Introduction
Lung cancer constitutes the leading cause of cancer
mortality and morbidity worldwide (1). The incidence of lung cancer has
markedly increased over the last decades in China. Surgery is
considered to be the treatment that offers the greatest potential
for cure for these patients (2).
Pulmonary resection has a direct negative impact on pulmonary
function and quality of life (QOL), especially on the QOL related
to aspects directly linked to pulmonary function. The aim of the
present study was to evaluate the effects of a systematic
rehabilitation program on the QOL in patients undergoing lung
resection of malignant lung lesions.
Patients and methods
Patients
Between April 2008 and May 2011, 56 consecutive lung
cancer patients with non-small cell lung cancer (NSCLC) were
enrolled in this study. Of the 56 cases, 48 underwent different
modes of surgery including pneumonectomy, lobectomy/bilobectomy or
sleeve lobectomy via open thoracotomy and 8 cases were excluded
from this study for non-compliance with surgical treatment or were
rejected. Patients (n=48) comprised 41 males and 7 females, with a
mean age of 56 years (range, 41–75 years). The present study was
approved by the Research Ethics Committees of the Third Xiangya
Hospital of the Central South University (Changsha, China). Written
informed consent was provided by the patients included.
Methods
The participants received standard medical and
nursing care involving a clinical pathway. Certain patients
received 4–6 cycles of standard chemotherapy. Radiation therapy was
delivered by a 6 megavolt (MeV) photon linear accelerator following
a standard protocol. Radiation was administered by 2.0 Gy daily
fractions, 5 times/week at a total dose of 60 Gy/6 weeks. The
experimental group received the systematic rehabilitation program,
including breathing control, breathing exercises, relaxation
training, progressive exercises and additional incorporating
physiotherapy programs during the preoperative period until
discharge and at 6 months after the surgical procedure. The
patients completed health-related QOL parameters prior to as well
as 3 and 6 months after surgery. QOL was assessed using the Chinese
version of the European Organization for the Research and Treatment
of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30)
(3), which investigates a
patient’s ability to fulfill the activities of everyday life. EORTC
QLQ-C30 is a 30-entry cancer-specific questionnaire that
incorporates six functioning scales (Global, physical, role,
cognitive, emotional and social), nine symptom scales (fatigue,
pain, nausea/vomiting, dyspnea, insomnia, loss of appetite,
constipation, diarrhea and financial difficulties), and one global
health status/QOL scale. The measures are scored from 0 to 100. For
the functional and global health status/QOL scales, a high value
reflects a better level of functioning, while a low value is
representative of low or disappointing function. However, higher
scores of the symptom scales indicate more severe problems.
Statistical analysis
Data were analyzed using the biological statistics
analysis software SPSS 13.0 Package. Application of the constituent
ratio of the χ2 test/Fisher’s test was used for data
analysis. QOL score prior to treatment was compared using the
Student’s t-test, while longitudinal data were compared using
repeated measures analysis of variance (ANOVA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 48 patients, classified into experimental
and control groups, were included in this study. The average age of
the patients in the experimental group was 57 years and in the
control group 55 years. The χ2 and Fisher’s tests showed
no statistically significant difference between the two groups in
terms of age, gender, behavior, clinical stage, adjuvant therapy
and Karnofsky scores. The two groups were well-balanced concerning
patient characteristics (Table I).
The patients underwent surgery, 21 patients received concurrent
chemotherapy, 16 external-beam radiation therapy and 11 concurrent
chemoradiotherapy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Groups
| | |
|---|
| Characteristics | Experimental | Control | Statistical
value | P-value |
|---|
| Age (years) | | | | |
| Range | 41–75 | 42–74 | | |
| Mean ± SD | 57.2±8.9 | 55.9±8.5 | t=0.517 | 0.304 |
| Gender | | | | |
| Male | 20 | 19 | | |
| Female | 4 | 5 |
χ2=0.137 | 0.712 |
| Behavior | | | | |
| Smoking | 16 | 18 | | |
| No-smoking | 8 | 6 |
χ2=0.403 | 0.525 |
| Clinical stage | | | | |
| II | 7 | 8 | | |
| IIIa | 12 | 11 | | |
| IIIb | 5 | 5 |
χ2=0.110 | 0.946 |
| Adjuvant therapy | | | | |
| Concurrent
chemotherapy | 15 | 16 | | |
| Concurrent
chemoradiotherapy | 9 | 8 |
χ2=0.091 | 0.763 |
| Karnofsky score | | | | |
| Range | 60–90 | 60–90 | | |
| Median | 80 | 80 | Z=0.010a | 0.992 |
Analysis of QOL at baseline
Basic QOL on the scale of 6 functional areas and 4
symptom domains were compared prior to treatment in the two groups.
Analysis of the results prior to intervention showed that the two
groups were homogeneous concerning QOL (Table II).
 | Table IIQOL baseline scores of the two patient
groups (mean ± SD). |
Table II
QOL baseline scores of the two patient
groups (mean ± SD).
| Groups
| | |
|---|
| EORTC QLQ-C30
scales | Experimental | Control | t-value | P-value |
|---|
| Function | | | | |
| Global | 58.3±26.3 | 60.3±21.6 | −0.288 | 0.387 |
| Physical | 64.3±22.4 | 65.4±31.2 | −0.140 | 0.445 |
| Role | 60.2±30.9 | 60.7±30.4 | −0.057 | 0.478 |
| Emotional | 45.6±36.6 | 46.5±38.2 | −0.083 | 0.467 |
| Cognitive | 64.0±21.4 | 60.7±30.5 | 0.434 | 0.333 |
| Social | 70.2±25.0 | 66.4±20.2 | 0.579 | 0.283 |
| Symptom | | | | |
| Fatigue | 20.8±13.5 | 26.3±9.8 | −1.615 | 0.057 |
|
Nausea/vomiting | 35.6±14.1 | 42.7±12.6 | −0.863 | 0.196 |
| Pain | 42.3±21.2 | 38.5±17.6 | 0.676 | 0.251 |
| Dyspnea | 18.2±19.7 | 16.9±18.7 | 0.234 | 0.408 |
| Insomnia | 9.4±8.7 | 11.2±10.4 | −0.650 | 0.259 |
| Appetite loss | 27.0±5.4 | 24.5±7.3 | 1.349 | 0.092 |
| Constipation | 10.4±6.5 | 12.0±7.8 | −0.772 | 0.222 |
| Diarrhea | 12.3±9.8 | 10.8±9.2 | 0.547 | 0.294 |
| Financial
difficulties | 10.6±8.2 | 9.5±6.8 | −0.542 | 0.238 |
Analysis of QOL at 3 months after the
rehabilitation process
Three months after the rehabilitation process, the
experimental group demonstrated an increase in the general QOL
functional scales and a decrease of symptom scales compared to the
control group. These changes were statistically significant in the
functional scales of global health (P<0.01), physical
(P<0.01), role (P<0.01) and emotional functions (P<0.05),
symptom scales of fatigue (P<0.01) and appetite loss (P=0.001)
(Table III).
 | Table IIIFunctional and symptom outcomes
derived from EORTC QLQ-C30 in the experimental and control groups 3
months after the intervention (mean ± SD). |
Table III
Functional and symptom outcomes
derived from EORTC QLQ-C30 in the experimental and control groups 3
months after the intervention (mean ± SD).
| Groups
| | |
|---|
| EORTC QLQ-C30
scales | Experimental | Control | t-value | P-value |
|---|
| Function | | | | |
| Global | 78.6±24.7 | 58.1±22.3 | 3.018 | 0.002 |
| Physical | 83.8±24.9 | 64.2±21.5 | 2.913 | 0.003 |
| Role | 76.6±20.4 | 60.2±19.8 | 2.826 | 0.003 |
| Emotional | 64.5±21.8 | 50.6±18.6 | 2.376 | 0.011 |
| Cognitive | 75.6±34.3 | 76.7±28.0 | −0.122 | 0.452 |
| Social | 70.0±22.3 | 65.6±20.4 | 0.713 | 0.240 |
| Symptom | | | | |
| Fatigue | 18.3±10.5 | 30.2±19.4 | −2.643 | 0.006 |
|
Nausea/vomiting | 40.2±22.8 | 36.7±28.9 | 0.466 | 0.322 |
| Pain | 30.8±25.5 | 34.3±18.5 | −0.544 | 0.294 |
| Dyspnea | 35.6±14.1 | 41.4±20.2 | −1.153 | 0.127 |
| Insomnia | 37.2±15.0 | 36.9±10.8 | 0.0080 | 0.468 |
| Appetite
loss | 4.2±4.7 | 12.1±11.4 | −3.139 | 0.001 |
| Constipation | 8.4±4.6 | 7.5±9.1 | 0.432 | 0.334 |
| Diarrhea | 22.0±8.5 | 25.6±9.2 | −1.408 | 0.083 |
| Financial
difficulties | 12.4±10.9 | 14.7±12.6 | −0.676 | 0.251 |
Analysis of QOL at 6 months after the
rehabilitation process
Six months after the intervention, the experimental
group demonstrated an increase in the general QOL functional scales
compared to the control group. These changes were statistically
significant in the functional scales of global health (P=0.001),
physical (P<0.01), role (P=0.001) and emotional functions
(P<0.01). Regarding symptom scales, the symptoms were also
reduced compared to the control group. This decrease was
significant in the scales of fatigue (P<0.001), pain
(P<0.001), dyspnea (P<0.001), insomnia (P<0.001), appetite
loss (P<0.001) and constipation (P<0.001). As a result, the
two groups demonstrated a statistically significant difference in
10 domains. The experimental group demonstrated a significant
recovery (Table IV).
 | Table IVFunctional and symptom outcomes
derived from EORTC QLQ-C30 in the experimental and control groups 6
months after the intervention (mean ± SD). |
Table IV
Functional and symptom outcomes
derived from EORTC QLQ-C30 in the experimental and control groups 6
months after the intervention (mean ± SD).
| Groups
| | |
|---|
| EORTC QLQ-C30
scales | Experimental | Control | t-value | P-value |
|---|
| Function | | | | |
| Global | 80.2±22.5 | 56.4±25.3 | 3.444 | 0.001 |
| Physical | 84.5±28.7 | 64.5±22.6 | 2.682 | 0.005 |
| Role | 86.2±24.5 | 63.4±21.0 | 3.461 | 0.001 |
| Emotional | 68.4±21.2 | 54.4±16.5 | 2.553 | 0.007 |
| Cognitive | 78.5±22.4 | 75.4±27.2 | 0.431 | 0.334 |
| Social | 86.7±43.3 | 82.0±36.3 | 0.408 | 0.343 |
| Symptom | | | | |
| Fatigue | 10.2±7.2 | 40.3±16.5 | −8.191 | 0.000 |
|
Nausea/vomiting | 20.5±14.1 | 26.4±13.2 | −1.496 | 0.071 |
| Pain | 18.7±16.5 | 38.6±20.7 | −3.683 | 0.000 |
| Dyspnea | 16.4±8.2 | 46.5±20.1 | −6.793 | 0.000 |
| Insomnia | 15.6±12.8 | 38.2±21.6 | −4.410 | 0.000 |
| Appetite
loss | 2.1±1.2 | 9.7±8.2 | −4.493 | 0.000 |
| Constipation | 2.5±1.3 | 9.2±6.8 | −4.741 | 0.000 |
| Diarrhea | 22.0±8.5 | 25.6±9.2 | −1.408 | 0.083 |
| Financial
difficulties | 12.4±10.9 | 14.7±12.6 | −0.676 | 0.251 |
EORTC QLQ-C30 scales of variance analysis
results of the two groups
When compared to the control group, the experimental
group demonstrated a statistically significant impact on function
scales, such as the global, physical, role and emotional scale at
various time points (P<0.01) and between various time points
(P<0.001). No statistically significant difference was observed
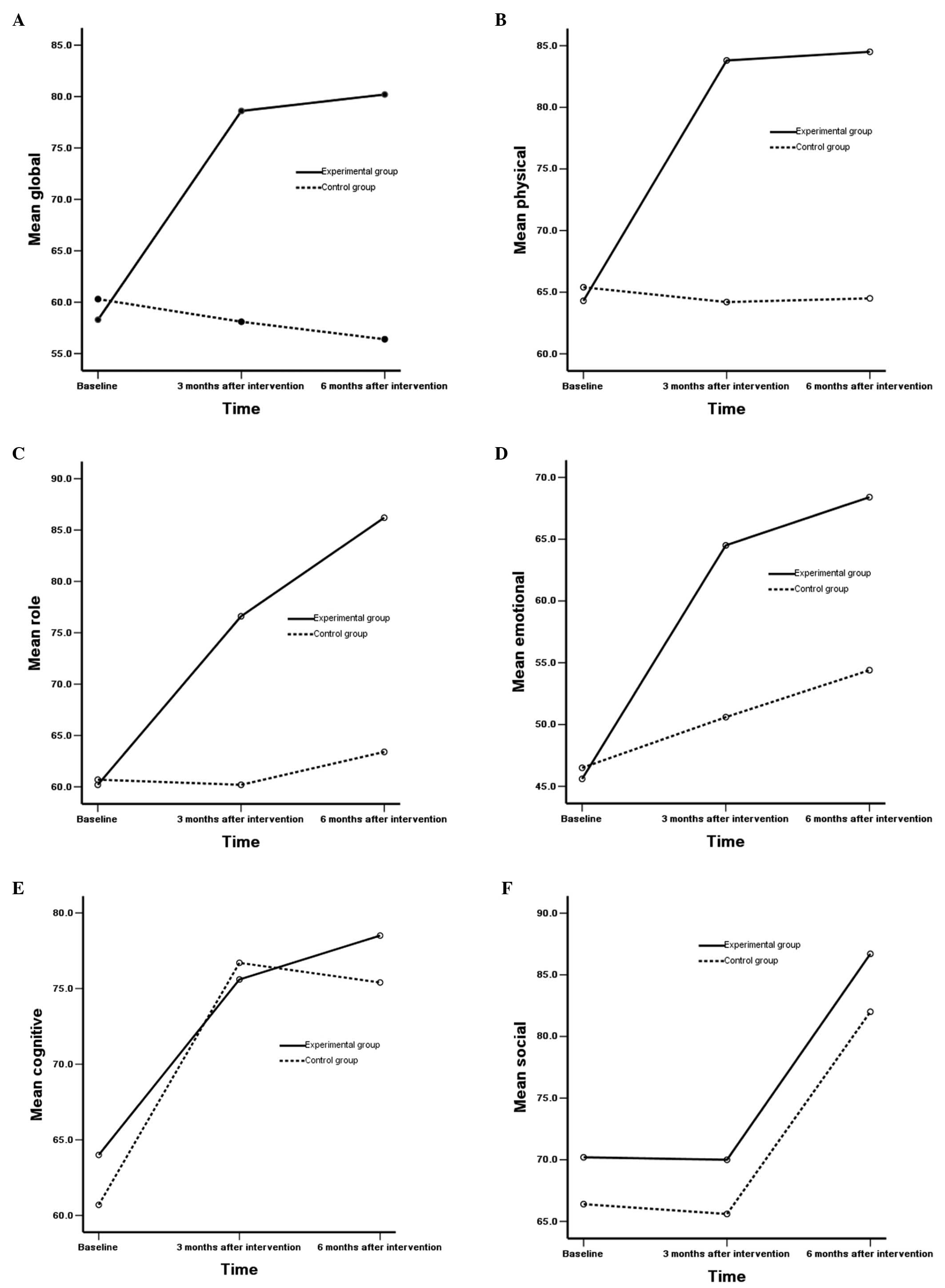
in the correlation between groups and time (P<0.05) (Table V). In addition, no statistically
significant differences were observed in the cognitive scale in
patients in the two groups and different time points (P>0.05),
while there were statistically significant differences in the
correlation between the groups and time (P<0.05). No
statistically significant differences were observed in the social
scale in patients in the two groups and different time points, and
no statistically significant differences were observed in the
correlation between groups and time (P>0.05) (Fig. 1A–F).
 | Table VEORTC QLQ-C30 scales of different
time and interaction variance analysis results of the two
groups. |
Table V
EORTC QLQ-C30 scales of different
time and interaction variance analysis results of the two
groups.
| EORTC QLQ-C30
scales |
FGroups | P-value |
FTime | P-value | FGroup ×
time | P-value |
|---|
| Function | | | | | | |
| Global | 9.332 | 0.004 | 10.659 | 0.000 | 3.190 | 0.046 |
| Physical | 8.652 | 0.005 | 11.337 | 0.000 | 3.668 | 0.029 |
| Role | 10.014 | 0.003 | 9.723 | 0.000 | 3.244 | 0.044 |
| Emotional | 7.568 | 0.008 | 8.352 | 0.000 | 3.874 | 0.024 |
| Cognitive | 2.087 | 0.155 | 2.036 | 0.136 | 3.572 | 0.032 |
| Social | 1.875 | 0.178 | 2.613 | 0.079 | 1.841 | 0.164 |
| Symptom | | | | | | |
| Fatigue | 3.557 | 0.066 | 1.899 | 0.156 | 5.294 | 0.007 |
|
Nausea/vomiting | 11.229 | 0.002 | 8.664 | 0.000 | 1.211 | 0.303 |
| Pain | 12.364 | 0.001 | 10.256 | 0.000 | 4.968 | 0.009 |
| Dyspnea | 10.872 | 0.002 | 7.681 | 0.001 | 3.179 | 0.046 |
| Insomnia | 9.639 | 0.003 | 6.846 | 0.002 | 1.233 | 0.296 |
| Appetite
loss | 12.615 | 0.001 | 3.117 | 0.049 | 3.289 | 0.042 |
| Constipation | 3.243 | 0.078 | 2.699 | 0.073 | 1.657 | 0.196 |
| Diarrhea | 2.068 | 0.157 | 2.897 | 0.060 | 3.605 | 0.031 |
| Financial
difficulties | 4.142 | 0.076 | 3.212 | 0.072 | 2.258 | 0.264 |
When compared to the control group, the experimental
group demonstrated a statistically significant impact on symptom
scales, such as nausea/vomiting, pain, dyspnea, insomnia and
appetite loss at various time points (P<0.01) and between
various time points (P<0.01). However, there were no
statistically significant differences in the correlation between
groups and time (P>0.05). There were no statistically
significant differences in the fatigue, constipation and diarrhea
scales in patients in the two groups and various time points
(P>0.05). Moreover, there were statistically significant
differences in the correlation of the fatigue, pain, dyspnea,
appetite loss and diarrhea scales between groups and time
(P<0.01), while no statistically significant differences were
observed in the correlation of nausea/vomiting, insomnia and
constipation scales (P>0.05) (Fig.
1G–O).
Discussion
Lung cancer is one of the most common malignant
tumors. Dysfunction of lung cancer is associated with complex
interaction of general and local factors, as well as combined
modality therapy (4). These
associated therapeutic modalities, advances in surgery,
radiotherapy and chemotherapy, as well as cancer itself, continue
to yield varied long-term outcomes of adverse physical/functional
impairments that markedly reduce a patient’s ability to tolerate
exercise. Poor exercise tolerance predisposes increased
susceptibility to additional common age-related dysfunctions, poor
QOL (5) and potential premature
death. Therefore, provision of the appropriate treatment and care
programs for controlling the disease and improving the patient QOL
remains a fundamental issue. A multidimensional therapeutic
approach is recommended including pulmonary rehabilitation
(6,7) and an adequate level of physical
activity (8,9) in order to improve exercise tolerance,
health-related QOL as well as surgical candidacy and to decrease
surgical morbidity. Recent studies have shown that physical
activity may have important benefits on lung cancer patients and
survivors (10–12), regardless of the disease stage
(13) or limited physical activity
(14,15). Limited physical activity, also
known as ‘de-conditioning’, may cause the heart and muscles to
regress and become less efficient. Regular exercise is ideal for
cancer patients or survivors in general, with recent studies
indicating improvements in cardiorespiratory fitness, appetite,
cancer-related fatigue and depression (16–18).
Individualized exercise rehabilitation programs for postoperative
lung cancer patients constitute a great method to improve fitness,
which in turn improves cardiac function, as well as oxygen-carrying
capacity, physical functioning, muscle strength and endurance.
Rehabilitation activity has been shown to improve fitness levels
and health status, leading to the improvement of health-related QOL
(19,20).
Numerous epidemiological studies have demonstrated
that regular physical activity reduces the risk of colon cancer and
potentially decreases the risk of endometrium, post- and
premenopausal breast cancer, prostate, lung as well as pancreas
cancer (21,22). A comprehensive rehabilitation
program is essential to improve health status and to enhance QOL in
cancer patients. Thus, the questions included concerned the
preoperative rehabilitation scheme and the lifetime period of
maintenance rehabilitation. No statistically significant difference
was observed in patient QOL in the control group during 6 months
and it can be indicated that patients in this group were stable,
although certain fields, such as dyspnea, they worsened. Prior to
rehabilitation intervention, no statistically significant
difference was observed between the two groups. However, 3 months
after the intervention, the experimental group demonstrated a
statistically significant improvement in six fields, while 6 months
later, improvement was observed in 11 fields compared to the
control group. Therefore, it can be concluded that systematic
rehabilitation intervention had a positive effect on the
experimental group and that patients’ QOL in the experimental group
improved significantly. The trend of improving results in the
experimental group indicates that time is crucial in assessing the
effect of rehabilitation intervention on QOL.
A consistent proportion of patients undergoing lung
resection exhibited an important postoperative deterioration of
their QOL. Thus, patients who received preoperative counseling and
rehabilitation training would benefit from physical and emotional
supportive programs, while others demonstrated a higher risk of a
relevant physical deterioration and worse mental health, such as
pain, pulmonary infection, cardiopulmonary dysfunction, delayed
wound healing and slower recovery, decreased physical fitness and
QOL after lung resection. Concurrent chemoradiotherapy also induced
late side-effects, including organ malfunction, chronic fatigue,
pain or premature death in lung cancer survivors. Future systematic
rehabilitation knowledge is likely to help lung cancer survivors,
their healthcare providers and caregivers by providing evidence for
establishing clinical recommendations to enhance their long-term
survival and health-related QOL. Thus, the 10 patients in the
control group exhibited medical complications and health status
deterioration, while the patients who underwent the systematic
rehabilitation program had beneficial effects on a wide variety of
physical fitness and QOL end-points including functional capacity,
muscular strength, flexibility, fatigue, nausea, diarrhea, pain,
physical and functional well-being, as well as overall QOL. This
has been crucial in overcoming pain and distress, health status and
health-related QOL.
Physical exercise has been of vital importance in
the rehabilitation of oncology patients. However, the issue of how
to provide guidelines for exercise prescription to the specific
needs of cancer patients at various stages of the disease process
has yet to be fully elucidated. Regardless of an individual being
newly diagnosed, currently under treatment or in a long- or
short-term survival phase, rehabilitation activity and exercise are
effective adjuvant interventions for them. However, exercise
prescription must be highly individualized due to the extreme
variability of the cancer effects and treatment regimens on
functional capacity. Exercise dosage was based on the Surgeon
General’s 1996 recommendations (23) and the American College of Sports
Medicine (24). Concerning
exercise mode, walking and cycling are recommended as safe and
generally well-tolerated exercise modes involving large muscle
groups, with a recommended frequency of 3–5 times/week. Patients
with an elevated degree of decondition should begin with daily
sessions of shorter duration and lower intensity. In general,
moderate intensity exercise (50–75% heart rate reserve, Ratings of
Perceived Exertion 11–14) (25)
sessions of 20- to 30-min duration are recommended, with
modifications as required, including extremely short exercise bouts
(3–5 min) followed by rest periods. The key to determine an optimal
exercise dosage was not only one, such parameters would presumably
be demanding for late-stage (i.e., II and III) cancer patients, as
well as for those experiencing especially debilitating treatment
side-effects of chemotherapy, radiation and other aspects. This
should be determined, not only by the subsequent course of the
disease, but by patient motivation and abilities as well. For
example, a patient with stage II lung cancer might be unable to
contemplate walking due to muscle disuse atrophy, surgical lung
excision and dyspnea 1 week after surgery. Treatment could focus
simply on breathing and associated dyspnea with vicinity of 1 MET.
Patients are able to tolerate slow walking without frustration,
anxiety or fatigue 2 weeks after surgery. After 4 weeks, patients
were focused on better rehabilitation goals and were able to
initiate or sustain moderate levels of activity, such as jogging
and swimming.
QOL is an important factor with physical well-being
and is as meaningful to patients as the actual length of life. Two
validated QOL instruments were EORTC QLQ-C30 and Short Form-36
Health Survey (SF-36). EORTC provided a more detailed evaluation of
lung-specific symptoms. For this reason, EORTC should be regarded
as the instrument of choice for measuring QOL in patients with
pulmonary resection for NSCLC (3).
Generally, the rehabilitation effect appeared to be
a positive effect on the functional scales of general QOL in the
experimental group 3 and 6 months after the intervention. The
effects on the QOL were correlated with the desired response and
were detectable after longer time periods.
In conclusion, systematic rehabilitation programs
have been involved in the comprehensive management of patients
undergoing lung resection of malignant lung lesions and improved
pulmonary vessels as well as QOL. Preliminary evidence in this area
supports the theory that systematic rehabilitation programs may be
considered to be incorporated into the multidisciplinary management
of patients diagnosed with lung cancer especially regarding their
long-term survival.
Acknowledgements
This study was supported by the
Natural Science Foundation of Hunan, China (grant no.
08jj6010).
References
|
1.
|
Becerra M, John G, Drepper M, et al:
Hospital-based internal medicine: a review of 2011. Rev Med Suisse.
8:259–263. 2012.(In French).
|
|
2.
|
Carr LL, Finigan JH and Kern JA:
Evaluation and treatment of patients with non-small cell lung
cancer. Med Clin North Am. 95:1041–1054. 2011.
|
|
3.
|
Cocks K, King MT, Velikova G, Martyn
St-James M, Fayers PM and Brown JM: Evidence-based guidelines for
determination of sample size and interpretation of the European
Organisation for the Research and Treatment of Cancer Quality of
Life Questionnaire Core 30. J Clin Oncol. 29:89–96. 2011.
|
|
4.
|
Soo RA, Kawaguchi T, Loh M, et al:
Differences in outcome and toxicity between Asian and caucasian
patients with lung cancer treated with systemic therapy. Future
Oncol. 8:451–462. 2012.
|
|
5.
|
Sloan JA, Zhao X, Novotny PJ, et al:
Relationship between deficits in overall quality of life and
non-small-cell lung cancer survival. J Clin Oncol. 30:1498–1504.
2012.
|
|
6.
|
Qiao Y, Qiu X and Zhou Q: Pulmonary
rehabilitation in the management of patients with lung cancer.
Zhongguo Fei Ai Za Zhi. 14:744–748. 2011.(In Chinese).
|
|
7.
|
Glattki GP, Manika K, Sichletidis L, Alexe
G, Brenke R and Spyratos D: Pulmonary rehabilitation in non-small
cell lung cancer patients after completion of treatment. Am J Clin
Oncol. 35:120–125. 2012.
|
|
8.
|
Jones LW: Physical activity and lung
cancer survivorship. Recent Results Cancer Res. 186:255–274.
2011.
|
|
9.
|
Andersen AH, Vinther A, Poulsen LL and
Mellemgaard A: Do patients with lung cancer benefit from physical
exercise? Acta Oncol. 50:307–313. 2011.
|
|
10.
|
Jones LW, Eves ND, Peterson BL, et al:
Safety and feasibility of aerobic training on cardiopulmonary
function and quality of life in postsurgical non-small cell lung
cancer patients: a pilot study. Cancer. 113:3430–3439. 2008.
|
|
11.
|
Nagamatsu Y, Iwasaki Y, Hayashida R, et
al: Factors related to an early restoration of exercise capacity
after major lung resection. Surg Today. 41:1228–1233. 2011.
|
|
12.
|
Benzo R, Wigle D, Novotny P, et al:
Preoperative pulmonary rehabilitation before lung cancer resection:
results from two randomized studies. Lung Cancer. 74:441–445.
2011.
|
|
13.
|
Op den Kamp CM, Langen RC, Minnaard R, et
al: Pre-cachexia in patients with stages I–III non-small cell lung
cancer: systemic inflammation and functional impairment without
activation of skeletal muscle ubiquitin proteasome system. Lung
Cancer. 76:112–117. 2012.
|
|
14.
|
Peddle-McIntyre CJ, Bell G, Fenton D,
McCargar L and Courneya KS: Feasibility and preliminary efficacy of
progressive resistance exercise training in lung cancer survivors.
Lung Cancer. 75:126–132. 2012.
|
|
15.
|
Quist M, Rørth M, Langer S, et al: Safety
and feasibility of a combined exercise intervention for inoperable
lung cancer patients undergoing chemotherapy: a pilot study. Lung
Cancer. 75:203–208. 2012.
|
|
16.
|
Litterini AJ and Jette DU: Exercise for
managing cancer-related fatigue. Phys Ther. 91:301–304. 2011.
|
|
17.
|
Rueda JR, Solà I, Pascual A and Subirana
Casacuberta M: Non-invasive interventions for improving well-being
and quality of life in patients with lung cancer. Cochrane Database
Syst Rev. 7:CD0042822011.
|
|
18.
|
Granger CL, McDonald CF, Berney S, Chao C
and Denehy L: Exercise intervention to improve exercise capacity
and health related quality of life for patients with non-small cell
lung cancer: a systematic review. Lung Cancer. 72:139–153.
2011.
|
|
19.
|
Arbane G, Tropman D, Jackson D and Garrod
R: Evaluation of an early exercise intervention after thoracotomy
for non-small cell lung cancer (NSCLC), effects on quality of life,
muscle strength and exercise tolerance: randomised controlled
trial. Lung Cancer. 71:229–234. 2011.
|
|
20.
|
Jones LW, Hornsby WE, Goetzinger A, et al:
Prognostic significance of functional capacity and exercise
behavior in patients with metastatic non-small cell lung cancer.
Lung Cancer. 76:248–252. 2012.
|
|
21.
|
Na HK and Oliynyk S: Effects of physical
activity on cancer prevention. Ann N Y Acad Sci. 1229:176–183.
2011.
|
|
22.
|
Emaus A and Thune I: Physical activity and
lung cancer prevention. Recent Results Cancer Res. 186:101–133.
2011.
|
|
23.
|
Erlichman J, Kerbey AL and James WP:
Physical activity and its impact on health outcomes. Paper 1: the
impact of physical activity on cardiovascular disease and all-cause
mortality: an historical perspective. Obes Rev. 3:257–271.
2002.
|
|
24.
|
Schmitz KH, Courneya KS, Matthews C, et
al: American College of Sports Medicine: American College of Sports
Medicine roundtable on exercise guidelines for cancer survivors.
Med Sci Sports Exerc. 42:1409–1426. 2010.
|
|
25.
|
Russell WD: On the current status of rated
perceived exertion. Percept Mot Skills. 84:799–808. 1997.
|