Introduction
Esophageal cancer is the eighth most common type of
cancer worldwide, with an estimated 481,645 new cases in 2008, and
the sixth most common cause of cancer-related mortality, with
406,533 deaths in 2008 (1).
Esophageal squamous cell carcinomas (ESCCs) are more common in
Asian countries, including Japan, compared to Western countries,
where adenocarcinomas in the lower third of the esophagus are
commonly encountered. According to the National Cancer Center data
base (2), 11,746 Japanese patients
succumbed to esophageal cancer in 2008, which was equivalent to
3.26% of the total deaths from malignant neoplasms in Japan.
Esophageal cancer is highly aggressive and has a
poor prognosis due to early metastasis to the lymph nodes, as well
as metastasis to distant organs (3–5).
Surgery has been considered as the mainstay of treatment for
patients with confirmed, locoregionally confined esophageal
carcinoma. However, until a decade ago the 3- or 5-year survival
rate was <30% worldwide, according to previous studies using
several different approaches (6,7–10).
In Japan, the survival rate has shown improvement over the last two
decades since three-field lymphadenectomy was introduced in the
early 1980s by Isono et al (11) and Ando et al (12), a modality that is now widely used.
In comparison, en bloc esophagectomy, also known as extensive
esophagectomy, is performed in Western countries (13–15).
However, even with extensive radical surgery involving the
esophagus, locoregional or distant recurrences have been observed
in 8–32% of patients (16–25). The estimated 5-year survival rates
for esophageal cancer treated with curative intent were 34–53%
(11,26–28).
To improve the survival of locoregional esophageal carcinoma,
multimodal therapy comprising chemotherapy and/or radiotherapy in
combination with radical surgery has been developed. An approach
involving neoadjuvant chemoradiotherapy (CRT) followed by
esophagectomy, known as trimodality therapy (29,30),
is mainly used. It offers the potential advantages of tumor
downstaging, reduced dissemination of malignant cells during
surgery and prevention of micrometastasis. Nine randomized trials
were conducted on patients with confirmed locoregionally confined
esophageal cancer who received preoperative CRT compared to surgery
alone (30–38). Two of these studies demonstrated an
improved outcome, despite the limited patient sample (30,38),
whereas the remaining studies showed no survival benefits in the
trimodality therapy group. Therefore, the benefits of preoperative
CRT remain controversial. In addition, there is no ongoing or
planned randomized study that is related to preoperative CRT for
ESCC in Japan. Since 1996, preoperative CRT using 5-fluorouracil
(5-FU) and cisplatin (CDDP) combined with radical surgery has been
employed for the treatment of advanced esophageal cancers, with
reported increased resectability, reduced incidence of local
recurrence and distant metastasis and a more favorable prognosis
for CRT responders (39).
Additionally, we have reported that preoperative CRT in UICC stage
II/III (non-T4) ESCC contributed to tumor shrinkage, leading to
higher resectability and longer patient survival (40).
In the present retrospective study on patients with
resectable esophageal cancer who underwent extensive radical
esophagectomy, we investigated whether increased survival benefits
were obtained from neoadjuvant CRT plus surgery, compared to
surgery alone.
Patients and methods
Patients
Patients with histologically confirmed ESCC who had
not undergone treatment previously were considered eligible for
inclusion in the present study. Endoscopy and CT scan and/or
endoscopic ultrasound examination were mandatory for determining
clinical stage (II, III or IV disease) in patients with resectable
disease according to the UICC TNM Classification of Malignant
Tumors (41). The eligibility
criteria for the present study were as follows: age <80 years,
adequate organ function (white blood cell count ≥3,500, hemoglobin
≥10 g/dl, aspartate aminotransferase/alanine aminotransferase ≤2×
upper limit of normal, platelet count ≥100,000/mm3,
serum creatinine ≤1.3 mg/dl) and a performance status (Eastern
Cooperative Oncology Group) of <2 at the time of admission.
Eighty-eight patients were enrolled in our study. Of these, 52
patients received preoperative CRT followed by esophagectomy (Group
A) and 36 patients received esophagectomy alone (Group B) between
August, 1997 and June, 2011 at the Departments of Surgery of Hyogo
College of Medicine (Nishinomiya, Japan) and Nara Hospital, Kinki
University School of Medicine (Ikoma, Japan). Informed consent was
obtained from the patients.
Neoadjuvant CRT followed by
esophagectomy
Neoadjuvant radiotherapy (1 fraction/day) was
performed for 5 days per week (Monday to Friday) using a linear
accelerator (Mevatron KD2; Toshiba, Kawasaki, Japan). Patients
received 20 fractions of 2 Gy up to a total radiation dose of 40
Gy. The radiation field encompassed the primary tumor volume (as
defined by endoscopy, esophagography and CT scans) with a 3-cm
margin in each cephalad and caudal direction and 4-cm horizontal
margins. If lymph node metastasis was detected using CT, the
radiation field was extended to include the primary tumor and the
metastatic lesions. Concurrent chemotherapy consisted of 5-FU (500
mg/m2/day) administration by a 120-h continuous
intravenous infusion starting on day 1 and CDDP (15–20 mg/day) by a
2-hour intravenous infusion on days 1–5 and repeated after 3 weeks.
Esophagectomy was planned for 4–7 weeks following completion of
CRT. The majority of patients underwent thoracotomy, laparotomy and
cervicotomy in order to perform esophagectomy with two- or
three-field lymphadenectomy and gastroesophageal anastomosis at the
left side of the neck. Radical resection (R0) was defined as the
removal of the macroscopic tumors, no evidence of distant
metastasis, absence of a microscopic residual tumor, free resection
margins and lymphadenectomy extending beyond the involved nodes.
Resection was defined as non-radical when microscopic (R1) or
macroscopic (R2) residual tumor was identified according to the TNM
criteria (41).
Evaluation of response after CRT
The effects of CRT on the primary tumor and the
metastatic nodes were assessed at 2–3 weeks after the completion of
radiotherapy, using chest CT scanning, barium esophagography and/or
upper gastrointestinal endoscopy and/or ultrasonography. The
response to therapy was defined according to the criteria of the
Japanese Society of Esophageal Disease (42) as follows: i) complete response
(CR), defined as 100% regression of cancer; ii) partial response
(PR), defined as >50% regression of the primary tumor and
metastatic nodes; iii) progressive disease (PD), defined as an
increase of 25% in the size of the primary tumor or metastatic
nodes or the appearance of new lesions; and iv) no change (NC)
defined as a decrease of <50% in the size of the primary tumor
and metastatic nodes and no evidence of tumor progression.
Toxicities were classified according to the National Cancer
Institute Common Terminology Criteria (NCI CTC) guidelines
(43).
Esophagectomy (surgery alone)
Esophagectomy was performed through a small
thoracotomy (∼10 cm) using thoracoscopy-assisted esophagectomy with
two- or three-field lymphadenectomy including the upper
mediastinum. The reconstruction was routinely performed using the
retrosternal root and gastroesophageal anastomosis at the left side
of the neck. The degree of radical resections (R) was similarly
assessed according to the TNM system (41).
Locoregional failure and distant
metastasis
After the first recurrence was noted, any additional
recurrence identified within 1 month was considered to have
occurred simultaneously. Locoregional recurrences were defined as
anastomotic recurrences or recurrences that occurred either in the
mediastinum or the upper abdomen at the site of previous esophageal
resection and nodal clearance. Distant recurrences were defined as
hematogenous or other types (in the pleura or peritoneum).
Cervical, celiac axis and para-aortic nodal metastases were
classified as distant metastasis according to TNM system (41).
Statistical analysis
The differences between the two groups (A and B) in
terms of patient characteristics, postoperative complications and
recurrence patterns were evaluated using the Fisher’s exact test.
Overall survival (OS) was defined as the time from the date of
initial treatment to patient death or to the date of the last
available information on the vital status. Disease-free survival
(DFS) was defined as the length of time after treatment during
which no cancer was detected. Differences between the cumulative
survival rates of the patient groups were calculated using the
log-rank test for comparisons of the Kaplan-Meier survival curves.
P<0.05 was considered to indicate a statistically significant
difference. Univariate analyses were used to assess patient
characteristics and other prognostic factors. The Cox proportional
hazards model was used to determine differences in survival between
the two treatment groups and subgroups. Statistical analyses were
performed using STATISTICA software, version 06J (StatSoft, Tulsa,
OK, USA) and SPSS statistics, version 16 (SPSS Japan Inc., Tokyo,
Japan).
Results
Patient characteristics
The patient characteristics are summarized in
Table I. Eighty-eight patients
were evaluated in this study, including 52 patients in Group A
(neoadjuvant CRT+surgery) and 36 patients in Group B (surgery
alone). The tumors were histologically confirmed as ESCCs. No
statistical differences were observed in age, male/female ratio,
location of primary tumor, lymph node metastasis or clinical stage.
As regards the depth of tumor invasion, there was a tendency for
deeper invasion in Group A compared to that in Group B patients
(P<0.001). R0 resection was performed in 42 patients in Group A
and the patients in Group B. Ten patients in Group A underwent R1
resection. Patients with positive cervical and/or celiac nodes
(M1a, M1b) were classified as clinical stage IV. One patient in
Group A had a double cancer of the esophagus and lower pharynx. She
received radiotherapy to the neck preoperatively, at a total dose
of 60 Gy, in order to preserve the pharynx and larynx.
 | Table I.Clinicopathological
characteristics. |
Table I.
Clinicopathological
characteristics.
| Variables | Group A | Group B | P-value |
|---|
| Age (years) | | | |
| Mean | 60.11 | 64.6 | NS |
| Gender | | | |
| Male | 42 | 32 | NS |
| Female | 10 | 4 | |
| Location of primary
tumor | | | |
| Upper
esophagus | 7 | 2 | NS |
| Middle
esophagus | 29 | 13 | |
| Lower
esophagus | 16 | 19 | |
| Abdominal | 0 | 2 | |
| Depth of tumor
invasion | | | |
| T1b | 0 | 1 | <0.001 |
| T2 | 2 | 14 | |
| T3 | 34 | 21 | |
| T4 | 16 | 0 | |
|
N-classification | | | |
| N0 | 34 | 17 | NS |
| N1 | 11 | 19 | |
| M1a | 2 | 0 | |
| M1b | 5 | 0 | |
| Clinical stage | | | |
| II | 27 | 22 | NS |
| III | 18 | 14 | |
| IV | 7 | 0 | |
Response to neoadjuvant CRT and
toxicity
The clinical response of the primary tumor and the
metastatic nodes is provided in Table
II. Lymph node metastasis was observed in 18 patients by CT
scans or upper gastrointestinal ultrasonography. The patients with
NC and PD for metastatic nodes had celiac or neck lymph node
metastasis, respectively. At the radiologists’ suggestion, these
fields were excluded from the primary radiation field to avoid
postoperative complications. The clinical response (CR+PR) to
neoadjuvant CRT for the primary tumor and the metastatic nodes was
86.5 and 44.4%, respectively. The collective clinical response of
the primary tumor and metastatic nodes was 80.8%. Major toxicology
profiles were summarized in medical records.
 | Table II.Effects of preoperative CRT on the
primary tumor and the metastatic nodes. |
Table II.
Effects of preoperative CRT on the
primary tumor and the metastatic nodes.
| Response | Primary tumor | Metastatic
nodes | Clinical response
rate (Primary tumor and metastatic nodes) |
|---|
| CR | 16 | 4 | 14 |
| PR | 29 | 4 | 28 |
| NC | 7 | 8 | 8 |
| PD | 0 | 2 | 2 |
| Response rate | 86.5% | 44.4% | 80.8% |
Leukocytopenia exceeding grade 3 was observed in
21.1% of patients, grade 1/2 general fatigue was observed in 30.8%,
grade 1/2 nausea in 28.9%, grade 2/3 loss of appetite in 23.1% and
grade 2 liver dysfunction in 3.8% of the patients, respectively.
There was no reported CRT-related mortality.
Surgery and postoperative
complications
Eighty-five patients (51 patients in Group A and 34
in Group B) underwent esophagectomy via right thoracotomy with two-
or three-field lymphadenectomy in both groups. One patient in Group
A underwent lower esophagectomy with proximal subtotal gastrectomy
via left thoracotomy and jejunal reconstruction was performed.
Furthermore, one patient in Group A received a total esophagectomy
with laryngectomy. Additionally, one patient in Group A received
ileocecal replacement since he had previously undergone gastrectomy
for early gastric cancer. Two patients in Group B underwent lower
esophagectomy with proximal subtotal gastrectomy via left
thoracotomy and jejunal reconstruction was performed. Postoperative
complications from medical records are summarized in Table III. Leakage following
esophagogastrostomy was observed in 4 patients (7.5%) in Group A, a
lower incidence compared to Group B (P=0.027). Two patients in
Group A underwent additional surgery to restore the continuity of
the alimentary tract. One patient received jejunal interposition
between the gastric tube and the neck of the esophagus and another
patient received skin flap transplantation of the latissimus dorsi
muscle.
 | Table III.Postoperative complications in the
two groups. |
Table III.
Postoperative complications in the
two groups.
| Group A | Group B | P-value |
|---|
| Blood loss (ml),
mean | 528 | 684 | NS |
| Respiratory failure
(%) | 5.7 | 13.8 | NS |
| Anastomotic leakage
(%) | 7.5 | 25 | 0.027 |
| Recurrent nerve
palsy (%) | 3.8 | 5.25 | NS |
| 30-day mortality
(%) | 0 | 2.78 | NS |
| Hospital death
(%) | 1.9 | 8.3 | NS |
Pathological response of the primary
tumor
Sixteen out of the 52 patients that received
neoadjuvant CRT (30.8%) showed no residual tumor in the resected
esophagus, which was compatible with pathological CR.
Recurrence pattern
Comparisons of incidence and type of disease
recurrence between the two treatment groups are provided in
Table IV. The incidence of
simultaneous locoregional and distant recurrence was significantly
higher in Group B compared to that in Group A (P=0.0474).
 | Table IV.Site of recurrence in 88 esophageal
squamous cell carcinoma patients. |
Table IV.
Site of recurrence in 88 esophageal
squamous cell carcinoma patients.
| Site | Group A No.
(%) | Group B No.
(%) | P-value |
|---|
| Locoregional
failure | 4/52 (7.69) | 5/36 (13.9) | NS |
| Distant
metastasis | 15/52 (28.8) | 7/36 (19.4) | NS |
| Local and distant
simultaneously | 2/52 (3.85) | 6/36 (16.7) | 0.0474 |
DFS and OS
The median follow-up period was 44.8 months in Group
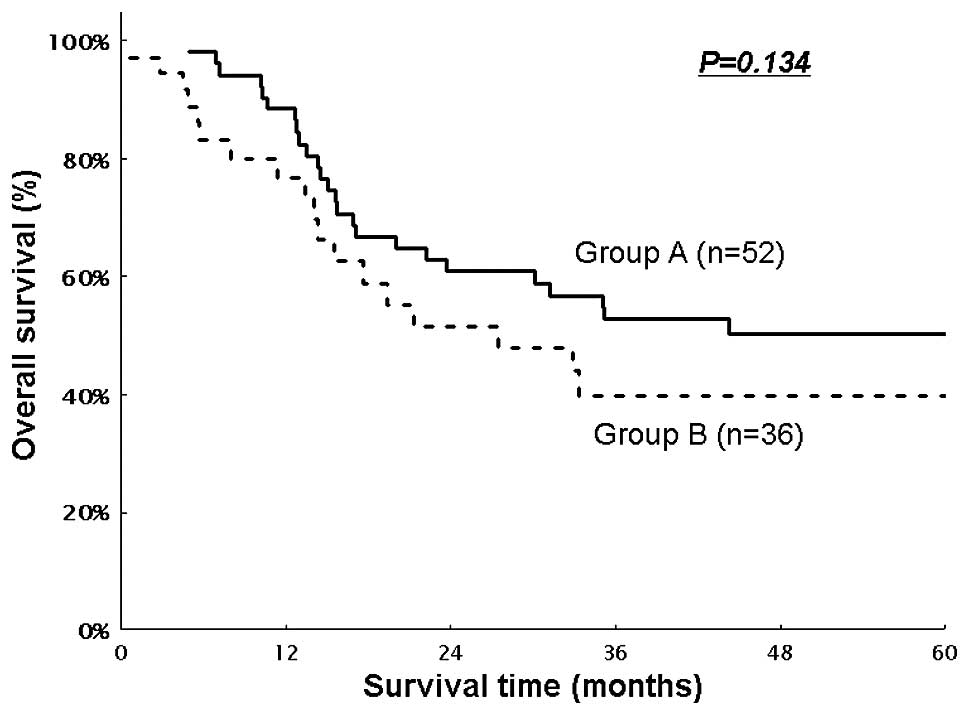
A and 24.6 months in Group B. The OS for Groups A and B, including
hospital deaths, is shown in Fig.
1. The median survival time (MST) was not achieved in Group A
and was 27.4 months in Group B. The estimated 3- and 5-year OS
rates were 52.7 and 50.3%, respectively, in Group A and 39.9 and
39.9%, respectively, in Group B. There was no significant
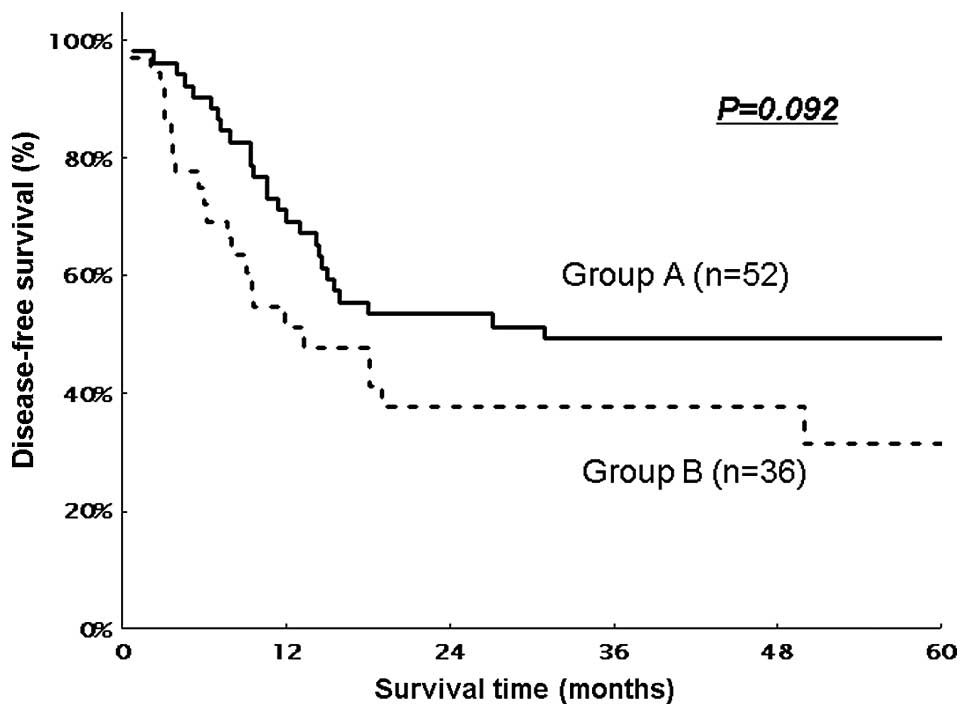
difference between the two groups (P=0.134). The DFS in the two
groups is shown in Fig. 2. The MST
was 30.97 months in Group A and 13.37 months in Group B. The
estimated 3- and 5-year DFS rates were 49.4 and 49.4%,
respectively, in Group A and 37.7 and 37.4%, respectively, in Group
B. Group A exhibited a tendency for a higher DFS rate compared to
Group B, although there was no significant difference between the
two groups (P=0.092). In the subgroup analysis of the patients with
stage II/III disease, Group A exhibited a significantly improved OS
rate compared to Group B (5-year OS, 59% for Group A vs. 39.9% for
Group B, P=0.043). Similarly, a higher DFS rate was observed in
Group A compared to Group B (5-year DFS, 57.2% for Group A vs.
31.4% for Group B, P=0.025).
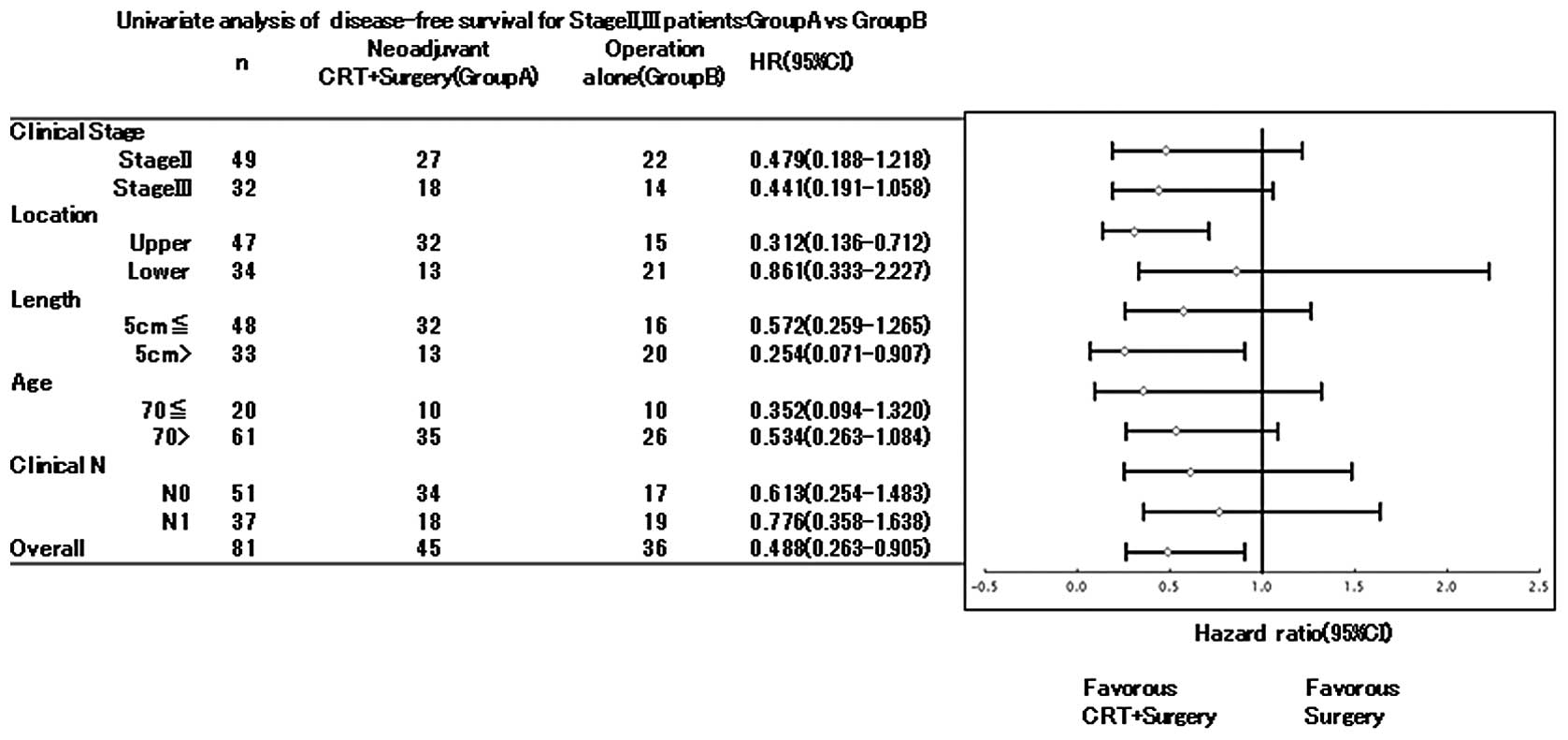
Subgroup analysis
The results of the subgroup analysis for OS and DFS
according to clinical stage, lymph node status, tumor depth of
invasion, tumor location and resectability of stage II/III patients
are shown in Table V. Patients
with N0 tumors that received neoadjuvant CRT (Group A) had a
significantly prolonged DFS compared to patients with N1 tumors and
Group B patients. As regards OS, Group A exhibited a significantly
prolonged survival rate and patients with N0 tumors showed a
tendency for improved survival (P= 0.057). Subgroup analysis
regarding DFS is shown in Fig. 3.
Patients with tumors located in the upper esophagus and tumors ≥5
cm in length exhibited a higher survival rate in Group A compared
to Group B [hazard ratio (HR), 0.312 and 0.136–0.712, respectively,
for tumor location, HR, 0.254 and 0.071–0.907, respectively, for
tumor length].
 | Table V.Univariate analysis of survival for
stage II/III esophageal cancer patients. |
Table V.
Univariate analysis of survival for
stage II/III esophageal cancer patients.
| Variables | No. | Disease-free
survival
| P-value | Overall survival
| P-value |
|---|
| Hazard ratio (95%
CI) | Hazard ratio (95%
CI) |
|---|
| CRT+surgery vs.
surgery alone | 45/36 | 0.488
(0.263–0.905) | 0.023a | 0.516
(0.269–0.989) | 0.046a |
| Men vs. women | 68/13 | 0.600
(0.235–1.530) | 0.285 | 0.723
(0.282–1.858) | 0.501 |
| Age (70> vs.
≥70) (years) | 20/61 | 0.792
(0.429–1.463) | 0.456 | 1.120
(0.580–2.163) | 0.735 |
| Clinical N0 vs.
N1 | 51/30 | 0.452
(0.245–0.836) | 0.011a | 0.532
(0.278–1.018) | 0.057 |
| bTumor location (upper vs.
lower) | 47/34 | 1.208
(0.652–2.239) | 0.548 | 1.316
(0.688–2.514) | 0.406 |
| Tumor length ≥5 vs.
<5 (cm) | 48/33 | 1.197
(0.638–2.262) | 0.579 | 1.546
(0.766–3.040) | 0.229 |
Discussion
In this retrospective study, we demonstrated that
neoadjuvant CRT with 5-FU and CDDP conferred an increased survival
benefit on patients with resectable stage II/III esophageal cancer,
compared to surgery alone. As regards preoperative CRT, we have
already shown that CRT in stage II/II (non-T4) patients contributed
to tumor shrinkage, leading to higher resectability and longer
survival (40). In this
retrospective study, we only evaluated patients with resectable
tumors, including T4 patients downstaged by neoadjuvant CRT. As
shown in Table II, the clinical
response rate for the primary tumor and the metastatic nodes
following CRT exceeded 80%. Therefore, neoadjuvant CRT proved to be
effective for stage II/III esophageal cancer patients. Over the
last few decades, surgical techniques and perioperative management,
as well as the overall prognosis of esophageal cancer patients
undergoing surgical resection, have significantly improved
(13,26,44).
In Japan, three-field lymph node dissection is currently considered
acceptable as a standard therapy for advanced esophageal SCC.
However, the survival benefit of three-field lymph node dissection
remains controversial (45–47).
There have been no randomized phase III trials on two- or
three-field lymph node dissection for ESCC in Japan. At present, we
omit the procedure of neck lymph node dissection when there is no
lymph node enlargement detected on preoperative CT scans. Our data
demonstrated that the 5-year OS rate for surgery alone in stage
II/III patients was 39.9%, whereas the current survival rates of
pathological stage IIA, IIB and III patients, classified according
to UICC criteria, are reported to be 51.5, 34.0 and 19.8%,
respectively, according to the comprehensive registry of esophageal
cancer in Japan (48). CRT for
esophageal cancer has been developed mainly in the United States,
since an RTOG study revealed that clinical benefits from CRT were
superior to those from radiotherapy alone in the patients with
localized carcinoma of the esophagus (49,50).
There have been two contradictory reports on stage II/III patients
regarding the comparison of trimodality therapy compared to surgery
alone (33,38). Tepper et al (38) evaluated patients in a neoadjuvant
CRT group and demonstrated a survival advantage compared to surgery
alone, supporting trimodality therapy as a standard of care for the
patients with stage IIa-III esophageal cancer; however, this trial
included a limited number of patients and the 5-year survival rate
was significantly lower (39% in the trimodality group vs. 10% in
the surgery only group) (38).
However, Apinop et al failed to demonstrate a clinical
advantage due to trimodality therapy compared to surgery alone in
69 patients with stage IIb-III ESCC. This study also included a
limited patient sample and the 5-year survival rate was low (24% in
the trimodality group vs. 10% in the surgery alone group) (33). Furthermore, neither study addressed
the details of operative procedures, which may be different in
Japan regarding lymph node dissection. Therefore, it is difficult
to assess the efficacy of trimodality therapy in the same
dimension. Burmeister et al (37) reported that trimodality therapy
improved DFS, unlike OS, in patients with stage I–III ESCC,
excluding adenocarcinoma (HR: 0.47 and 0.25–0.86, respectively)
(37). This subset analysis has
encouraged us to continue trimodality therapy for ESCC in Japan. We
also reported an improved prognosis, especially for patients with
tumors located higher and <5 cm in diameter. However, the
efficacy of CRT using our current regime may not suffice for tumors
>5 cm in diameter.
As regards the pattern of recurrence following
surgical resection, we demonstrated a more frequent simultaneous
locoregional and distant recurrence in patients after surgery
alone. Neoadjuvant CRT has been reported to control tumor
micrometastasis and to inhibit locoregional and distant metastasis
(51). Locoregional recurrence and
distant metastasis after radical esophagectomy with two- or
three-field lymph node dissection have been found to vary from 11.3
to 32.6% and the incidence of simultaneous locoregional recurrence
and distant metastasis has been reported to range from 1.1 to 13.9%
(19–25). The major complications following
esophagectomy with radical lymph node dissection were anastomotic
leakage, recurrent nerve palsy and respiratory complications.
Previous Japanese studies have indicated hospital mortality in
2.2–12.3% of the patients who underwent two- or three-field lymph
node dissection, anastomotic leakage in 11–39%, recurrent nerve
palsy in 9–76% and respiratory complications in 8–32% (26,27,52,53).
The incidence of postoperative complications after surgery alone in
our study were in accordance with these data. Even when CRT was
administered in combination with esophagectomy with extended lymph
node dissection, the incidence of postoperative complications did
not increase in our study. In Japan, neoadjuvant chemotherapy (CDDP
plus 5-FU) followed by esophagectomy is currently considered the
standard treatment for stage II/III esophageal cancer, although
this was considered disputable by previous randomized studies
(54–56). We strongly recommend that the the
efficacy of CRT is objectively evaluated in Japan by additional
JCOG studies.
In conclusion, treatment with neoadjuvant CRT
consisting of 5-FU and CDDP did not contribute to a better
prognosis in patients with resectable ESCC. However, it may be
beneficial for patients with stage II/III disease. Additional large
prospective randomized controlled trials involving preoperative CRT
are required to elucidate whether this treatment may improve the
prognosis of ESCC patients.
References
|
1.
|
GLOBOCAN 2008, All Cancers (excluding
non-melanoma skin cancer) Incidence and Mortality Worldwide.
Available: http://globocan.iarc.fr/factsheets/cancers/all.asp.
Accessed August 26, 2012.
|
|
2.
|
Cancer Statistics in Japan-2011.
Available: http://ganjoho.jp/public/statistics/backnumber/2011_jp.html.
Accessed August 26, 2012.
|
|
3.
|
Goseki N, Koike M and Yoshida M:
Histopathologic characteristics of early stage esophageal
carcinoma. A comparative study with gastric carcinoma. Cancer.
69:1088–1093. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Roth JA and Putnam JB Jr: Surgery for
cancer of the esophagus. Semin Oncol. 21:453–461. 1994.PubMed/NCBI
|
|
5.
|
Sugimachi K, Inokuchi K, Kuwano H, Kai H,
Okamura T and Okudaira Y: Patterns of recurrence after curative
resection for carcinoma of the thoracic part of the esophagus. Surg
Gynecol Obstet. 157:537–540. 1983.PubMed/NCBI
|
|
6.
|
Karl RC, Schreiber R, Boulware D, Baker S
and Coppola D: Factors affecting morbidity, mortality, and survival
in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg.
231:635–643. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Orringer MB, Marshall B and Iannettoni MD:
Transhiatal esophagectomy: clinical experience and refinements. Ann
Surg. 230:392–403. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chu KM, Law SY, Fok M and Wong J: A
prospective randomized comparison of transhiatal and transthoracic
resection for lower-third esophageal carcinoma. Am J Surg.
174:320–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lieberman MD, Shriver CD, Bleckner S and
Burt M: Carcinoma of the esophagus. Prognostic significance of
histologic type. J Thorac Cardiovasc Surg. 109:130–139. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ellis FH Jr, Heatley GJ, Krasna MJ,
Williamson WA and Balogh K: Esophagogastrectomy for carcinoma of
the esophagus and cardia: a comparison of findings and results
after standard resection in three consecutive eight-year intervals
with improved staging criteria. J Thorac Cardiovasc Surg.
113:836–848. 1997.
|
|
11.
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surgery. 232:225–232. 2000.PubMed/NCBI
|
|
13.
|
Altorki N and Skinner D: Should en bloc
esophagectomy be the standard of care for esophageal carcinoma? Ann
Surg. 234:581–587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lerut T, Nafteux P, Moons J, et al:
Three-field lymphadenectomy for carcinoma of the esophagus and
gastroesophageal junction in 174 R0 resections: impact on staging,
disease-free survival, and outcome: a plea for adaptation of TNM
classification in upper-half esophageal carcinoma. Ann Surg.
240:962–974. 2004. View Article : Google Scholar
|
|
15.
|
Lerut T, Coosemans W, Decker G, et al:
Extended surgery for cancer of the esophagus and gastroesophageal
junction. J Surg Res. 117:58–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Altorki NK, Girardi L and Skinner DB: En
bloc esophagectomy improves survival for stage III esophageal
cancer. J Thorac Cardiovasc Surg. 114:948–956. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Collard JM: Exclusive radical surgery for
esophageal adenocarcinoma. Cancer. 91:1098–1104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Clark GW, Peters JH, Ireland AP, et al:
Nodal metastasis and sites of recurrence after en bloc
esophagectomy for adenocarcinoma. Ann Thorac Surg. 58:646–654.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chen G, Wang Z, Liu XY and Liu FY:
Recurrence pattern of squamous cell carcinoma in the middle
thoracic esophagus after modified Ivor-Lewis esophagectomy. World J
Surg. 31:1107–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nakagawa S, Kanda T, Kosugi S, Ohashi M,
Suzuki T and Hatakeyama K: Recurrence pattern of squamous cell
carcinoma of the thoracic esophagus after extended radical
esophagectomy with three-field lymphadenectomy. J Am Coll Surg.
198:205–211. 2004. View Article : Google Scholar
|
|
21.
|
Matsubara T, Ueda M, Takahashi T, Nakajima
T and Nishi M: Localization of recurrent disease after extended
lymph node dissection for carcinoma of the thoracic esophagus. J Am
Coll Surg. 182:340–346. 1996.PubMed/NCBI
|
|
22.
|
Bhansali MS, Fujita H, Kakegawa T, et al:
Pattern of recurrence after extended radical esophagectomy with
three-field lymph node dissection for squamous cell carcinoma in
the thoracic esophagus. World J Surg. 21:275–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hulscher JB, van Sandick JW, Tijssen JG,
Obertop H and van Lanschot JJ: The recurrence pattern of esophageal
carcinoma after transhiatal resection. J Am Coll Surg. 191:143–148.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kyriazanos ID, Tachibana M, Shibakita M,
et al: Pattern of recurrence after extended esophagectomy for
squamous cell carcinoma of the esophagus. Hepatogastroenterology.
50:115–120. 2003.PubMed/NCBI
|
|
25.
|
Mariette C, Balon JM, Piessen G, Fabre S,
Van Seuningen I and Triboulet JP: Pattern of recurrence following
complete resection of esophageal carcinoma and factors predictive
of recurrent disease. Cancer. 97:1616–1623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–373. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kato H, Watanabe H, Tachimori Y and Iizuka
T: Evaluation of neck lymph node dissection for thoracic esophageal
carcinoma. Ann Thorac Surg. 51:931–935. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Altorki N, Kent M, Ferrara C and Port J:
Three-field lymph node dissection for squamous cell and
adenocarcinoma of the esophagus. Ann Surg. 236:177–183. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Urba SG, Orringer MB, Perez-Tamayo C,
Bromberg J and Forastiere A: Concurrent preoperative chemotherapy
and radiation therapy in localized esophageal adenocarcinoma.
Cancer. 69:285–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Walsh TN, Noonan N, Hollywood D, Kelly A,
Keeling N and Hennessy TP: A comparison of multimodal therapy and
surgery for esophageal adenocarcinoma. N Engl J Med. 335:462–467.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Nygaard K, Hagen S, Hansen HS, et al:
Pre-operative radio-therapy prolongs survival in operable
esophageal carcinoma: a randomized, multicenter study of
pre-operative radiotherapy and chemotherapy. The second
Scandinavian trial in esophageal cancer. World J Surg.
16:1104–1110. 1992. View Article : Google Scholar
|
|
32.
|
Le Prise E, Etienne PL, Meunier B, et al:
A randomized study of chemotherapy, radiation therapy, and surgery
versus surgery for localized squamous cell carcinoma of the
esophagus. Cancer. 73:1779–1784. 1994.PubMed/NCBI
|
|
33.
|
Apinop C, Puttisak P and Preecha N: A
prospective study of combined therapy in esophageal cancer.
Hepatogastroenterology. 41:391–393. 1994.PubMed/NCBI
|
|
34.
|
Bosset JF, Gignoux M, Triboulet JP, et al:
Chemoradiotherapy followed by surgery compared with surgery alone
in squamous-cell cancer of the esophagus. N Engl J Med.
337:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Urba SG, Orringer MB, Turrisi A,
Iannettoni M, Forastiere A and Strawderman M: Randomized trial of
preoperative chemo-radiation versus surgery alone in patients with
locoregional esophageal carcinoma. J Clin Oncol. 19:305–313.
2001.PubMed/NCBI
|
|
36.
|
Lee JL, Park SI, Kim SB, et al: A single
institutional phase III trial of preoperative chemotherapy with
hyperfractionation radiotherapy plus surgery versus surgery alone
for resectable esophageal squamous cell carcinoma. Ann Oncol.
15:947–954. 2004. View Article : Google Scholar
|
|
37.
|
Burmeister BH, Smithers BM, Gebski V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
resectable cancer of the oesophagus: a randomised controlled phase
III trial. Lancet Oncol. 6:659–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Fujiwara Y, Kamikonya N, Inoue T, et al:
Chemoradiotherapy for T3 and T4 squamous cell carcinoma of the
esophagus using low-dose FP and radiation: A preliminary report.
Oncol Rep. 14:1177–1182. 2005.PubMed/NCBI
|
|
40.
|
Fujiwara Y, Yoshikawa R, Kamikonya N, et
al: Trimodality therapy of esophagectomy plus neoadjuvant
chemoradiotherapy improves the survival of clinical stage II/III
esophageal squamous cell carcinoma patients. Oncol Rep. 28:446–452.
2012.
|
|
41.
|
Sobin LH and Wittekin CH: International
Union Against Cancer (UICC). TNM classification of malignant
tumors. 5th edition. John Wiley & Sons, Inc.; New York:
1997
|
|
42.
|
Japanese Society for Esophageal Diseases:
Guidelines for clinical and pathologic studies on carcinoma of the
esophagus. 9th edition. Kanehara & Co., Ltd.; Tokyo: 2001
|
|
43.
|
National Cancer Institute: Cancer Therapy
Evaluation Program, Common Terminology Criteria for Adverse Events.
Version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Accessed August 28, 2012.
|
|
44.
|
Muller JM, Erasmi H, Stelzner M, Zieren U
and Pichlmaier H: Surgical therapy of oesophageal carcinoma. Br J
Surg. 77:845–857. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Kakegawa T: Study on rational surgery for
thoracic esophageal cancer based on the esophageal lymphatic
drainage. Annual Report of the Cancer Research Ministry of Health
and Welfare 1990 Tokyo: National Cancer Center; pp. 285–289. 1991,
(In Japanese).
|
|
46.
|
Iizuka T: Report of 4th Meeting of ISDE
TNM. Research Committee; Kyoto. 1992
|
|
47.
|
Watanabe H, Kato H and Tachimori Y:
Significance of extended systemic lymph node dissection for
thoracic esophageal carcinoma in Japan. Recent Results Cancer Res.
155:123–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Ide H: Comprehensive Registry of
Esophageal Cancer in Japan (1998, 1999) and Long-term Results of
Esophagectomy in Japan (1988–1997). 3rd edition. The Japanese
Society for Esophageal Disease; Chiba: 2002
|
|
49.
|
Herskovic A, Martz K, al-Sarraf M, et al:
Combined chemotherapy and radiotherapy compared with radiotherapy
alone in patients with cancer of the esophagus. N Engl J Med.
326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Yoshikawa R, Nakano Y, Tao L, et al:
Hedgehog signal activation in oesophageal cancer patients
undergoing neoadjuvant chemoradiotherapy. Br J Cancer.
98:1670–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Igaki H, Tachimori Y and Kato H: Improved
survival for patients with upper and/or middle mediastinal lymph
node metastasis of squamous cell carcinoma of the lower thoracic
esophagus treated with 3-field dissection. Ann Surg. 239:483–490.
2004. View Article : Google Scholar
|
|
53.
|
Fujita H, Kakegawa T, Yamana H, et al:
Mortality and morbidity rates, postoperative course, quality of
life, and prognosis after extended radical lymphadenectomy for
esophageal cancer. Comparison of three-field lymphadenectomy with
two-field lymphadenectomy. Ann Surg. 222:654–662. 1995. View Article : Google Scholar
|
|
54.
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
55.
|
Kitagawa Y, Ando N, Nakamura K, Shibata T
and Fukuda H: The role of adjuvant chemotherapy for localized
squamous cell esophageal cancer: current Japanese standard and the
unending role of the drawing board. Ann Surg Oncol. 19:1425–1427.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Ajani JA and Swisher SG: Preoperative
chemotherapy for localized squamous cell carcinoma of the
esophagus? We should go back to the drawing board! Ann Surg Oncol.
19:3–4. 2012.PubMed/NCBI
|