Med. 380:989–990.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki T, Seto T, Yamanaka T, Kunitake N,
Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, et
al: A randomised phase II trial of S-1 plus cisplatin versus
vinorelbine plus cisplatin with concurrent thoracic radiotherapy
for unresectable, locally advanced non-small cell lung cancer:
WJOG5008L. Br J Cancer. 119:675–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al KEYNOTE-024 Investigators, : Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furugaki K, Fukumura J, Iwai T, Yorozu K,
Kurasawa M, Yanagisawa M, Moriya Y, Yamamoto K, Suda K, Mizuuchi H,
et al: Impact of bevacizumab in combination with erlotinib on
EGFR-mutated non-small cell lung cancer xenograft models with T790M
mutation or MET amplification. Int J Cancer. 138:1024–1032. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scagliotti GV, Shuster D, Orlov S, von
Pawel J, Shepherd FA, Ross JS, Wang Q, Schwartz B and Akerley W:
Tivantinib in combination with erlotinib versus erlotinib alone for
EGFR-Mutant NSCLC: An exploratory analysis of the phase 3 MARQUEE
study. J Thorac Oncol. 13:849–854. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JG and Wu R: Combination
erlotinib-cisplatin and Atg3-mediated autophagy in erlotinib
resistant lung cancer. PLoS One. 7:e485322012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Virtuoso LP, Anderson CD and Egilmez
NK: Regulatory rebound in IL-12-treated tumors is driven by
uncommitted peripheral regulatory T cells. J Immunol Volume?
1293–1300. 2015. View Article : Google Scholar
|
|
27
|
Caetano MS, Zhang H, Cumpian AM, Gong L,
Unver N, Ostrin EJ, Daliri S, Chang SH, Ochoa CE, Hanash S, et al:
IL-6 blockade reprograms the lung tumor microenvironment to limit
the development and progression of K-ras mutant lung cancer. Cancer
Res. 76:3189–3199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G and Lu B: Patients come first - the
direction of lung cancer treatment. J Thorac Dis. 10:E714–E715.
2018. View Article : Google Scholar : PubMed/NCBI
|