Introduction
Chemotherapy-induced complications are distressing
reactions (1). The incidence,
prevalence and severity of the complications are associated with
several factors. Notably, the emetic risk of the chemotherapy is
correlated with the specific drug, dose, schedule and route of
administration, as well as with patient variables. Among the
complications, chemotherapy-induced nausea and vomiting (CINV) has
been reduced by metoclopramide, serotonin (5-HT3) antagonists and
corticosteroids without additional severe toxicity (2). Nevertheless, the control of acute and
delayed CINV with these antiemetic therapies has not been proven to
be sufficient, especially in patients receiving highly (HEC) and
moderately emetogenic chemotherapy (MEC). As a result, more
effective antiemetic treatments are still required (3,4).
This has led to the development of a new class of antiemetic
agents, such as aprepitant, an antagonist of the neurokinin-1
(NK-1) receptor. The addition of aprepitant to 5-HT3 receptor
antagonist and dexamethasone in cisplatin-based chemotherapy
markedly reduces acute and delayed emesis (5). This three-drug combination has also
been investigated, with favorable results, in patients receiving a
combination of an anthracycline and cyclophosphamide-based regimen,
and these studies were funded by pharmaceutical companies (6,7).
The aim of this study was to determine the incidence
of chemotherapy-induced complications. Pharmacists, who had no
conflicts of interest with any pharmaceutical companies, completed
a questionnaire regarding their perceptions of the incidence of
complications.
Materials and methods
Study description
This prospective observational study was conducted
at Mito Kyodo General Hospital, Mito, Ibaraki, Japan, between 2011
and 2012. A survey asking pharmacists to predict the incidence of
complications including CINV and appetite loss following HEC or MEC
was used. Patients administered HEC or MEC regimens at our
Institution were recruited. Eligible patients were adults,
administered HEC or MEC [emetic levels 3–5 of chemotherapy as
defined by Hesketh et al(5)] (Table
I). Patients were ineligible in case they were unable to
complete the questionnaires. Written and signed informed consent
was obtained from the patients. The protocol was approved by the
Hospital’s Ethics Committee.
 | Table ICharacteristics of patients with lung
cancer. |
Table I
Characteristics of patients with lung
cancer.
| Characteristics | Value |
|---|
| Age (years), median
(range) | 69 (60–82) |
| Gender, n (%) | |
| Male | 25 (80.6) |
| Female | 6 (19.4) |
| Treatment regimens, n
(%) | |
| CDDP-regimens | 6 (19.4) |
| CBDCA-regimens | 21 (67.7) |
| Non-platinum
regimens | 4 (12.9) |
| Complications, n
(%) | |
| Nausea | 10 (32.3) |
| Vomiting | 3 (9.7) |
| Constipation | 24 (77.4) |
| General
fatigue | 22 (71.0) |
| Appetite loss | 21 (67.7) |
| Dysesthesia | 6 (19.4) |
| Stomatitis | 4 (12.9) |
| Diarrhea | 4 (12.9) |
Antiemetic therapy
Patients administered antiemetic treatment with a
three-drug regimen that included aprepitant (125 mg/day on day 1,
80 mg/day on days 2–3), dexamethasone (6.6 mg/day drip infusion on
days 1–3) and granisetron (3 mg drip infusion on day 1). Rescue
therapy was administered when necessary. Cisplatin-based regimens
were considered HEC regimens, carboplatin-based regimens were
evaluated as MEC and non-platinum regimens were considered MEC
regimens.
Study measurements
Pharmacists completed a questionnaire regarding
their perceptions of the incidence of complications including CINV
in their own practices subsequent to chemotherapy, despite the use
of adequate antiemetic therapy.
Based on previous studies (8–11),
seven complications on days 1–7 were evaluated in this study. The
extent of the complications were: nausea (none, slightly but no
effect on eating, hard to eat, cannot eat and drink), vomiting
(number of vomiting episodes per day), appetite (no change,
slightly decreased, half decreased, considerably decreased),
stomatitis (none, slightly but no effect on eating, effect on
eating, hard to eat, cannot eat), constipation (none, slightly but
without medication, slightly with medication, moderate with
medication, with abdominal pain), diarrhea (none, slightly but
without medication, slightly with medication, moderate with
medication, with abdominal pain), dysesthesia (none, slightly,
moderately, hard to work) and general fatigue (none, slightly,
moderately, hard, severe). Patients were instructed to use a diary
to report each episode of complication. In this study, we evaluated
each complication when present, whether a modest or considerable
degree was observed.
Results
The total number of patients who completed the
questionnaires and diaries was 31. The patient characteristics are
shown in Table I. The majority of
enrolled patients were male (80.6%). The median age of the 31
patients was 69 years (range, 60–82 years). Six (19.4%) patients
were administered CDDP-, 21 (67.7%) CBDCA- and 4 (12.9%)
non-platinum regimens. There were no severe complications requiring
additional medication.
Control of nausea and emesis
The percentage of patients reporting nausea/day
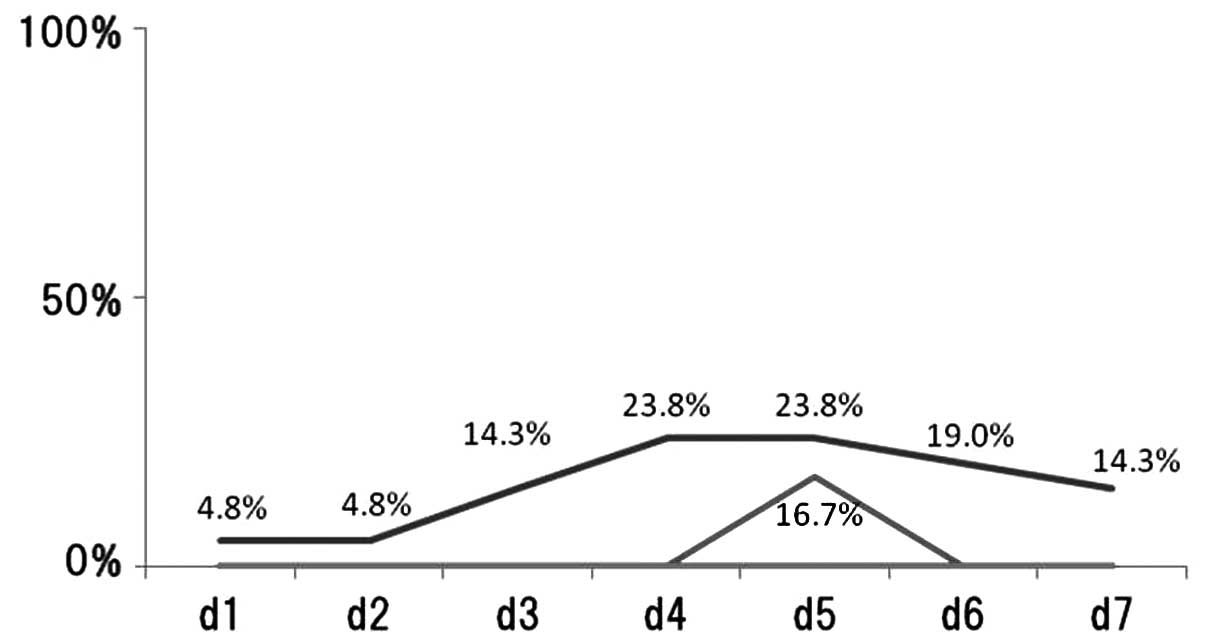
following chemotherapy treatment was recorded (Fig. 1). Regarding nausea, 10 (32.3%) of
the 31 patients exhibited the complication between days 1–7. In
patients treated with CDDP-regimens, the incidence of nausea on
each day was: day 1, 4.8%; day 2, 4.8%; day 3, 14.3%; day 4, 23.8%;
day 5, 23.8%; day 6, 19.0% and day 7, 14.3%. In patients treated
with CBDCA-regimens, the incidence of nausea on each day was: days
1–4, 0%; day 5, 16.7% and day 6–7, 0%. In patients treated without
platinum-regimens, the incidence of nausea on days 1–7 was 0%.
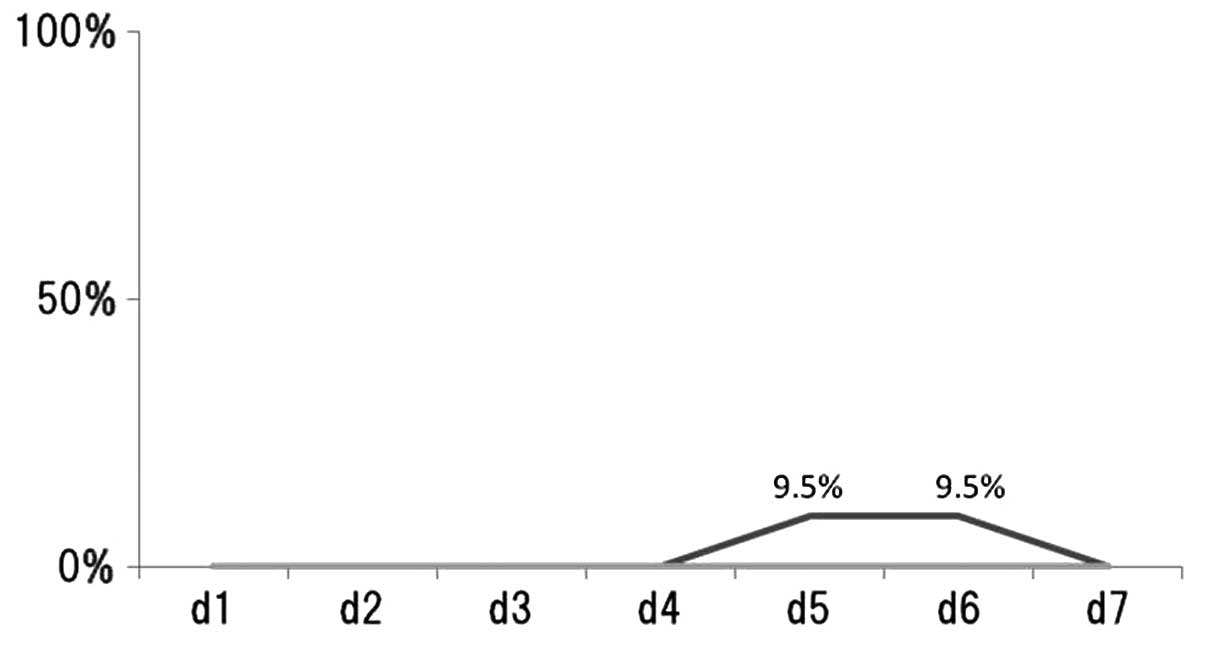
The percentage of patients reporting vomiting/day
following chemotherapy treatment was recorded (Fig. 2). Vomiting was observed in 3 (9.7%)
of the 31 patients (Table I). In
patients treated with CDDP-regimens, 9.5% exhibited vomiting on
days 5 and 6. However, none of the patients treated with
CBDCA-regimens and without platinum-regimens exhibited vomiting on
days 1–7.
Other complications
Three of the most common complications were
constipation, general fatigue and appetite loss. The incidence of
these complications was 77.4, 71.0 and 67.7%, respectively. The
incidence of dysesthesia, stomatitis and diarrhea was 19.4, 12.9
and 12.9%, respectively.
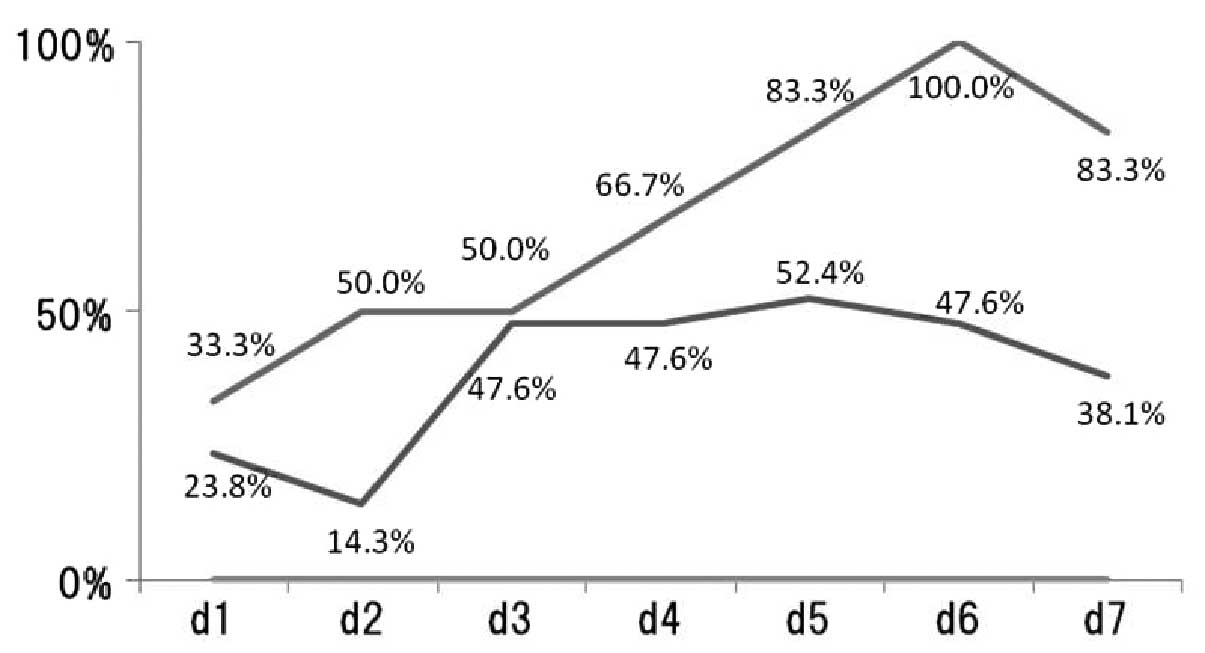
The percentage of patients reporting appetite
loss/day following chemotherapy treatment was recorded (Fig. 3). In patients treated with
CDDP-regimens, the incidence of appetite loss each day was: day 1,
33.3%; day 2, 50%; day 3, 50%; day 4, 66.7%; day 5, 83.3%; day 6,
100% and day 7, 83.3%. In patients treated with CBDCA-regimens, the
incidence of appetite loss each day was: day 1, 23.8%; day 2,
14.3%; day 3, 47.6%; day 4, 47.6%; day 5, 52.4%; day 6, 47.6% and
day 7, 38.1%.
Discussion
Recently, a new class of antiemetic agents has been
developed. The NK-1 receptor antagonist aprepitant, when combined
with 5-HT3 receptor antagonist and dexamethasone, are linked to a
significant reduction in acute and delayed emesis in patients
administered platinum-based regimens (12). Although CINV has been one of the
most significant adverse events of chemotherapy, its impact appears
to be decreased by metoclopramide, 5-HT3 antagonists and
corticosteroids (13,14). Several studies have assessed the
accuracy with which physicians and nurses perceive the control of
nausea and emesis among their own patients. These studies concluded
that these healthcare providers underestimate the incidence of the
delayed nausea as well as emesis subsequent to HEC or MEC (15–17).
Nevertheless, the majority of these studies were completed prior to
accepting aprepitant as a standard treatment and introducing it
into daily practice for the prevention of CINV (13–17).
In their study, Majem et al(18) assessed control rates in their own
practice following the introduction of aprepitant with a view to
evaluate the accuracy with which physicians and nurses perceive the
incidence of CINV (18). In our
study, incidence rates of complications including CINV were
assessed by pharmacists, who had no conflict of interests with any
pharmaceutical companies. To the best of our best knowledge, this
is the first study to evaluate incidence rates of complications
including CINV assessed by pharmacists.
Even in the era of new antiemetic agents, a number
of patients receiving CDDP-based regimens and those treated with
CBDCA-based regimens continued to experience nausea, although
<10% of patients treated with CDDP-based regimens had vomiting.
Prior to the current era of new antiemetics, healthcare providers,
such as physicians and nurses overestimated the control of delayed
nausea and emesis (15,16). Previously, Majem et
al(18) reported that the
control rate of CINV was 66.7% in 95 patients (87% administered
HEC). They described that predictions of the control rate of CINV
by healthcare providers were more accurate compared to those
previously reported for HEC regimens with CDDP (18). In our patients, the control rate of
CINV was 67.7%, which was almost identical to the findings reported
by Majem et al(18). In
their study, Hilarius et al(19) reported that CINV was worse in women
and in younger patients. In that study, 69% of the patients were
female, with a mean age of 56 years (19). In our patients, there was a high
percentage of male patients (80.6%), with a mean age of 69
years.
In addition, the regulation of other complications,
such as constipation, general fatigue and appetite loss remained
poor, as observed in the present study. No promising drugs have
been found for chemotherapy-induced general fatigue or for appetite
loss. The precise mechanism of chemotherapy-induced general fatigue
has yet to be elucidated, and its effective treatment has not been
established at present. In chemotherapy-induced constipation, there
may be several mechanisms in various antitumor drugs, and
therefore, no decisive drug has been found for this complication.
However, regarding appetite loss, a number of recent studies
demonstrated that certain herbal medicines may modulate ghrelin,
which is considered a pivotal signal to the brain to stimulate
feeding (20). Rikkunshito is one
of these promising herbal drugs for the control of appetite loss
(21–23) and a clinical trial for this drug is
currently being delineated.
There were certain limitations to this study,
including the small sample size, possible selection bias and lack
of a standardized antiemetic regimen. However, this study has shown
that CDDP-regimen-induced CINV as well as constipation, general
fatigue and appetite loss continue to be problems. These findings
suggest that the current management of patients receiving
chemotherapy repeatedly should carefully be considered.
Additionally, the use of scales, such as pharmacist-reported
outcome instruments assessing the impact of CINV on the daily
function of patients and assessing frequency, severity and duration
of postchemotherapy-related complications are likely to help
physicians to better manage chemotherapy.
In conclusion, CINV as well as constipation, general
fatigue and appetite loss continue to be problems for patients
receiving chemotherapy. Their incidence is underestimated by
physicians and nurses. A better assessment of the incidence of
these chemotherapy-related complications by medical oncologists and
physicians as well as medical staff, is essential for their
adequate control.
References
|
1.
|
Peters BG: An overview of chemotherapy
toxicities. Top Hosp Pharm Manage. 14:59–88. 1994.
|
|
2.
|
Van Ryckeghem F and Van Belle S:
Management of chemotherapy-induced nausea and vomiting. Acta Clin
Belg. 65:305–310. 2010.
|
|
3.
|
Grunberg SM and Hesketh PJ: Control of
chemotherapy-induced emesis. N Engl J Med. 329:1790–1796. 1993.
|
|
4.
|
Hickok JT, Roscoe JA, Morrow GR, King DK,
Atkins JN and Fitch TR: Nausea and emesis remain significant
problems of chemotherapy despite prophylaxis with
5-hydroxytryptamine-3 antiemetics: a University of Rochester James
P. Wilmot Cancer Center Community Clinical Oncology Program Study
of 360 cancer patients treated in the community. Cancer.
97:2880–2886. 2003.
|
|
5.
|
Hesketh PJ, Grunberg SM, Gralla RJ, et al:
Aprepitant Protocol 052 Study Group: The oral neurokinin-1
antagonist aprepitant for the prevention of chemotherapy-induced
nausea and vomiting: a multinational, randomized, double-blind,
placebo-controlled trial in patients receiving high-dose cisplatin
- the Aprepitant Protocol 052 Study Group. J Clin Oncol.
21:4112–4119. 2003.
|
|
6.
|
Warr DG, Grunberg SM, Gralla RJ, et al:
The oral NK(1) antagonist aprepitant for the prevention of acute
and delayed chemotherapy-induced nausea and vomiting: pooled data
from 2 randomised, double-blind, placebo controlled trials. Eur J
Cancer. 41:1278–1285. 2005.
|
|
7.
|
Warr DG, Hesketh PJ, Gralla RJ, et al:
Efficacy and tolerability of aprepitant for the prevention of
chemotherapy-induced nausea and vomiting in patients with breast
cancer after moderately emetogenic chemotherapy. J Clin Oncol.
23:2822–2830. 2005.
|
|
8.
|
Osoba D: Health-related quality-of-life
assessment in clinical trials of supportive care in oncology.
Support Care Cancer. 8:84–88. 2000.
|
|
9.
|
Barford KL and D’Olimpio JT: Symptom
management in geriatric oncology: practical treatment
considerations and current challenges. Curr Treat Options Oncol.
9:204–214. 2008.
|
|
10.
|
Stokman MA, Spijkervet FK, Boezen HM,
Schouten JP, Roodenburg JL and de Vries EG: Preventive intervention
possibilities in radiotherapy- and chemotherapy-induced oral
mucositis: results of meta-analyses. J Dent Res. 85:690–700.
2006.
|
|
11.
|
Park SB, Krishnan AV, Lin CS, Goldstein D,
Friedlander M and Kiernan MC: Mechanisms underlying
chemotherapy-induced neurotoxicity and the potential for
neuroprotective strategies. Curr Med Chem. 15:3081–3094. 2008.
|
|
12.
|
Navari RM, Reinhardt RR, Gralla RJ, et al:
Reduction of cisplatin-induced emesis by a selective
neurokinin-1-receptor antagonist. L-754, 030 Antiemetic Trials
Group. N Engl J Med. 340:190–195. 1999.
|
|
13.
|
Coates A, Abraham S, Kaye SB, et al: On
the receiving end-patient perception of the side-effects of cancer
chemotherapy. Eur J Cancer Clin Oncol. 19:203–208. 1983.
|
|
14.
|
Griffin AM, Butow PN, Coates AS, et al: On
the receiving end. V: patient perceptions of the side effects of
cancer chemotherapy in 1993. Ann Oncol. 7:189–195. 1986.
|
|
15.
|
Erazo Valle A, Wisniewski T, Figueroa
Vadillo JI, Burke TA and Martinez Corona R: Incidence of
chemotherapy-induced nausea and vomiting in Mexico: healthcare
provider predictions versus observed. Curr Med Res Opin.
22:2403–2410. 2006.
|
|
16.
|
Grunberg SM, Deuson RR, Mavros P, et al:
Incidence of chemotherapy-induced nausea and emesis after modern
antiemetics. Cancer. 100:2261–2268. 2004.
|
|
17.
|
Liau CT, Chu NM, Liu HE, Deuson R, Lien J
and Chen JS: Incidence of chemotherapy-induced nausea and vomiting
in Taiwan: physicians’ and nurses’ estimation vs. patients’
reported outcomes. Support Care Cancer. 13:277–286. 2005.
|
|
18.
|
Majem M, Moreno ME, Calvo N, et al:
Perception of healthcare providers versus patient reported
incidence of chemotherapy-induced nausea and vomiting after the
addition of NK-1 receptor antagonists. Support Care Cancer.
19:1983–1990. 2011.
|
|
19.
|
Hilarius DL, Kloeg PH, van der Wall E, van
den Heuvel JJ, Gundy CM and Aaronson NK: Chemotherapy-induced
nausea and vomiting in daily clinical practice: a community
hospital-based study. Support Care Cancer. 20:107–117. 2012.
|
|
20.
|
Yada T, Kohno D, Maejima Y, et al:
Neurohormones, rikkunshito and hypothalamic neurons interactively
control appetite and anorexia. Curr Pharm Des. May 23–2012.(Epub
ahead of print).
|
|
21.
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology.
134:2004–2013. 2008.
|
|
22.
|
Takeda H, Muto S, Nakagawa K, Ohnishi S
and Asaka M: Rikkunshito and ghrelin secretion. Curr Pharm Des. May
23–2012.(Epub ahead of print).
|
|
23.
|
Cheng KC, Li YX and Cheng JT: The use of
herbal medicine in cancer-related anorexia/cachexia treatment
around the world. Curr Pharm Des. May 23–2012.(Epub ahead of
print).
|