Introduction
As a common malignant tumor threatening human life
and health, lung cancer has increasing morbidity and mortality
rates (1). Approximately 85% of
patients with the disease are patients with non-small cell lung
cancer (NSCLC). Early lung cancer mostly treated by surgery has no
obvious signs and symptoms, so the patients diagnosed have been in
the middle and advanced stages when the tumor tissue has
metastasized. That is to say, the patients lose the best surgical
treatment time and have a low overall survival rate (2,3).
Clinically, lung cancer has been treated by platinum-based
chemotherapy. Although dose-limited platinum drugs cause toxic and
side effects such as nephrotoxicity and neurotoxicity, cisplatin
improves the overall survival of patients with lung cancer
metastasis (4). With the development
of molecular biology, targeted therapy is gradually used for the
treatment of lung cancer (5,6). Epidermal growth factor receptor (EGFR)
mutations are common drivers of lung cancer, and EGFR-tyrosine
kinase inhibitors (TKIs) are considered as the best choice for the
first-line treatment of advanced or recurrent non-squamous NSCLC
carrying activating EGFR mutations (7). EGFR-TKIs are more effective for
patients with EGFR mutant lung cancer than platinum-based
chemotherapy (8). Erlotinib as a
kind of EGFR-TKIs, is clinically effective for the treatment of
NSCLC (9). According to Korytowsky
et al (10), there is no
significant difference in efficacy between erlotinib and
chemotherapy (docetaxel or pemetrexed) for patients with advanced
NSCLC who have received platinum-based chemotherapy for no more
than 4 cycles and have disease progression during or after
chemotherapy.
In previous treatment, tumor cells were given high
level of consideration, while the influence of tumor
microenvironment on the efficacy was ignored (11). Interleukin-6 (IL-6), a cytokine that
exists in tumor microenvironment, is closely related to cancer cell
proliferation, angiogenesis and metastasis (12). IL-12 is one of the most effective
cytokines for mediating antitumor activity and has a pleiotropic
effect on immune cells forming tumor microenvironment. As a
therapeutic target for tumors, it establishes a link between innate
and adaptive immunities and plays a key role in shaping antitumor
or tumor immunity (13).
Previous studies have shown obvious benefits of
erlotinib and cisplatin in advanced lung cancer (14), but few studies exist on the specific
application of erlotinib combined with cisplatin and its effects on
IL-6 and IL-12. Lewis lung cancer mouse model is one of the tumor
models frequently used in studies. As a common model for studying
drug treatment of lung cancer, it is easy to model and has high
tumor formation rate (15).
Therefore, a mouse model of lung cancer was established in this
study to explore the effects of erlotinib combined with cisplatin
on the tumor growth, IL-6 and IL-12 of mice with Lewis lung cancer
(LLC).
Materials and methods
Animals and cells
Forty-four pure inbred SPF C57BL/6J mice [Shanghai
SLAC Laboratory Animal Co., Ltd., license no. SCXK (2003–0003)],
aged 6–8 weeks with a body weight of 20.13±2.16 g, were fed with
SPF granular chow in well ventilated clean facility. They have free
access to water and food. Indoor humidity was 45–64% and indoor
temperature was 20–24°C, with 12-h light (500 lx)/12-h dark (0 lx).
This experiment was carried out 1 week after acclimatization and
was approved by the Ethics Committee of the Hospital, with the
process following Guide for the Care and Use of Laboratory Animals
(16,17). LLC cell line of the mice was
introduced by Beina Chuanglian Biotechnology Research Institute
(item no. ATCCCRL-1642) and stored in liquid nitrogen.
Cell culture and preparation of animal
models
LLC cells were inoculated into a culture dish, added
with RPMI-1640 medium containing 10% fetal bovine serum (both from
Gibco BRL) and 1% mycillin/streptomycin, and then cultured in an
incubator (Thermo Electron Corporation) at 37°C with 5%
CO2. The culture fluid was changed once/2 days, and the
LLC cell line was digested with 2.5% pancreatin and then passaged.
Cells in logarithmic phase were taken to prepare a suspension with
a cell concentration of 2.80×107 ml−1 for
subsequent experiments.
Modeling, grouping and medication
The mice were fixed on the operating console for
routine skin disinfection then 0.2 ml (~2×106 living
cells) of the LLC cell suspension was subcutaneously injected into
the right axilla of mice with a 1 ml syringe, during aseptic
operation. The tumor formed around the 8th day and then grew to ~8
mm. At that time, 44 mice were randomized into groups A, B, C and
D. Mice in group A were given 30 mg/kg of normal saline, group B
was given 30 mg/kg of erlotinib (Roche Medical Electronics), group
C was given 3 mg/kg of cisplatin injection (Qilu Pharmaceutical
Co., Ltd., batch no. ALA1206023) and group D erlotinib (30 mg/kg)
combined with cisplatin (3 mg/kg). From the 2nd day after modeling,
the drugs were intraperitoneally injected once daily for 21
consecutive days.
Observational indexes and methods
The longest diameter (a) and the shortest diameter
(b) of the tumor were measured with vernier calipers at 1 day, 5
days, 9 days, 13 days, 17 days and 21 days after medication. The
tumor volume was estimated with reference to V=ab2/2
(18), and the tumor growth curve
was plotted.
Forty days later, the mice were sacrificed through
Cervical dislocation, with the eyeballs enucleated. The eyeballs
were removed and 0.5 ml of blood was taken with the EP tube. The
serum was separated by centrifugation at 1,500 × g at 4°C for 10
min. Upper serum (50 µl) was collected and stored in a refrigerator
at −80°C. The tumor was excised and measured for mass (average
tumor mass in group A - average tumor mass in each group after
medication)/average tumor mass in group A ×100% = tumor inhibition
rate (TIR).
Serum IL-6 and IL-12 levels were detected by
enzyme-linked immunosorbent assay (ELISA) according to the
instructions of mouse IL-6 and IL-12 ELISA kits (Shanghai Hengfei
Biotechnology Co., Ltd., CSB-E04639m-1, CSB-E07360m-1). All samples
and reagents were taken out in advance to balance with room
temperature. The ELISA plate was washed twice with 300 µl of
washing liquid, and discarded, the wells were dried with absorbent
paper. A well for the sample to be tested, a standard well and a
blank well were set up, in which 50 µl of the sample, standard
substance and sample diluent was respectively added, and then 50 µl
of biotin-labeled antibody was added. The plate was sealed with a
microplate sealer and incubated at 37°C for 1 h. After the liquid
was discarded, each well was added with 300 µl of the washing
liquid to wash the plate 5 times, and the wells were dried each
time after the washing. Each well was added with 100 µl of
streptavidin, and then the plate was sealed with a microplate
sealer and incubated at 37°C for 1 h. Each well was added with 300
µl of the washing liquid to wash the plate 5 times, and the wells
were dried each time after the washing. Each well was added with 50
µl of A and 50 µl of B working solutions, and then incubated in
dark at room temperature for 20 min. After that, 100 µl of stop
solution was added to each well. OD values of each well were
detected at 450 nm using a 680 fully automatic microplate reader
(Bio-Rad), to calculate IL-6 and IL-12 levels.
Statistical methods
SPSS 18.0 (IBM Corp.) was used for statistical
analysis, GraphPad Prism 7 for plotting figures. Count data were
expressed by the number of cases/percentage (n/%), and Chi-square
test was used for comparison of the count data between groups.
Measurement data were expressed by mean ± standard deviation (mean
± SD), and one-way analysis of variance (ANOVA) was used for
comparison of mean between multiple groups. After that, Dunnett
t-test was used for pairwise comparison, repeated measures ANOVA
for comparison of different time-points, Bonferroni for pairwise
comparison between different time-points within groups. P<0.05
indicates a statistically significant difference.
Results
Comparison of general conditions
There were no statistically significant differences
between groups A, B, C and D with respect to sex, age, body weight,
indoor temperature and indoor humidity (P>0.05) (Table I).
 | Table I.Comparison of general conditions
[n(%)]/(mean ± SD). |
Table I.
Comparison of general conditions
[n(%)]/(mean ± SD).
| Categories | Group A (n=11) | Group B (n=11) | Group C (n=11) | Group D (n=11) | F/χ2
value | P-value |
|---|
| Sex |
|
|
|
| 0.786 | 0.853 |
| Male
(%) | 7 (63.64) | 8 (72.73) | 7 (63.64) | 6 (54.55) |
|
|
| Female
(%) | 4 (36.36) | 3 (27.27) | 4 (36.36) | 5 (45.45) |
|
|
| Age (weeks) | 7.02±0.58 | 6.94±0.46 | 7.12±0.51 | 6.85±0.63 | 0.483 | 0.696 |
| Body weight (g) | 20.13±2.16 | 21.08±1.93 | 20.47±2.09 | 20.54±2.12 | 0.394 | 0.758 |
| Indoor humidity
(%) | 50.18±2.93 | 49.76±3.67 | 50.26±3.52 | 51.64±3.46 | 0.727 | 0.542 |
| Indoor temperature
(°C) | 22.05±1.24 | 22.67±0.94 | 22.38±1.05 | 22.03±1.27 | 0.792 | 0.505 |
Comparison of tumor volume
According to the tumor growth curve, the tumor
volume of mice in the four groups increased with time, and the
growth rate of group A was the fastest, followed by groups C, B and
D. According to repeated measures ANOVA, there was a statistically
significant difference in tumor volume between the four groups
after medication (P<0.05). At different time-points after
medication, tumor volume in group D was significantly lower than
that in groups A, B and C (P<0.05), and that in groups B and C
was significantly lower than that in group A (P<0.05), whereas
there was no significant difference between groups B and C
(P>0.05) (Fig. 1).
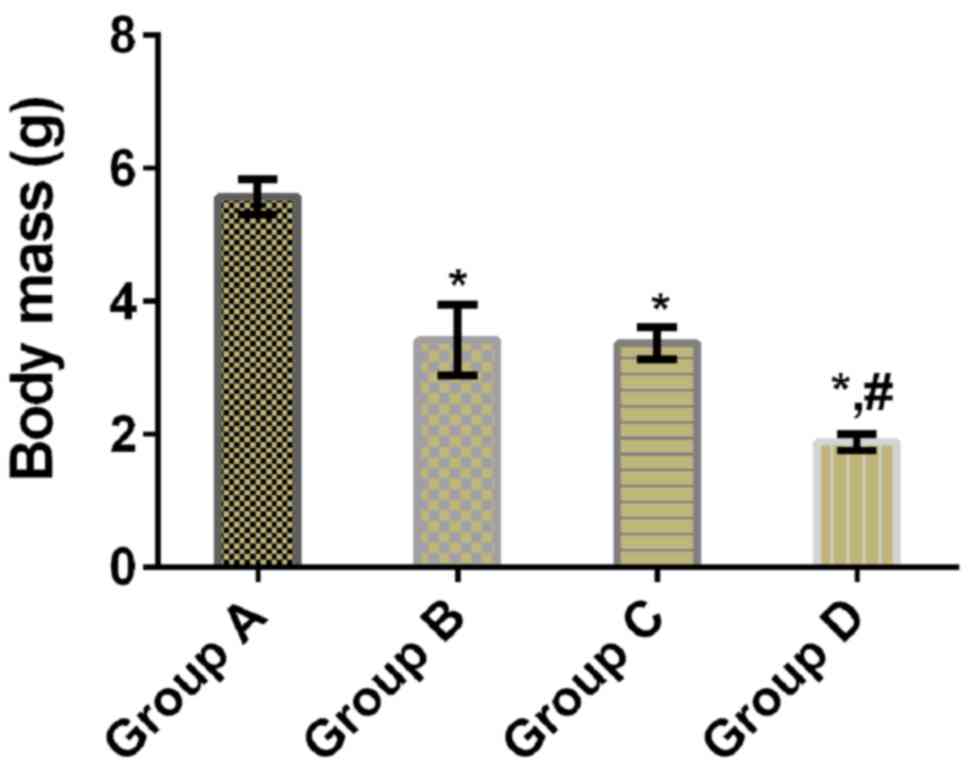
Comparison of tumor mass
There was a statistically significant difference in
tumor mass between the four groups (P<0.05). Tumor mass in
groups B, C and D was significantly lower than that in group A
(P<0.05), and that in group D was significantly lower than that
in groups B and C (P<0.05), whereas there was no significant
difference between groups B and C (P>0.05) (Table II and Fig. 2).
 | Table II.Comparison of tumor mass and TIR (mean
± SD). |
Table II.
Comparison of tumor mass and TIR (mean
± SD).
| Groups | n | Tumor mass/g | TIR (%) |
|---|
| Group A | 11 | 5.57±0.27 | – |
| Group B | 11 |
3.42±0.53a | 38.60 |
| Group C | 11 |
3.37±0.24a | 44.88 |
| Group D | 11 |
1.89±0.13a,b | 66.07 |
| F value | – | 242.000 | – |
| P-value | – | <0.001 | – |
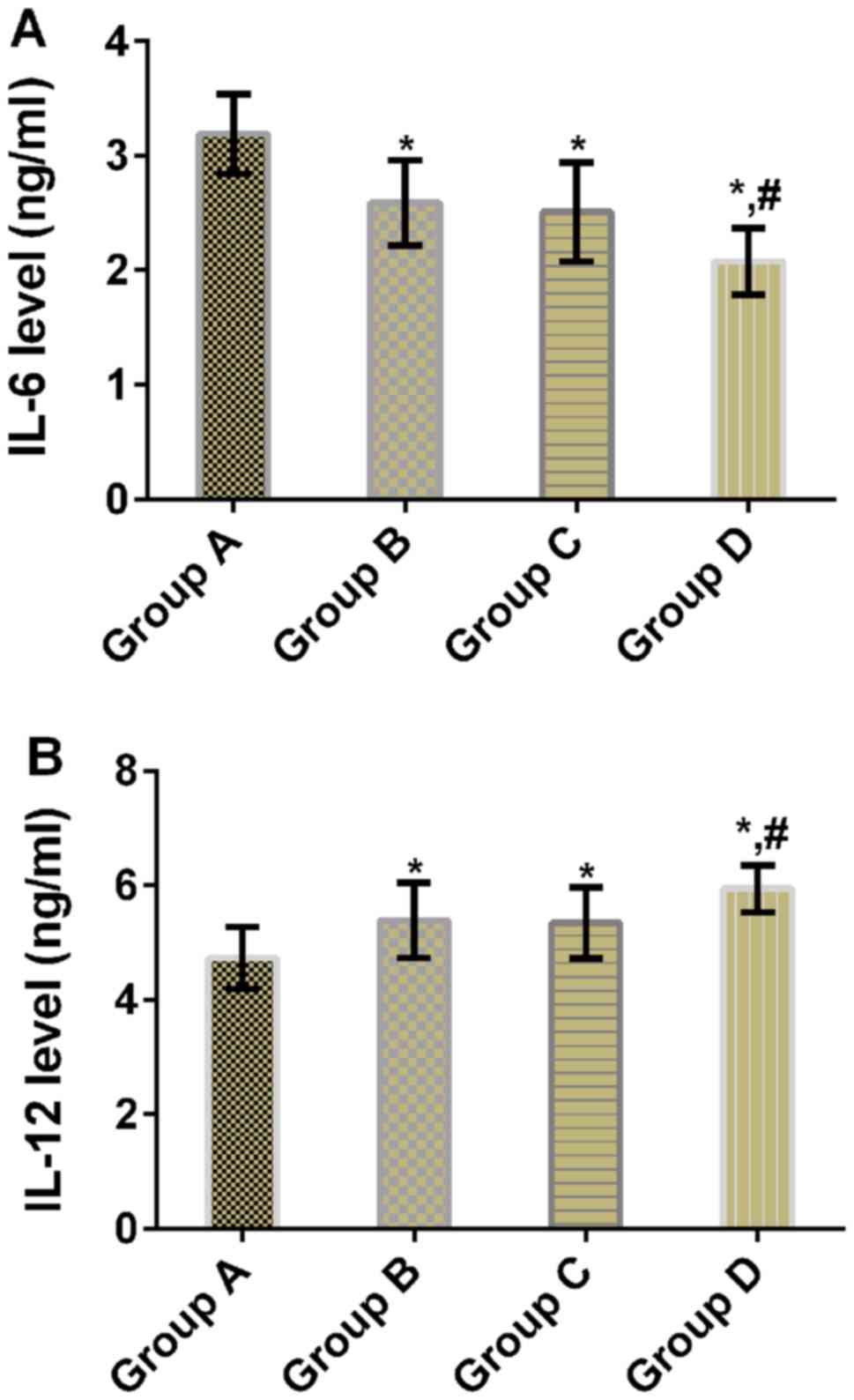
Comparison of IL-6 and IL-12 levels
after treatment
There were statistically significant differences in
IL-6 and IL-12 levels between the four groups (P<0.05). Compared
with group A, mice in groups B, C and D had significantly lower
IL-6 levels (P<0.05), but significantly higher IL-12 levels
(P<0.05). Compared with groups B and C, mice in group D had
significantly lower IL-6 level (P<0.05), but significantly
higher IL-12 level (P<0.05). There were no significant
differences in IL-6 and IL-12 levels between groups B and C
(P>0.05) (Table III and
Fig. 3).
 | Table III.Comparison of IL-6 and IL-12 levels
(mean ± SD). |
Table III.
Comparison of IL-6 and IL-12 levels
(mean ± SD).
| Groups | n | IL-6 (ng/ml) | IL-12 (ng/ml) |
|---|
| Group A | 11 | 3.19±0.35 | 4.73±0.54 |
| Group B | 11 |
2.59±0.37a |
5.39±0.66a |
| Group C | 11 |
2.51±0.43a |
5.34±0.62a |
| Group D | 11 |
2.08±0.29a,b |
5.94±0.41a,b |
| F value | – | 17.390 | 8.415 |
| P-value | – | <0.001 | <0.001 |
Discussion
Most patients with lung cancer are in the advanced
stage when diagnosed. Those who undergo chemotherapy and
radiotherapy for the treatment of unresectable advanced lung cancer
have poor median progression-free survival time and 5-year overall
survival time (19). Patients with
advanced lung cancer are mostly treated by platinum drugs, but some
of the patients have poor tolerance and therapeutic effects.
Therefore, lung cancer has no cure although chemotherapeutics are
continuously updated (20).
First-line treatment decisions for advanced lung
cancer are currently based on sensitive EGFR mutations (21). In recent years, EGFR-TKIs represented
by erlotinib, which specifically inhibits EGFR signaling pathway
and further inhibits tumor growth are increasingly valued in the
comprehensive treatment of lung cancer (22). According to Scagliotti et al
(23), erlotinib combined with
gefitinib is a tolerable regimen and has better clinical efficacy
than erlotinib alone in chemotherapy for patients with EGFR-mutant
NSCLC. Therefore, the combination of erlotinib and other drugs is
effective for patients with advanced lung cancer. It has been shown
that erlotinib-cisplatin combination is effective for
erlotinib-resistant cancer by targeting (downregulating)
Atg3-mediated autophagy and inducing apoptotic cell death (24). However, scarce research exists on the
effects of erlotinib combined with cisplatin for lung cancer in
vivo. Lewis lung cancer is a commonly used model for studying
drug therapy for lung cancer, so Lewis lung cancer mice were used
in this study. Tumor volume in group D was significantly lower than
that in groups A, B and C at different time-points after
medication, and tumor mass was significantly lower than that in
groups A, B and C, suggesting that erlotinib combined with
cisplatin significantly inhibits the tumor growth of mice with lung
cancer. Therefore, erlotinib combined with cisplatin may become a
new therapeutic regimen for lung cancer.
IL-6 is a main cytokine in tumor microenvironment,
and its high level shows the correlation of inflammations with
cancers. It promotes tumorigenesis through regulating markers and
signal transduction pathways (including apoptosis, survival,
proliferation, angiogenesis, invasion and metastasis) of cancers
(25). As a cytokine that stimulates
cellular immunity, IL-12 exerts effective antitumor activity
through immune stimulation and antiangiogenic mechanisms, and
promotes rapid reversal of immunosuppression in tumor
microenvironment (26). In a study
by Caetano et al (27), IL-6
was used as a therapeutic target for tumors, and its blocking not
only directly inhibited tumor cells, but also redirected the lung
microenvironment to antitumor phenotypes through changing the ratio
of tumor-promoting to antitumor immune cells. According to Li et
al (28), the antitumor activity
of IL-12 increases the immunoregulation of cytokines, inhibits the
growth of human lung adenocarcinoma and acts on normal bronchial
epithelial cells near tumors. In this study, mice in group D had
significantly lower IL-6 level but significantly higher IL-12
level, indicating that inhibition of IL-6 level and upregulation of
IL-12 level through improvement of the microenvironment may be a
therapeutic mechanism of erlotinib combined with cisplatin for lung
cancer. However, the specific regulatory mechanism remains to be
further studied.
This study confirmed the inhibitory effect of
erlotinib combined with cisplatin on the tumor growth of mice with
lung cancer, and preliminarily discussed its therapeutic
mechanism.
In conclusion, erlotinib combined with cisplatin can
inhibit the tumor growth of mice with LLC, and inhibition of IL-6
level and upregulation of IL-12 level may be one of its therapeutic
mechanisms.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Plan Projects of Guangdong Province of China (no.
2013B021800163).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ conceived the study and wrote the manuscript. JC
and HJ were responsible for ELISA. WZ, ZC and HW contributed to
analysis of observation indexes. The final version was read and
adopted by all the authors. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Zhejiang University (Hangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cetta F, Renieri A and Frullanti E:
Germline mutations in lung cancer and personalized medicine. Fam
Cancer. 17:429–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung cancer -
Recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al OAK Study Group, : Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaplan JA, Liu R, Freedman JD, Padera R,
Schwartz J, Colson YL and Grinstaff MW: Prevention of lung cancer
recurrence using cisplatin-loaded superhydrophobic nanofiber
meshes. Biomaterials. 76:273–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al CheckMate 026 Investigators, : First-line nivolumab in stage
IV or recurrent non-small-cell lung cancer. N Engl J Med.
376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H and Song Y: MDT is still important
in the treatment of early stage lung cancer. J Thorac Dis. 10
(Suppl 33):S3984–S3985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu S, Yu Y and Yang Y: Retrospect and
prospect for lung cancer in China: Clinical advances ofimmune
checkpoint inhibitors. Oncologist. 24 (Suppl 1):S21–S30. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korytowsky B, Radtchenko J, Nwokeji ED,
Tuell KW, Kish JK and Feinberg BA: Understanding total cost of care
in advanced non-small cell lung cancer pre- and postapproval of
immuno-oncology therapies. Am J Manag Care. 24 (Suppl
20):S439–S447. 2018.PubMed/NCBI
|
|
11
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tugues S, Burkhard SH, Ohs I, Vrohlings M,
Nussbaum K, Vom Berg J, Kulig P and Becher B: New insights into
IL-12-mediated tumor suppression. Cell Death Differ. 22:237–246.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garon EB, Siegfried JM, Stabile LP, Young
PA, Marquez-Garban DC, Park DJ, Patel R, Hu EH, Sadeghi S, Parikh
RJ, et al: Randomized phase II study of fulvestrant and erlotinib
compared with erlotinib alone in patients with advanced or
metastatic non-small cell lung cancer. Lung Cancer. 123:91–98.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doki Y, Murakami K, Yamaura T, Sugiyama S,
Misaki T and Saiki I: Mediastinal lymph node metastasis model by
orthotopic intrapulmonary implantation of Lewis lung carcinoma
cells in mice. Br J Cancer. 79:1121–1126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogden BE, Pang William W, Agui T and Lee
BH: Laboratory animal laws, regulations, guidelines and standards
in China Mainland, Japan, and Korea. ILAR J. 57:301–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karachaliou N, Fernandez-Bruno M, Bracht
JWP and Rosell R: Challenges and unanswered questions for the next
decade of immune-oncology research in NSCLC. Transl Lung Cancer
Res. 7:691–702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berland L, Heeke S, Humbert O, Macocco A,
Long-Mira E, Lassalle S, Lespinet-Fabre V, Lalvée S, Bordone O,
Cohen C, et al: Current views on tumor mutational burden in
patients with non-small cell lung cancer treated by immune
checkpoint inhibitors. J Thorac Dis. 11 (Suppl 1):S71–S80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YH: Durvalumab after chemoradiotherapy
in Stage III non-small-cell lung cancer. N Engl J Med. 380:989–990.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki T, Seto T, Yamanaka T, Kunitake N,
Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, et
al: A randomised phase II trial of S-1 plus cisplatin versus
vinorelbine plus cisplatin with concurrent thoracic radiotherapy
for unresectable, locally advanced non-small cell lung cancer:
WJOG5008L. Br J Cancer. 119:675–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al KEYNOTE-024 Investigators, : Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furugaki K, Fukumura J, Iwai T, Yorozu K,
Kurasawa M, Yanagisawa M, Moriya Y, Yamamoto K, Suda K, Mizuuchi H,
et al: Impact of bevacizumab in combination with erlotinib on
EGFR-mutated non-small cell lung cancer xenograft models with T790M
mutation or MET amplification. Int J Cancer. 138:1024–1032. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scagliotti GV, Shuster D, Orlov S, von
Pawel J, Shepherd FA, Ross JS, Wang Q, Schwartz B and Akerley W:
Tivantinib in combination with erlotinib versus erlotinib alone for
EGFR-Mutant NSCLC: An exploratory analysis of the phase 3 MARQUEE
study. J Thorac Oncol. 13:849–854. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JG and Wu R: Combination
erlotinib-cisplatin and Atg3-mediated autophagy in erlotinib
resistant lung cancer. PLoS One. 7:e485322012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Virtuoso LP, Anderson CD and Egilmez
NK: Regulatory rebound in IL-12-treated tumors is driven by
uncommitted peripheral regulatory T cells. J Immunol Volume?
1293–1300. 2015. View Article : Google Scholar
|
|
27
|
Caetano MS, Zhang H, Cumpian AM, Gong L,
Unver N, Ostrin EJ, Daliri S, Chang SH, Ochoa CE, Hanash S, et al:
IL-6 blockade reprograms the lung tumor microenvironment to limit
the development and progression of K-ras mutant lung cancer. Cancer
Res. 76:3189–3199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G and Lu B: Patients come first - the
direction of lung cancer treatment. J Thorac Dis. 10:E714–E715.
2018. View Article : Google Scholar : PubMed/NCBI
|