Introduction
Squamous cell carcinoma of the head and neck is the
sixth most common cancer worldwide, with approximately 600,000
patients developing this type of cancer annually (1). Despite recent advancements in cancer
treatment, the overall survival of patients with oral squamous cell
carcinoma (OSCC) has not improved significantly over the past 20
years. Cancer chemotherapy is an important alternative to surgery
and radiation in treating OSCC. However, although the conventional
anticancer medications are effective and useful, the resistance and
toxic responses of patients to these medications remain a
problem.
Herbal products contain a wide variety of chemical
compounds with potent biological effects, including anticancer
activity (2–5). Identification of the active
components in herbal products and their mechanism of action is of
ever-growing interest in the field of pharmaceutical medication for
clinical use development worldwide. In 2010, our department
initiated a collaborative project aiming to identify new
chemopreventive and chemotherapeutic agents in natural products
with the Tokyo University of Marine Science and Technology, having
provided more than 400 bio-active herbal products. Subsequent to
the examination of these products by cytotoxic and growth
inhibition assays, Scutellaria baicalensis (S. baicalensis)
root extract demonstrating a potential anticancer activity was
thoroughly examined.
Traditionally, the dried roots of S.
baicalensis have been used in the Chinese herbal medicine
‘Huang Qin’ to treat a variety of diseases including viral
hepatitis, inflammatory diseases and bacterial infections (3,6,7). The
S. baicalensis root extract contains three major flavonoids:
baicalin, baicalein and wogonin. In previous studies, these
flavonoids were shown to have the potential to arrest certain tumor
cell lines, while inhibiting tumor angiogenesis (8–11).
However, the anticancer effects of this compound, including the
crude root extract, havenever been studied in the framework of
OSCC. The present study aimed to investigate the effects of the
S. baicalensis root extract on OSCC cell proliferation,
cell-cycle progression and apoptosis.
Materials and methods
Preparation of Scutellaria baicalensis
and the reagents
The roots of S. baicalensis were collected
and imported from China. A specimen was deposited in the herbarium
of the Tokyo University of Marine Science and Technology. Dry
powdered roots (100 g) of S. baicalensis were extracted and
then concentrated until dry at 1 mg/ml under reduced pressure.
Cis-diamminedichloroplatinum [(CDDP) brand name, Randa®]
was purchased from Nippon Kayaku (Tokyo, Japan). CDDP was used as
the positive control in each in vitro assay system
(proliferation and DNA fragmentation assay), since CDDP is the most
promising antitumor (antiproliferative and apoptosis-inducing)
medication for the treatment of OSCC.
Cell culture and hypoxic condition
OSCC cell lines (HSC2,3,4, SAS) derived from a human
oral squamous cell carcinoma were cultured at 37°C with 5%
CO2 in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). Human umbilical
vein endothelial cells (HUVECs) derived from human umbilical cords
were purchased from Lonza Biologics (Basel, Switzerland) and used
between passages 3 and 7. These cells were cultured in endothelial
cell basal medium (EBM)-2 complete medium (Lonza Biologics).
Hypoxia experiments were performed for the indicated times in a
humidified INVIVO2 workstation (Ruskinn Technology,
South Wales, UK), calibrated to deliver 5% CO2, 2%
O2 and 93% N2 at 37°C.
Proliferation assay
Monolayer- and anchorage-independent growth assays
were performed as previously described (12). The monolayer cell proliferation was
measured using an MTT assay kit (Roche Diagnostics Corp.,
Indianapolis, IN, USA) that measures a purple formazan compound
produced by viable cells (13).
The anchorage-independent growth was measured using a commercial
kit, CytoSelect™ 96-Well In Vitro Tumor Sensitivity Assay
(Cell Biolabs, Inc., San Diego, CA, USA), according to the
manufacturer’s instructions.
Extracellular matrix (ECM)-related
assay
Adhesion assays were carried out using the
CytoSelect™ 48-Well Cell Adhesion Assay (Cell Biolabs, Inc.). SAS
cells (1×105) were seeded onto the wells and were
incubated at 37°C for 60 min in a tissue culture incubator prior to
washing and staining. Gelatin zymography was performed according to
the method described previously (14). SAS cells were cultured as described
in the ‘Collection of conditioned medium’ subsection, and the
conditioned medium was harvested and lyophilized. Equivalent
amounts of samples were dissolved in a Tris-HCl buffer containing
30% glycerol, 7.7% SDS and 0.3% bromophenol blue at pH 6.8.
Collection of conditioned medium
Prior to use in experiments, HUVECs were maintained
in an EBM-2 medium without hydrocortisone for at least 24 h. The
removal of hydrocortisone was necessary, since it inhibits
metalloproteinase production. Once passed and plated, endothelial
cells grew normally even in the absence of hydrocortisone.
The SAS cells were inoculated at a density of
1×106 cells in 10-cm diameter dishes and cultured for 60
h in DMEM containing 10% FBS. The cells were replenished with fresh
medium and cultured for 24 h. The cells were then replenished with
fresh medium and placed under normoxic or hypoxic conditions for 12
h. The cell culture supernatant and the cell layer fraction were
harvested for subsequent analysis.
Western blot analysis
Western blot analysis of fraction markers was
carried out, according to the method described in a previous study
(15). Anti-phospho-p42/p44
mitogen-activated protein kinase [MAPK; extracellular
signal-regulated kinase (ERK)1/2] and anti-phospho-p38 were
obtained from Promega (Madison, WI, USA), while anti-p42/p44 MAPK
(ERK1/2), anti-phospho-c-Jun NH2-terminal kinase and anti-c-Jun
NH2-terminal kinase were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-p38 obtained from
Calbiochem (Bad Soden, Germany). CDK4, CDC2, and anti-Bcl-2
antibodies and peroxidase-conjugated secondary antibody were
purchased from Santa Cruz Biotechnology, Inc.
DNA fragmentation analysis
DNA fragmentation electrophoretic analysis and
terminal deoxyuridine nick-end labeling (TUNEL) assay were carried
out as described previously (15).
Cells were observed at 24 h subsequent to treatment with S.
baicalensis root extract for the two assays.
Tube formation analysis
Tube formation of endothelial cells was performed as
described previously (16).
Matrigel (50 μl) was pipetted into 96-well dishes and then
polymerized. HUVECs incubated in EBM-2 medium without vascular
endothelial growth factor (VEGF) and hydrocortisone for 6 h were
harvested subsequent to trypsin treatment, while various
concentrations of S. baicalensis were added to the cells
prior to their being seeded and plated onto a layer of Matrigel at
a density of 3×105 cells/well, followed by the addition
of 10 μg/ml VEGF. The cultures were photographed after 12 h
(×10, ×40 magnifications).
Statistical analysis
Unless otherwise specified, the experiments were
repeated at least twice, with similar results. Statistical analysis
was carried out using the Student’s t-test. Data were shown as the
means ± standard deviations. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of S. baicalensis root
extracts on the growth of several human oral squamous cell
carcinoma cell lines
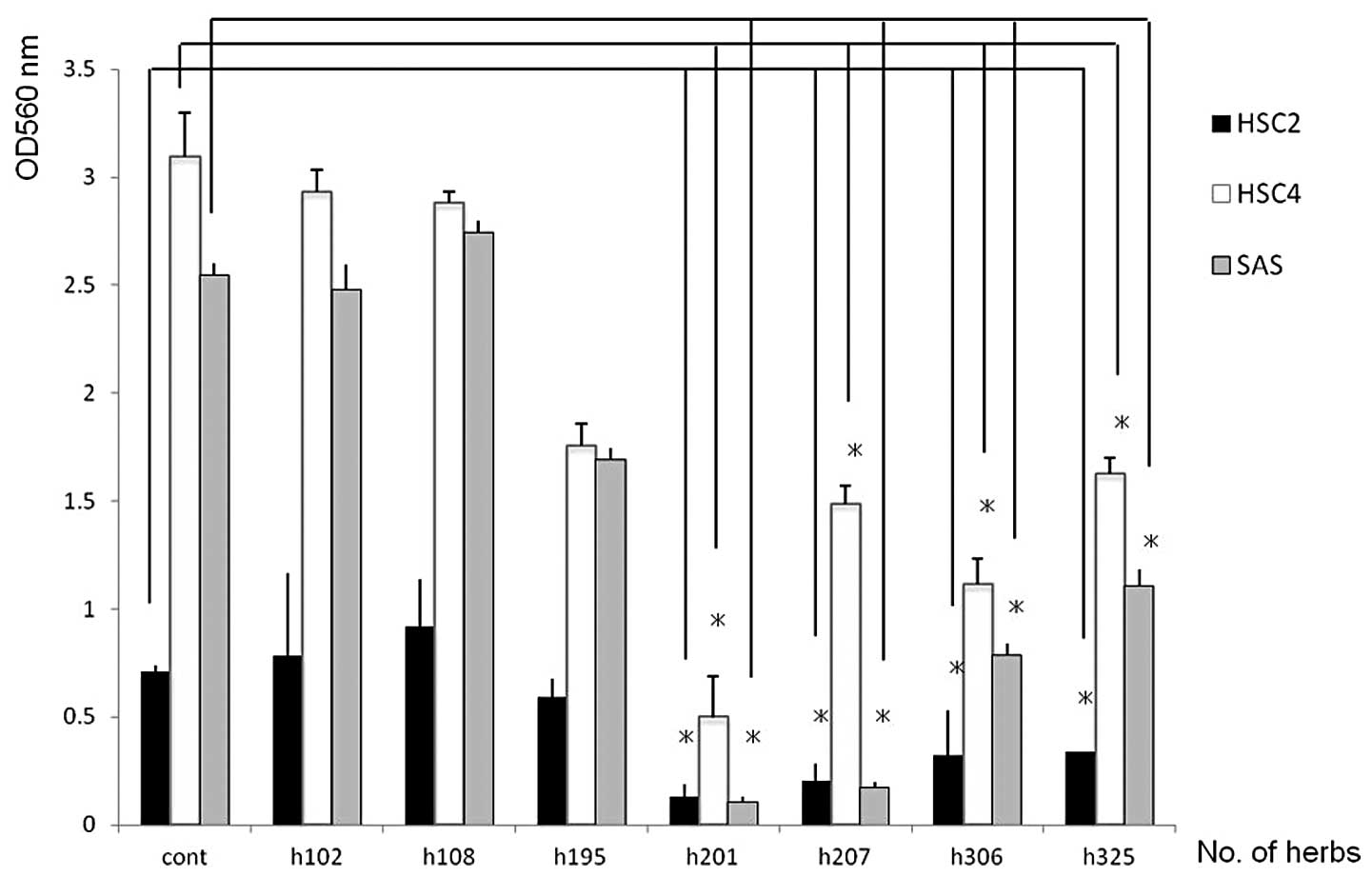
According to a series of screening analyses using
MTT assays, seven herbal products (h102, h108, h195, h201, h207,
h306 and h325) were available out of >400 bio-active herbal
products. In the cell lines tested, h201 induced significant growth
inhibition at a dose of 100 μg/ml compared to other herb
products (Fig. 1). The active
compound in the h201 herbal product was identified as the S.
baicalensis root extract.
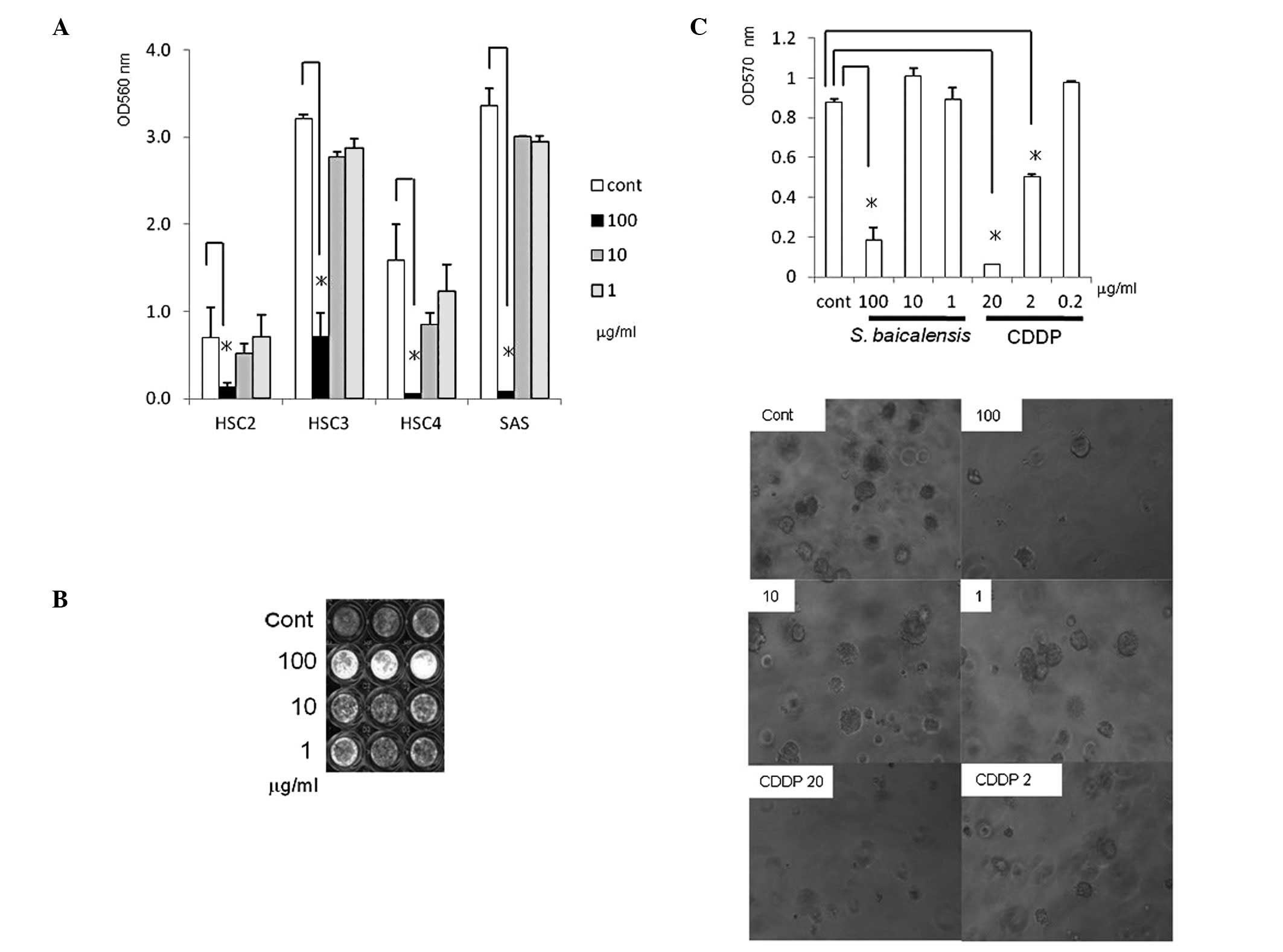
Effects of S. baicalensis on the growth
of OSCC cells
The S. baicalensis root extract inhibited
monolayer- and anchorage-independent growth in OSCC cells. To
examine the growth-inhibitory activity of S. baicalensis
extracts in several human OSCC cell lines, additional MTT assays
were performed at various concentrations of the S.
baicalensis extract (1–100 μg/ml). The S.
baicalensis extract at a dose of 100 μg/ml showed
significant inhibition of monolayer proliferation in cells from the
four cell lines (Fig. 2A). Similar
results were obtained in SAS cells using crystal violet staining
(Fig. 2B). The S.
baicalensis extract was also able to markedly inhibit
anchorage-independent growth at the same dose (Fig. 2C).
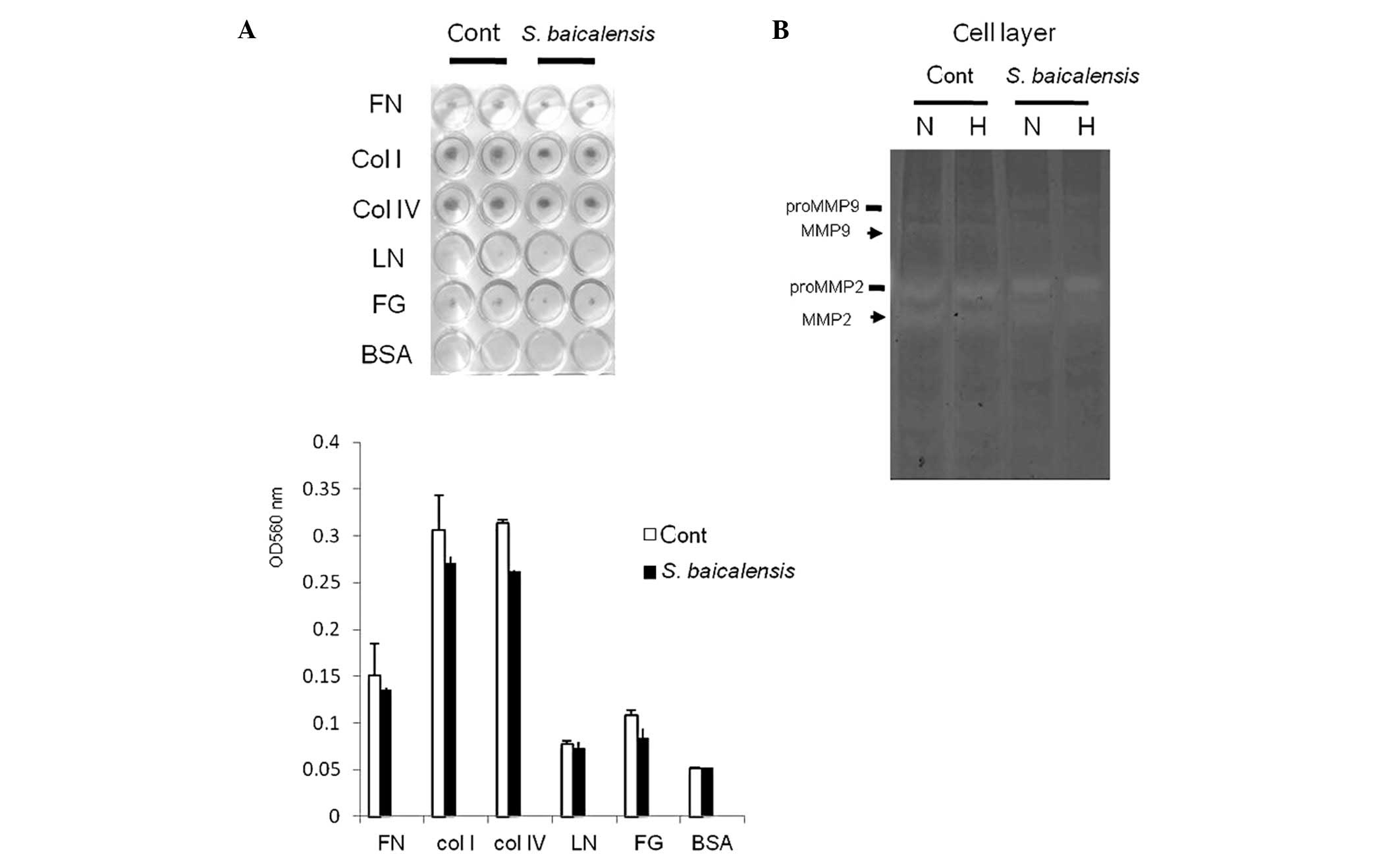
Effect of S. baicalensis on the ECM
adhesion and proteolytic activities in SAS cells
The S. baicalensis root extract did not
affect adhesion to ECM despite its proteolytic activities of matrix
metalloproteinases in SAS cells. Given that in order to
metastasize, malignant tumor cells require several distinct
cellular functions including migration, adhesion, detachment and
ECM proteolysis, the ECM adhesion and the proteolytic activities of
cells incubated with 100 μg/ml of S. baicalensis were
then examined using a cell adhesion assay and gelatin zymography.
S. baicalensis had no effect on cell adhesion to ECM
(Fig. 3A). Hypoxia converted
proMMP-2 into its active form in SAS cells, whereas the levels of
proteolytic activities detected at a dose of 100 μg/ml of
S. baicalensis were inhibited in the hypoxic-SAS cell
samples compared to normoxic ones (Fig. 3B).
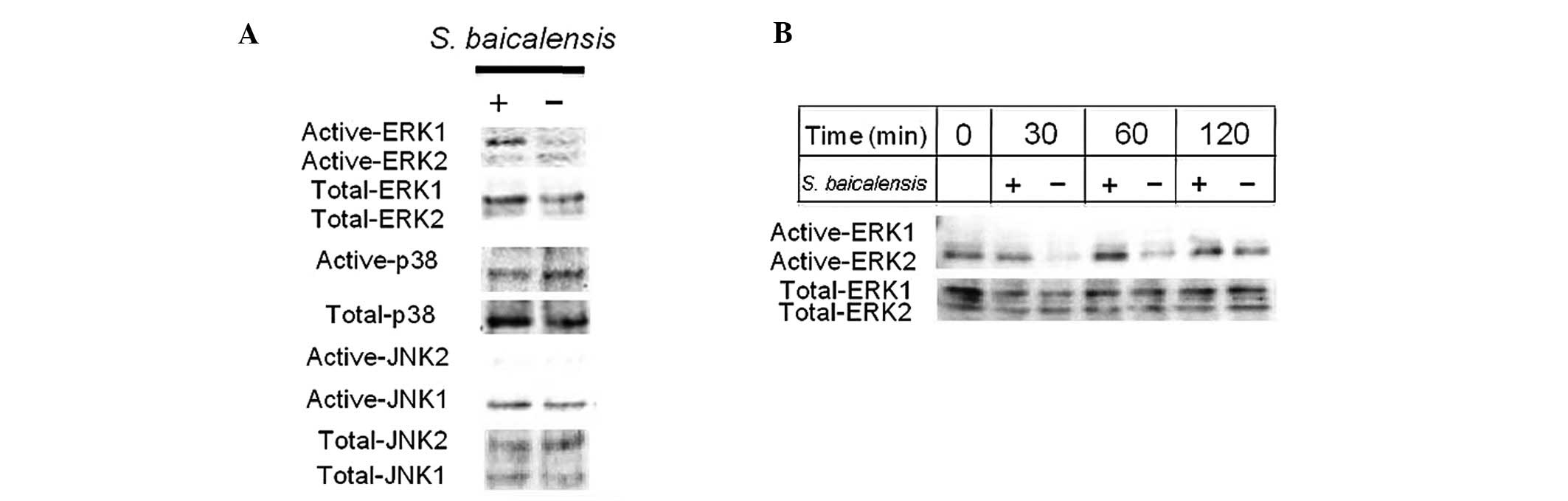
Effect of S. baicalensis on the
activation of three subgroups of MAPKs
To understand the molecular mechanism whereby S.
baicalensis inhibits cell proliferation, initially the effects
of S. baicalensis on signal transduction were investigated.
As a result, S. baicalensis at a dose of 100 μg/ml
induced the phosphorylation of ERK as early as at 30 min (Fig. 4).
S. baicalensis induces apoptotic cell
death
To investigate the molecular mechanism underlying
the observed growth-suppressing effects of S. baicalensis,
assays were conducted to detect the induction of cell apoptosis in
S. baicalensis-treated SAS cells. G1/S-transition proteins
(CDK4 and its partner cyclin D1) were depleted within 6 h
subsequent to stimulation by S. baicalensis (Fig. 5A). Furthermore, an increase in the
cleaved poly (ADP-ribose) polymerase (PARP) expression was observed
at 12–24 h. The apoptosis-related protein Bcl-2 expression also
decreased markedly, within 12–24 h. DNA fragmentation was observed
in SAS cells treated with S. baicalensis at a dose of 100
μg/ml (Fig. 5B). Apoptosis
was directly confirmed by a significant increase in TUNEL-positive
cells (Fig. 5C).
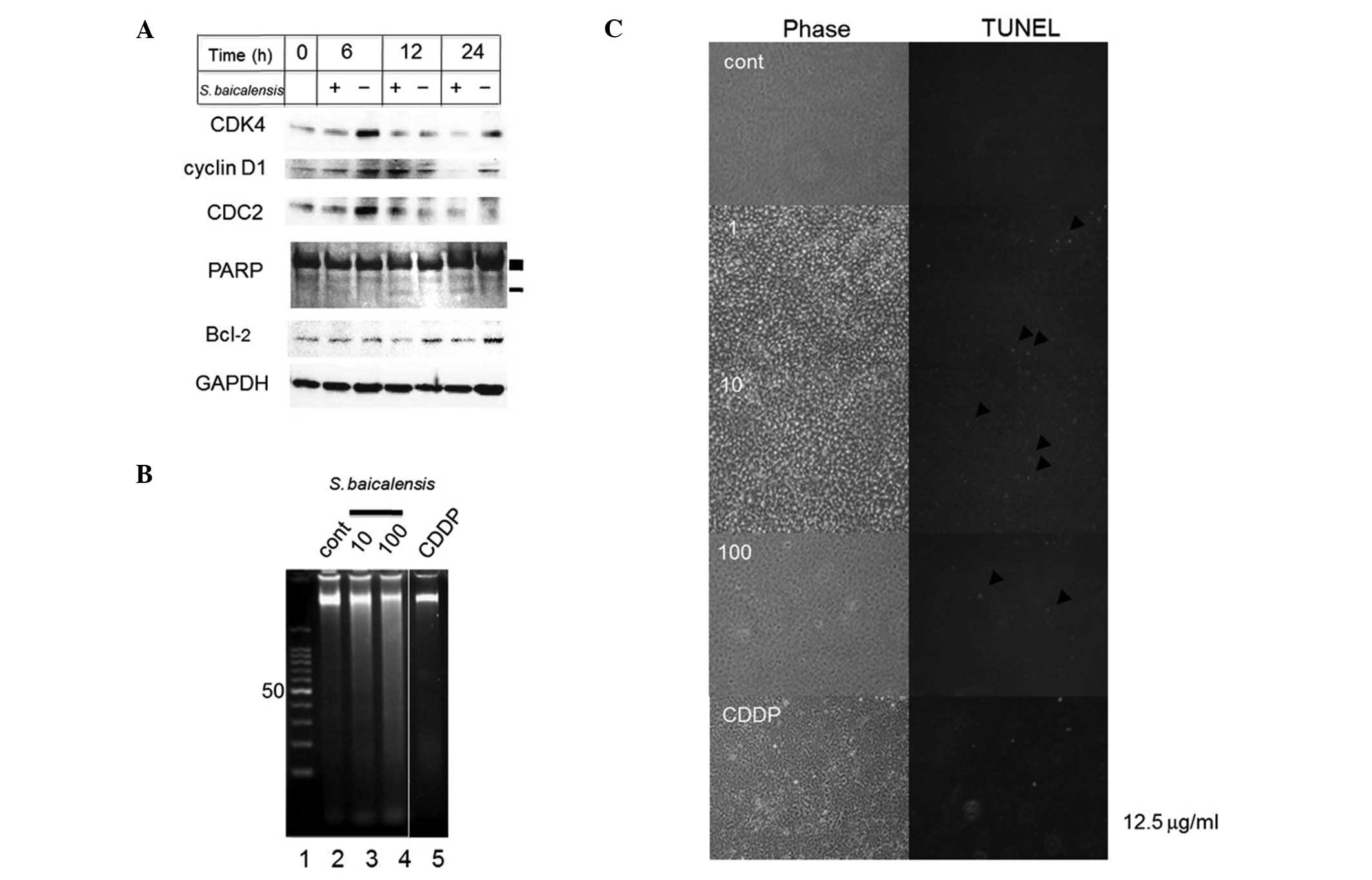 | Figure 5Cell cycle and pro-apoptotic effect of
S. baicalensis in SAS cells are shown. (A) Immunoblotting
analysis of G1/S (CDK4, cyclin D1), G2/M (CDC2) cell cycle
transition proteins and apoptotic-related protein (Bcl-2, PARP: ▪,
full length and -, cleaved fragment) expression. (B) The DNA
fragmentation was analyzed by gel electrophoresis, as described in
Materials and methods. Lane 1, DNA marker; lane 2, normal sample;
lanes 3 and 4, S. baicalensis-exposed samples; lane 5,
CDDP-exposed samples (12.5 μg/ml) is the positive control.
(C) TUNEL staining is shown. SAS cells were treated with either
S. baicalensis (1, 10 or 100 μg/ml) or the vehicle
for the indicated periods, then were observed by phase-contrast
microscopy (Phase) and fluorescence TUNEL staining (TUNEL).
Arrowheads indicate typically apoptotic cells showing the
concentration or fragmentation of nuclei. |
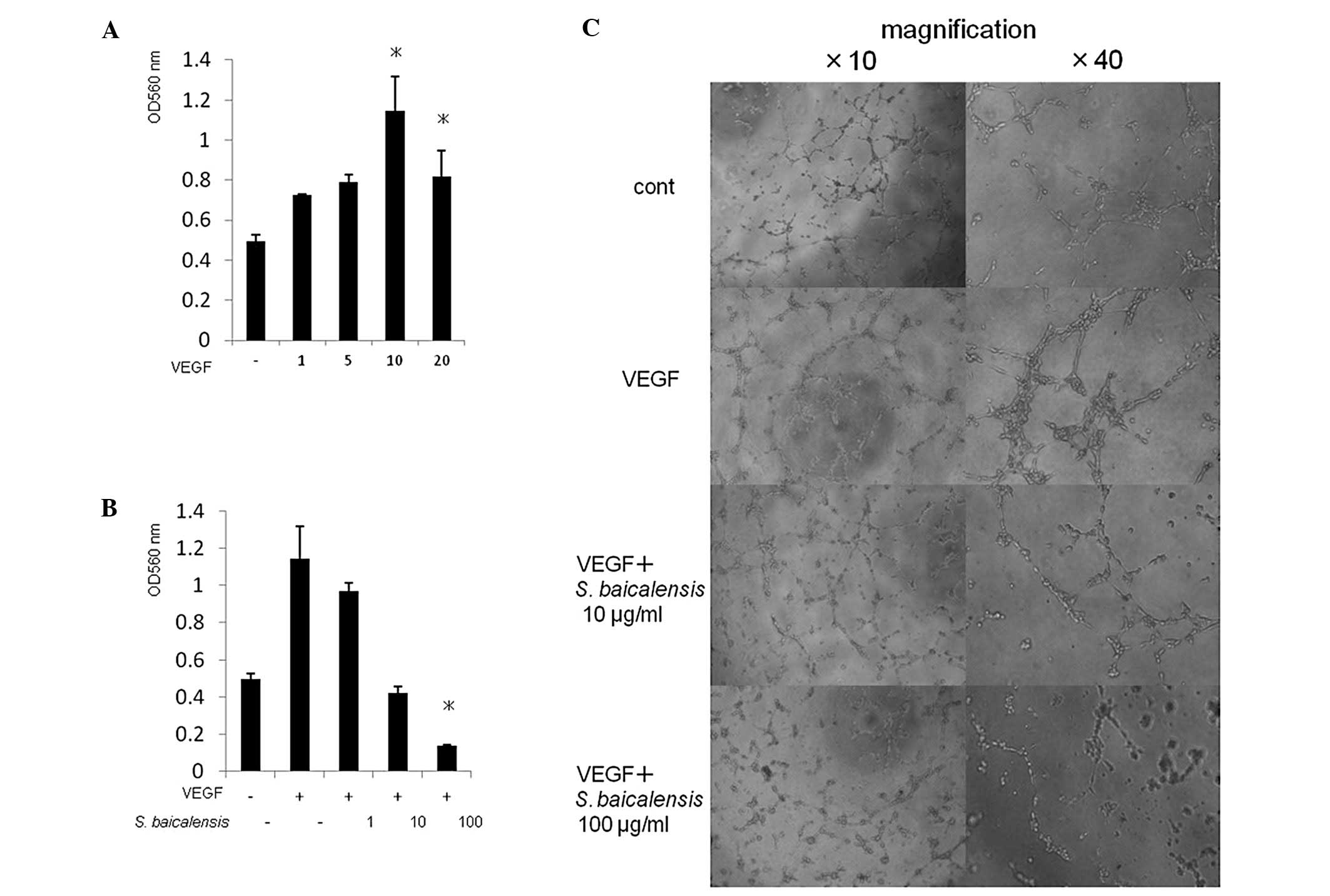
S. baicalensis inhibits VEGF-induced
proliferation and tube formation of endothelial cells
To determine the anti-angiogenic activity of S.
baicalensis in vitro, its inhibitory effect on the VEGF-induced
proliferation of endothelial cells was evaluated. VEGF (10 ng/ml)
significantly increased proliferation of HUVECs, resulting in
complete blockage by S. baicalensis (100 μg/ml)
(Fig. 6A and B). When kept in a
EBM-2 medium without hydrocortisone and VEGF, and then placed on
Matrigel in the presence of VEGF, HUVECs were induced by VEGF to
form elongated and robust tube-like structures organized by a
larger number of cells, compared to the control. S.
baicalensis effectively abrogated the tube formation induced by
VEGF in a concentration-dependent manner (Fig. 6C).
Discussion
Oral cancer treatment essentially aims to identify
chemopreventive and therapeutic agents that selectively target OSCC
cells without cytotoxic effects on healthy cells and tissues.
Although there are various chemotherapeutic agents used to treat
OSCC, treatment with currently existing chemotherapeutic
medications, such as CDDP and 5-fluorouracil (5-FU) does not always
substantially induce a positive response, since tumor cells are not
dependent on a single receptor or signal transduction pathway for
growth and progression. Therefore, finding new compounds
demonstrating anticancer effects on OSCC is of utmost importance.
These compounds may provide new and more effective treatment
regimens for the treatment of OSCC (17–21).
In the present study, we examined a large number of
herbal products and found the S. baicalensis extracts to
have potent anti-proliferative and apoptotic effects on human OSCC
cell lines. While most of the previous studies on S.
baicalensis have focused on severe acute respiratory syndrome
(SARS) (22–24), additional studies have demonstrated
that flavonoids such as baicalin, baicalein and wogonin derived
from S. baicalensis showed antitumor and anti-angiogenic
effects on various cancer cell lines (25–27).
By contrast, the effects of these flavonoids are not
anti-proliferative in all the cell lines, suggesting that they
exert either pro- or anti-apoptotic activity, depending on the cell
type. The anti-proliferative and apoptogenic properties of the
S. baicalensis root extract on OSCC cell lines may be
attributed to the pro-apoptotic activity of these flavonoids at a
late stage. A postulated mechanism for this is the inhibition of
cell cycle regulatory proteins (CDK4, cyclin D1) within 6 h
(Fig. 5A), preventing the release
of E2F to mediate the G1-to-S transition, thus leading to a potent
cytostasis, despite the potential of the S. baicalensis root
extract to induce ERK, a survival protein, phosphorylation at an
early stage (as early as at 30 min) (Fig. 4B).
Despite the expectations, combined evidence drawn
from the ECM-related assay suggested that S. baicalensis
does not affect the expression of any of the adhesion receptors,
such as integrin, which mediates cell-ECM interactions despite
having an effect on MMP-2-associated proteolytic activity. The
cascade of invasion and metastasis through adhesion molecules or
protease activity on OSCC cells is likely to be affected in part by
S. baicalensis.
Angiogenesis is a useful target of cancer treatment,
since most angiogenesis inhibitors specifically target the newly
formed vasculature in tumors, as opposed to the quiescent blood
vessels, thus reducing the possibility of side effects (16). At a cellular level, S.
baicalensis completely suppressed the stimulatory effect of
VEGF on endothelial cell tube formation, suggesting that S.
baicalensis suppresses angiogenesis via the differentiated
inhibition of human endothelial cells. These data have shown that
the S. baicalensis strategy has dual molecular properties
through the prevention of tumor-induced angiogenensis and the
direct cytotoxic effect on tumor cells. Although the half-maximal
inhibition was relatively high, the potential properties of S.
baicalensis leading to the blockage of OSCC tumor blood supply
are likely to be beneficial for future chemo-preventive and
therapeutic agents used against this disease.
Whether or not flavonoids derived from S.
baicalensis are useful in the chemoprevention and/or
chemotherapy of human OSCC cells has yet to be determined. At
present, additional studies are being conducted aiming to identify
the active flavonoid component of the extracts, as well as the
precise molecular mechanism whereby the flavonoid-induced
inhibition of the human OSCC cell growth in mice bearing OSCC cell
xenografts occurs.
In conclusion, based on the data provided in the
present study, S. baicalensis is suggested to induce
cytostasis and apoptosis in human OSCC cells. Consequently, S.
baicalensis might be a promising chemopreventive and
therapeutic agent for the treatment of OSCC.
Acknowledgements
The authors would like to thank Drs
Yasumasa Yoshizawa, Hikari Tsukamoto and Arisa Yasuda for their
helpful suggestions, and Ms. Miho Yoshihara for her secretarial
assistance. This study was supported by Grants-in-aid for the
Scientific Research from the Japan Society for the Promotion of
Science (to S.K. and Y.M.), and the High-Technology Research Center
Project of the Ministry of Education, Culture, Sports, Science and
Technology (to S.S.).
References
|
1.
|
Shiga K, Tateda M, Katagiri K, et al:
Distinct feature of second primary malignancies in head and neck
cancer patients in Japan. Tohoku J Exp Med. 225:5–12. 2011.
|
|
2.
|
Zhu H, Zhang Y, Ye G, Li Z, Zhou P and
Huang C: In vivo and in vitro antiviral activities of
calycosin-7-O-beta-D-glucopyrano-side against coxsackie virus B3.
Biol Pharm Bull. 32:68–73. 2009.
|
|
3.
|
Kubo M, Kimura Y, Odani T, Tani T and
Namba K: Studies on Scutellaria radix. Part II: The antibacterial
substance. Planta Med. 43:194–201. 1981.
|
|
4.
|
Huang YL, Kou JP, Ma L, Song JX and Yu BY:
Possible mechanism of the anti-inflammatory activity of ruscogenin:
role of intercellular adhesion molecule-1 and nuclear
factor-kappaB. J Pharmacol Sci. 108:198–205. 2008.
|
|
5.
|
Tan W, Lu J, Huang M, et al: Anti-cancer
natural products isolated from Chinese medicinal herbs. Chin Med.
6:272011.
|
|
6.
|
Huang RL, Chen CC, Huang HL, et al:
Anti-hepatitis B virus effects of wogonin isolated from
Scutellaria baicalensis. Planta Med. 66:694–698. 2000.
|
|
7.
|
Huang WH, Lee AR and Yang CH:
Antioxidative and anti-inflammatory activities of
polyhydroxyflavonoids of Scutellaria baicalensis GEORGI.
Biosci Biotechnol Biochem. 70:2371–2380. 2006.
|
|
8.
|
Fas SC, Baumann S, Zhu JY, et al: Wogonin
sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced
apoptosis. Blood. 108:3700–3706. 2006.
|
|
9.
|
Liu JJ, Huang TS, Cheng WF and Lu FJ:
Baicalein and baicalin are potent inhibitors of angiogenesis:
inhibition of endothelial cell proliferation, migration and
differentiation. Int J Cancer. 106:559–565. 2003.
|
|
10.
|
Wang W, Guo QL, You QD, et al: The
anticancer activities of wogonin in murine sarcoma S180 both in
vitro and in vivo. Biol Pharm Bull. 29:1132–1137. 2006.
|
|
11.
|
Lu N, Gao Y, Ling Y, et al: Wogonin
suppresses tumor growth in vivo and VEGF-induced angiogenesis
through inhibiting tyrosine phosphorylation of VEGFR2. Life Sci.
82:956–963. 2008.
|
|
12.
|
Li C, Yazawa K, Kondo S, Mukudai Y, Sato
D, Kurihara Y, Kamatani T and Shintani S: The root bark of
Paeonia moutan is a potential anticancer agent in human oral
squamous cell carcinoma cells. Anticancer Res. 32:2625–2630.
2012.
|
|
13.
|
Nagumo T, Takaoka S, Yoshiba S, et al:
Antitumor activity of suberoylanilide hydroxamic acid against human
oral squamous cell carcinoma cell lines in vitro and in vivo. Oral
Oncol. 45:766–770. 2009.
|
|
14.
|
Kondo S, Kubota S, Shimo T, et al:
Connective tissue growth factor increased by hypoxia may initiate
angiogenesis in collaboration with matrix metalloproteinases.
Carcinogenesis. 23:769–776. 2002.
|
|
15.
|
Yasuda Y, Kondo S, Nagumo T, et al:
Anti-tumor activity of dehydroxymethylepoxyquinomicin against human
oral squamous cell carcinoma cell lines in vitro and in vivo. Oral
Oncol. 47:334–339. 2011.
|
|
16.
|
Kondo S, Tanaka N, Kubota S, et al: Novel
angiogenic inhibitor DN-9693 that inhibits post-transcriptional
induction of connective tissue growth factor (CTGF/CCN2) by
vascular endothelial growth factor in human endothelial cells. Mol
Cancer Ther. 5:129–137. 2006.
|
|
17.
|
Zhang DY, Wu J, Ye F, et al: Inhibition of
cancer cell proliferation and prostaglandin E2 synthesis by
Scutellaria baicalensis. Cancer Res. 63:4037–4043. 2003.
|
|
18.
|
Scheck AC, Perry K, Hank NC and Clark WD:
Anticancer activity of extracts derived from the mature roots of
Scutellaria baicalensis on human malignant brain tumor
cells. BMC Complement Altern Med. 6:272006.
|
|
19.
|
Wang CZ, Li XL, Wang QF, Mehendale SR and
Yuan CS: Selective fraction of Scutellaria baicalensis and
its chemopreventive effects on MCF-7 human breast cancer cells.
Phytomedicine. 17:63–68. 2010.
|
|
20.
|
Bonham M, Posakony J, Coleman I,
Montgomery B, Simon J and Nelson PS: Characterization of chemical
constituents in Scutellaria baicalensis with antiandrogenic
and growth-inhibitory activities toward prostate carcinoma. Clin
Cancer Res. 11:3905–3914. 2005.
|
|
21.
|
Parajuli P, Joshee N, Rimando AM, Mittal S
and Yadav AK: In vitro antitumor mechanisms of various
Scutellaria extracts and constituent flavonoids. Planta Med.
75:41–48. 2009.
|
|
22.
|
Tayarani Najaran Z, Emami SA, Asili J,
Mirzaei A and Mousavi SH: Analyzing cytotoxic and apoptogenic
properties of Scutellaria litwinowii root extract on cancer
cell lines. Evid Based Complement Alternat Med.
2011:1606822011.
|
|
23.
|
Hsu CH, Hwang KC, Chao CL, et al: An
evaluation of the additive effect of natural herbal medicine on
SARS or SARS-like infectious diseases in 2003: A randomized,
double-blind, and controlled pilot study. Evid Based Complement
Alternat Med. 5:355–362. 2008.
|
|
24.
|
Adams LS, Seeram NP, Hardy ML, Carpenter C
and Heber D: Analysis of the interactions of botanical extract
combinations against the viability of prostate cancer cell lines.
Evid Based Complement Alternat Med. 3:117–124. 2006.
|
|
25.
|
Lin YT, Yang JS, Lin HJ, et al: Baicalein
induces apoptosis in SCC-4 human tongue cancer cells via a
Ca2+-dependent mitochondrial pathway. In Vivo.
21:1053–1058. 2007.
|
|
26.
|
Li-Weber M: New therapeutic aspects of
flavones: the anti-cancer properties of Scutellaria and its
main active constituents wogonin, baicalein and baicalin. Cancer
Treat Rev. 35:57–68. 2009.
|
|
27.
|
Liao HL and Hu MK: Synthesis and
anticancer activities of 5, 6, 7-trimethylbaicalein derivatives.
Chem Pharm Bull. 52:1162–1165. 2004.
|