Introduction
Brain metastases, including leptomeningeal
metastases (LMM), usually present late during the course of breast
cancer and are associated with an unfavorable prognosis. Several
previous studies demonstrated that the status of estrogen receptor
(ER), progesterone receptor (PR) and human epidermal growth factor
receptor type 2 (HER2) is altered at a certain point between the
emergence of the primary breast tumor and the development of
metastases (1–5). The mechanism of this discordance in
ER, PR and HER2 status between primary tumors and metastases has
not been fully elucidated. It was previously reported that the
majority of the tumors that do initially respond to targeted
therapies may eventually develop acquired resistance (6). Other possible mechanisms are a
genetic drift occurring during tumor progression (7) or intratumoral heterogeneity, wherein
the clone with the more aggressive phenotype initiates the
micrometastatic process (8,9). The
number of available pathoanatomical studies on the
brain-metastasizing type of breast cancer that evaluated the extent
to which the hormone and HER2 receptor discordance between paired
pathology samples of primary and metastatic breast cancer specimens
affect the prognosis is limited (1–5).
Furthermore, few studies reported the biological marker alterations
between primary tumors and brain metastases (5,10,11).
Post-operative adjuvant treatment decisions are commonly based on
the expression of ER, PR and HER2 of primary tumors. Moreover,
treatment decisions for recurrent brain tumor cases are generally
based on the ER and HER2 status of the primary tumors. Trastuzumab
is a humanized monoclonal antibody directed against the HER2/neu
oncoprotein and has the ability to inhibit tumor growth in breast
cancer patients overexpressing HER2 (12). However, the pharmacokinetics and
effect of trastuzumab on the brain after brain metastases have not
been determined. The aim of this study was to compare the
expression of ER, PR and HER2 in pathology samples from primary
tumors and brain metastases in order to evaluate whether the
previous therapy was able to modify this status and to determine
whether biomarker alterations affect prognosis after brain
metastases. We also investigated the effect of trastuzumab therapy
after brain metastases.
Materials and methods
Patients and tissue samples
Data were collected from 62 patients who were
initially diagnosed with breast cancer and underwent surgical
removal of brain metastases between 2000 and 2012 at the Osaka
Medical Center for Cancer and Cardiovascular Diseases and the
National Cancer Center Hospital, Japan. These patients had received
treatment for primary breast cancer between 1983 and 2011 and
undergone surgery for primary breast cancer and brain metastases.
Tumor samples were collected from the primary resected breast
cancer and metastatic brain lesions. However, not all the primary
tumor samples acquired during operation at other hospitals were
obtained. We only evaluated specimens considered sufficient for ER,
PR and HER2 status estimation.
The ER, PR and HER2 status was determined in the
samples from the primary and metastatic lesions. The first brain
metastatic-free survival time was defined as the time from the
first surgery for the primary tumor to the first detection of brain
metastasis on magnetic resonance imaging (MRI). The second brain
metastatic-free survival time was defined as the time from the
first surgery for brain lesions to the second occurrence of brain
metastases on MRI or patient death from any cause. LMM was
diagnosed by radiological findings or the cytological evaluation of
cerebrospinal fluid obtained by lumbar puncture. Detailed
information on the 62 patients is provided in Table I.
 | Table I.Characteristics of patients with
brain metastases from breast cancer. |
Table I.
Characteristics of patients with
brain metastases from breast cancer.
|
Characteristics | Patient no. | Years | % |
|---|
| Gender | | | |
| Female | 59 | | 95.2 |
| Male | 3 | | 4.8 |
| Age at onset | | | |
| Median | | 45.5 | |
| Range | | 31–76 | |
| Age at first | | | |
| brain
metastases | | | |
| Median | | 51 | |
| Range | | 35–79 | |
| RPA
classification | | | |
| Class 1 | 14 | | 22.6 |
| Class 2 | 40 | | 64.5 |
| Class 3 | 8 | | 12.9 |
| Radiotherapy | | | |
| WBRT | 34 | | 54.8 |
| WBRT + LBRT | 3 | | 4.8 |
| WBRT + SRS | 13 | | 21.0 |
| LBRT | 9 | | 14.5 |
| LBRT + SRS | 1 | | 1.6 |
| None | 2 | | 3.2 |
| Second BM | | | |
| Local and
distant | 26 | | 41.9 |
| LMM | 10 | | 16.1 |
| No second
recurrence | 22 | | 35.5 |
| Unknown | 4 | | 6.5 |
| Median overall
survival | | 6.5 | |
| Median survival
time after BM | | 1.1 | |
| Median first
BM-free survival | | 4.0 | |
| Median second brain
BM-free survival | | 0.6 | |
This study was approved by the Institutional Review
Board of each center.
Histopathological analysis
Surgical specimens were fixed in 10% formalin and
embedded in paraffin. Hematoxylin and eosin-stained specimens were
examined in order to determine the histological tumor type.
Multiple serial sections were subjected to immunohistochemical
analysis to assess local staining. Furthermore, tissue sections
were subjected to 15 min of microwave heating to activate antigens
in a retrieval solution consisting of 0.1 mol/l sodium citrate (pH
6.0), followed by immunostaining of the specimens with the
streptavidin-biotin-peroxidase complex method (Vectastain; Vector
Laboratories, Burlingame, CA, USA). Human monoclonal antibodies
were used against ER (clone 1D5; Dako, Carpinteria, CA, USA) or PR
(clone PgR636; Dako) with the streptavidin-biotin method and were
considered positive if ≥10% of the nuclei in the invasive component
of the tumor were stained (13,14).
The HER2/neu status, as assessed using the HercepTest assay (Dako),
was scored by the pathologists at each center on a scale of 0 to
3+, according to the Dako scoring system. HER2/neu positivity was
defined as HER2/neu 3+ or HER2/neu 2+ and fluorescence in
situ hybridization positivity.
Statistical analysis
Metastatic-free survival and overall survival (OS)
times were calculated with the Kaplan-Meier method and differences
between groups were compared using the log-rank test (JMP software
version 8; SAS Institute Inc., Cary, NC, USA).
Results
Metastatic-free survival and OS time
The 62 patients underwent resections of the first
brain metastases. The median age at the first brain metastasis was
51 years. Thirty-four patients received whole-brain radiotherapy
(WBRT). Three patients received WBRT and local brain radiotherapy
(LBRT) and 13 patients received WBRT and stereotactic radiosurgery
(SRS). Nine patients received LBRT and one received LBRT plus SRS.
Two patients were observed without radiotherapy following surgical
resection of the first brain metastases (Table I). Five of the nine patients who
only received LBRT developed a second brain recurrence negative for
LMM whereas the remaining four patients did not develop second
brain metastases.
The median first and second brain metastatic-free
survival times, the survival time after brain metastases and the OS
time from the initial diagnosis of breast cancer for the 62 breast
cancer patients were 4.0, 0.6, 1.1 and 6.5 years, respectively
(Table I). The 5-year OS rate from
surgical resection of brain metastases was 11.1%. Patients with
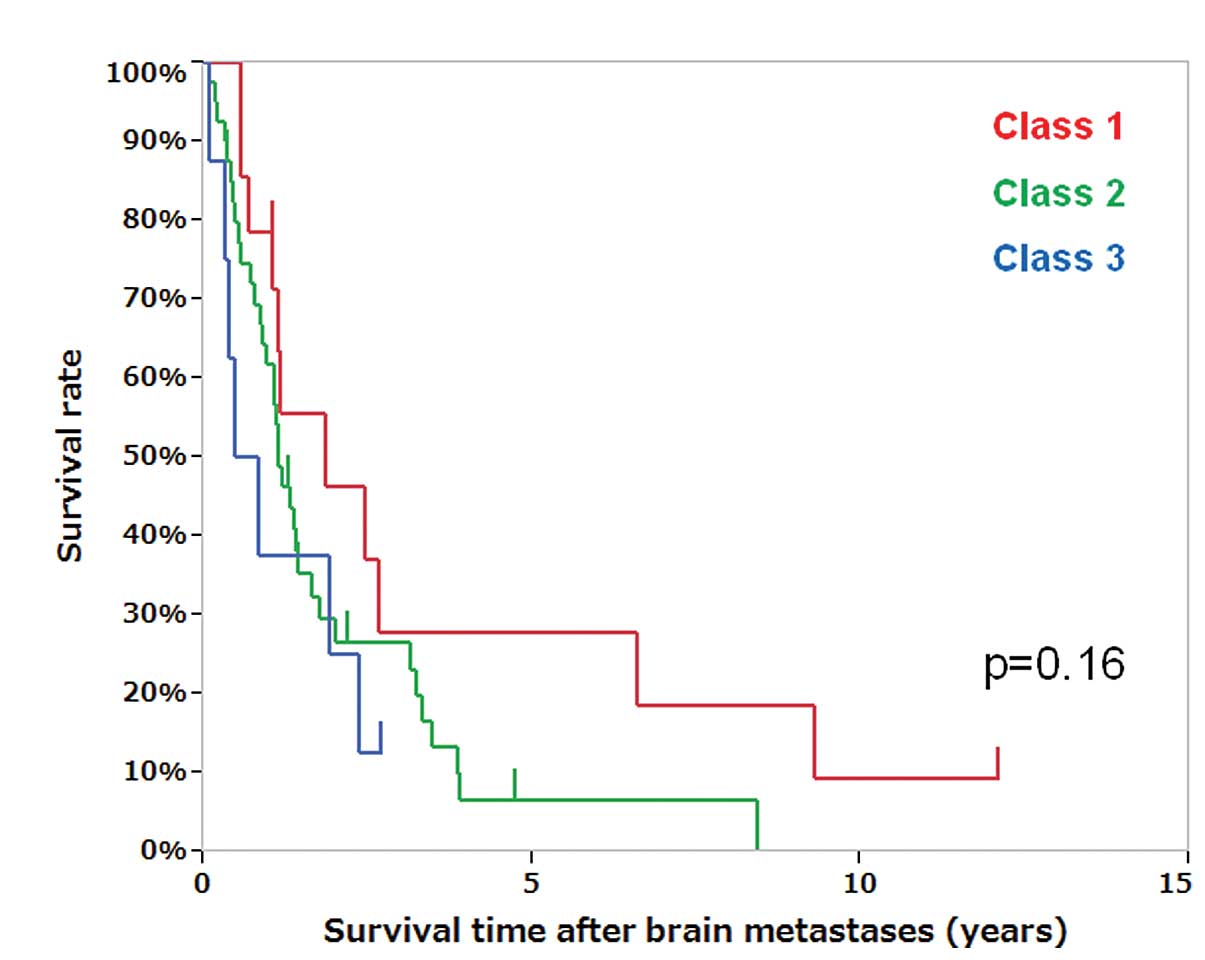
recursive partitioning analysis (RPA) (15) class I and II had a more favorable
OS compared to class III patients, although RPA classes did not
significantly differ in survival time after brain metastases
(Fig. 1, P=0.16).
Alteration of ER, PR and HER2 status in
primary tumor and brain metastases
The alterations in the ER and HER2 status in primary
tumors and brain metastases are presented in Table II. The positive rate of
immunohistochemical profiles of ER, PR and HER2 in the primary
tumors were 11.7% (7/60), 8.6% (5/58) and 41.4% (24/58),
respectively. The positive rates of immunohistochemical profiles of
ER, PR and HER2 in brain metastases were 16.1% (10/62), 11.3%
(7/62) and 43.5% (27/62), respectively. The rates of
immunohistochemical alteration of ER, PR and HER2 and
triple-negative status between primary and brain metastases were
21.7% (13/60), 10.3% (6/58), 12.1% (7/58) and 13.8% (8/58),
respectively. The immunohistochemical profiles for ER, PR and HER2
differed between the primary tumors and the brain metastases in 17
patients (29.3%; 17/58, Table
III). The discordance rates in the 17 patients were 76.5% for
ER, 35.3% for PR and 41.2% for HER2 (Table III). All the patients with ER or PR
alterations (positive-negative or negative-positive) had received
hormone therapy prior to the development of brain metastases. Two
of three patients with HER2 alteration (positive-negative) had
received trastuzumab therapy prior to the development of brain
metastases. A HER2-positive status was maintained in 88.9% (16/18)
of the patients who had received trastuzumab prior to the
development of brain metastases. All the patients with
negative-positive alteration in HER2 had received hormone therapy
but not trastuzumab prior to the development of brain metastases
(Table III). One of five patients
(20.0%) with triple-negative breast cancer (TNBC) did not exhibit
any status alterations in the brain metastases and had received
chemotherapy prior to the development of brain metastases.
 | Table II.Alterations in ER and HER2 status in
primary tumor and brain metastases. |
Table II.
Alterations in ER and HER2 status in
primary tumor and brain metastases.
| Primary breast
cancer ER/HER2 status | Brain metastases
ER/HER2 status
|
|---|
| (+/+) | (+/−) | (−/+) | (−/−) |
|---|
| (+/+) | 0 (0%) | 0 (0%) | 2 (3.4%) | 0 (0%) |
| (+/−) | 0 (0%) | 2 (3.4%) | 0 (0%) | 3 (5.2%) |
| (−/+) | 1 (1.7%) | 1 (1.7%) | 18 (31.0%) | 2 (3.4%) |
| (−/−) | 3 (5.2%) | 3 (5.2%) | 1 (1.7%) | 22 (37.9%) |
 | Table III.Discordance cases of
immunohistochemical profiles between primary tumors and brain
metastases. |
Table III.
Discordance cases of
immunohistochemical profiles between primary tumors and brain
metastases.
| Case no. | HER2
| ER
| PR
| Chemotherapy prior
to BM | Hormone therapy
prior to BM | Trastuzumab therapy
prior to BM |
|---|
| Primary | BM | Primary | BM | Primary | BM |
|---|
| 1 | − | − | − | + | − | − | − | + | − |
| 2 | − | − | − | + | − | − | − | + | − |
| 3 | − | − | − | + | − | + | − | + | − |
| 4 | − | + | − | − | − | − | + | + | − |
| 5 | − | + | − | + | − | − | − | + | − |
| 6 | − | + | − | + | − | + | − | + | − |
| 7 | − | + | − | + | − | + | − | + | − |
| 8 | − | − | + | + | − | + | + | + | − |
| 9 | − | − | + | − | + | − | − | + | − |
| 10 | − | − | + | − | + | + | − | + | − |
| 11 | − | − | + | − | + | + | − | + | − |
| 12 | + | − | − | − | − | − | + | − | − |
| 13 | + | − | − | − | − | − | + | − | + |
| 14 | + | − | − | + | − | − | − | + | + |
| 15 | + | + | − | + | − | − | − | + | − |
| 16 | + | + | + | − | − | − | + | + | + |
| 17 | + | + | + | − | + | − | + | + | − |
Clinical outcome with trastuzumab therapy
after brain metastases
Trastuzumab was administered to 18 of 24 patients
who had HER2-positive primary tumors prior to brain metastases and
to 10 of these 24 patients after brain metastases. Two patients
were started on trastuzumab after brain metastases.
The median first brain metastatic-free survival time
of patients with a positive HER2 status in the primary tumors with
(n=18) and without trastuzumab (n=6) was 4.2 and 5.3 years,
respectively, whereas that of patients with negative HER2 status
(n=34) was 4.0 years. The HER2 status in primary tumors with
trastuzumab therapy prior to brain metastases did not correlate
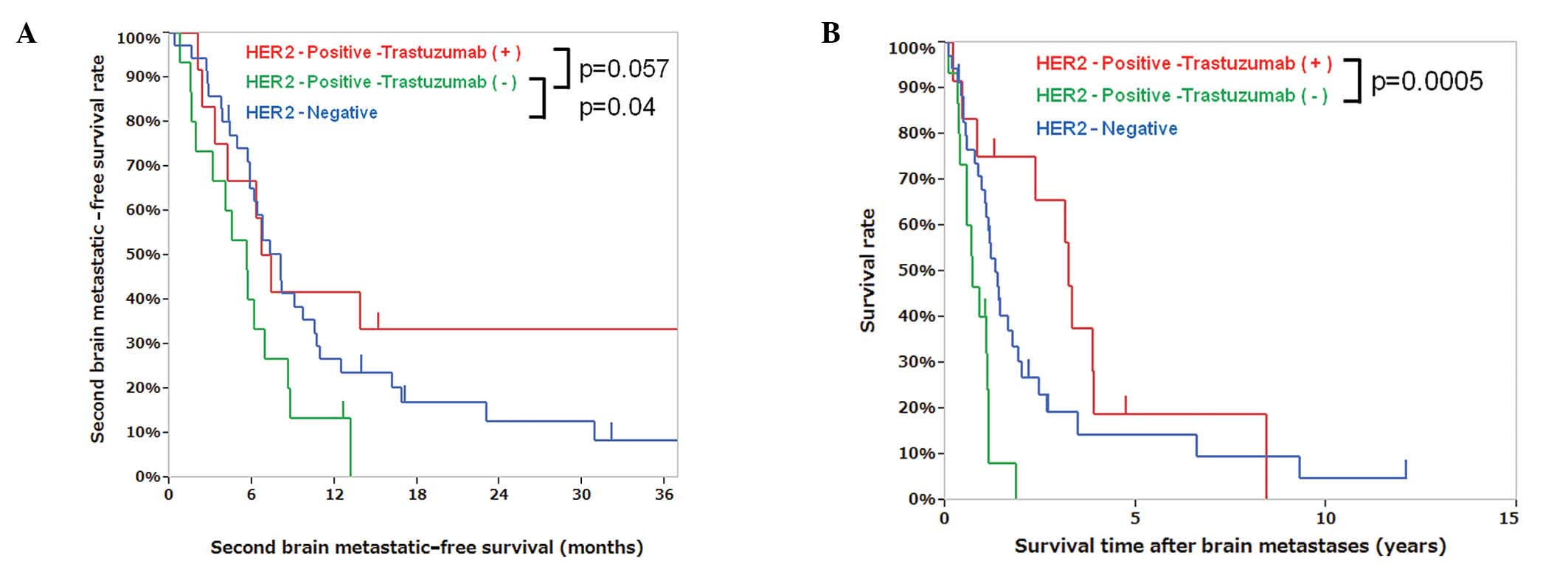
with the first brain metastatic-free survival time. The second
brain metastatic-free survival time of patients with positive HER2
status in brain lesions with (n=12) and without trastuzumab (n=15)
was 7.0 and 5.6 months, respectively (P=0.057), whereas that of
patients with negative HER2 status (n=35) was 8.0 months (Fig. 2A). The median OS from the first
brain metastasis in patients with positive HER2 status with and
without trastuzumab was 3.2 and 0.7 years, respectively (P=0.0005,
Fig. 2B, Table IV). The HER2 status in brain
metastases with trastuzumab therapy after brain metastases did not
correlate with the second brain metastatic-free survival time.
Compared to HER2-positive patients who did not receive trastuzumab
and those with HER2-negative brain metastases, the patients who had
HER2-positive brain metastases and received trastuzumab tended to
have longer second brain metastatic-free survival times (P=0.057)
and exhibited significantly longer survival times after brain
metastases (P=0.0005) (Fig. 2A and
B, Table IV). Patient
characteristics, including age at brain metastasis and proportion
of RPA classes, were not different in the HER2-positive group with
or without trastuzumab (Table IV).
The ER, PR and HER2 status of brain metastatic lesions exhibited no
correlation with survival time after brain metastases. There was no
difference in survival time after brain metastases between TNBC and
HER2-positive patients.
 | Table IV.Characteristics and survival time
according to HER2 status and trastuzumab therapy after brain
metastases. |
Table IV.
Characteristics and survival time
according to HER2 status and trastuzumab therapy after brain
metastases.
|
Characteristics | Positive HER2 with
trastuzumab | Positive HER2
without trastuzumab | Negative HER2 |
|---|
| Patient no. | 12 | 15 | 35 |
| Median age at first
brain metastasis, years (range) | 47 (35–67) | 54 (39–64) | 53 (35–79) |
| RPA class
1/2/3 | 0/10/2 | 4/9/2 | 10/21/4 |
| ER-brain metastases
+/− | 0/12 | 4/11 | 6/29 |
| PR-brain metastases
+/− | 1/11 | 2/13 | 4/31 |
| Any 2nd brain
metastases including LMM | 8 (67%) | 7 (47%) | 21 (60%) |
| (within 6 months
after surgery) | 3 (25%) | 5 (33%) | 8 (23%) |
| (within 12
months after surgery) | 6 (50%) | 7 (47%) | 17 (49%) |
| LMM | 0 (0%) | 4 (27%) | 6 (17%) |
| (within 6 months
after surgery) | 0 (0%) | 4 (27%) | 4 (11%) |
| 2nd brain
metastatic-free survival time, years (95% CI) | 0.6 (0.2–3.3) | 0.5 (0.1–0.6) | 0.7 (0.5–0.9) |
| Survival time after
brain metastases, years (95% CI) | 3.2 (0.4–3.9) | 0.7 (0.3–1.1) | 1.3 (0.9–1.8) |
Trastuzumab therapy and postoperative
LMM
The incidence of LMM after surgery for brain
metastases was 27% (4/15) in patients with HER2-positive central
nervous system (CNS) samples who did not receive trastuzumab and
17% (6/35) in patients with HER2-negative CNS samples. All the
patients with LMM exhibited neuronal death. Of five patients with
recurrent brain metastases who did not receive trastuzumab, four
patients (80%) developed LMM within 6 months after surgery for
brain metastases. However, none of the 12 patients who were
administered trastuzumab after surgery presented with LMM. Thus,
trastuzumab therapy after brain metastases decreased the incidence
of postoperative LMM (Chi-square test, P=0.053).
Discussion
Breast cancer is the second most common cause of
brain metastases. Brain metastases occur in 14–20% of breast cancer
patients and usually occur late in the progression of metastatic
disease (16). The results of the
present study have demonstrated that, among patients with
HER2-positive brain metastases, those who received trastuzumab had
longer survival times following surgery for brain metastases,
compared to those without trastuzumab treatment.
The prolongation of the OS time in HER2-positive
breast cancer patients with trastuzumab may be attributed to the
decrease in LMM and neuronal death and the effects of trastuzumab
on the control of systemic metastases (17–21).
A positive HER2 status in primary breast cancer was considered a
risk factor for the development of brain metastases (22). Although trastuzumab does not cross
the blood-brain barrier (BBB) and has no direct activity on brain
metastases, previous studies reported a survival benefit with
trastuzumab in HER2-positive patients with brain metastases, who
had a significantly longer survival compared to that of
HER2-negative patients (17–21),
which was consistent with our findings. Trastuzumab therapy prior
to brain metastases did not correlate with the first brain
metastatic-free survival time in our study, since trastuzumab
therapy was not associated with an increased incidence of brain
metastases (23,24). The patients with HER2-positive
brain metastases who received trastuzumab exhibited longer OS after
brain metastases compared to the patients with HER2-positive brain
metastases without trastuzumab treatment in our study. The
development of LMM was a rare manifestation encountered in 5–8.1%
of patients with HER2-positive primary tumors (17,20).
None of the patients who received trastuzumab therapy after surgery
for brain metastases presented with LMM in our study. Of note,
radiotherapy with doses of 20–30 Gy with a fraction size of 2 Gy
was reported to increase the permeability of the BBB and may
enhance the effect of chemotherapy (25). Thus, surgery followed by WBRT may
disrupt the BBB and facilitate the delivery of trastuzumab to the
brain in HER2-positive patients.
Hormone receptor and HER2 status are important
predictive markers in breast cancer. ER negativity was associated
with an increased risk of brain metastases (26–28)
and HER2 amplification/overexpression was shown to be a prognostic
and predictive factor for the development of brain metastases
(29). Approximately two-thirds of
early breast cancer patients were found to be ER-positive (30) and the HER2-positivity rate in early
breast cancer was reported to be ∼15% by a previous study (31). In our study, the ER and
HER2-positivity rate in primary tumors was lower (11.7%) and higher
(41.4%), respectively, compared to those reported by previous
studies on primary breast tumors.
Several previous studies demonstrated that the
discordance in biomarker expression between primary tumors and
metastases and the alteration of hormone receptor and HER2 status
is affected by adjuvant chemotherapy associated with hormone
therapy and may affect the prognosis (1–5,32,33).
The alterations were possibly attributed to a genetic drift or
clonal selection during tumor progression (7) or to the presence of intratumoral
heterogeneity in ER, PR and HER2 status (8,9). In
previous studies that investigated primary breast cancer and
distant metastases, the locoregional recurrence or lymph node
metastasis, including brain metastasis, exhibited discordance rates
of 0–37.5% (34–40). In our study, the
immunohistochemical profiles for ER, PR and HER2 differed between
the primary tumors and the brain metastases in 29.3% of the
patients. Prior hormone therapy or chemotherapy exerted an effect
on this discordance phenomenon (1–5,32,33).
All the patients with ER or PR alterations (positive-negative and
negative-positive) had received hormone therapy prior to the
development of brain metastases. Negative-positive alterations were
observed in 15.1% of ER-negative, 7.5% of PR-negative and 11.8% of
HER2-negative primary tumors in our study. The HER2 status was
highly maintained and the concordance rate between primary tumors
and systemic metastases was shown to be 97% by a previous study
(41). We demonstrated that 89% of
HER2-positive patients treated with trastuzumab prior to the
development of brain metastases maintained a positive status in
brain metastases. The possibility of the discordance rate in HER2
expression between primary and metastatic tumors was less frequent
compared to ER or PR (10,11,34–40,42);
however, the possibility of intratumoral heterogeneity must be
considered, with re-evaluation of the HER2 status in brain
metastases for further systemic treatment after brain metastases.
New HER2-targeted drugs, such as lapatinib, are able to cross the
BBB and thereby control brain metastases and other systemic breast
cancer metastases more effectively (43).
In conclusion, 11.8% of HER2-negative patients with
primary breast cancers had positive HER2 status alterations in the
brain metastases. Patients treated with trastuzumab after surgery
for brain metastases and radiotherapy exhibited a better prognosis.
Thus, the HER2 status in brain metastases requires re-evaluation
and the administration of trastuzumab or lapatinib in HER2-positive
patients should be considered even after brain metastases.
Acknowledgements
This study was supported by
Grant-in-Aid for Scientific Research from the Ministry of
Education, Science and Culture of Japan (no. 24791520 to Y.O., no.
24592180 to Y.N.).
References
|
1.
|
Bogina G, Bortesi L, Marconi M, Venturini
M, Lunardi G, Coati F, Massocco A, Manfrin E, Pegoraro C and
Zamboni G: Comparison of hormonal receptor and HER-2 status between
breast primary tumours and relapsing tumours: clinical implications
of progesterone receptor loss. Virchows Arch. 459:1–10. 2011.
View Article : Google Scholar
|
|
2.
|
Broom RJ, Tang PA, Simmons C, Bordeleau L,
Mulligan AM, O’Malley FP, Miller N, Andrulis IL, Brenner DM and
Clemons MJ: Changes in estrogen receptor, progesterone receptor and
Her-2/neu status with time: discordance rates between primary and
metastatic breast cancer. Anticancer Res. 29:1557–1562. 2009.
|
|
3.
|
Guarneri V, Giovannelli S, Ficarra G,
Bettelli S, Maiorana A, Piacentini F, Barbieri E, Dieci MV, D’Amico
R, Jovic G and Conte P: Comparison of HER-2 and hormone receptor
expression in primary breast cancers and asynchronous paired
metastases: impact on patient management. Oncologist. 13:838–844.
2008. View Article : Google Scholar
|
|
4.
|
Nishimura R, Osako T, Okumura Y, Tashima
R, Toyozumi Y and Arima N: Changes in the ER, PgR, HER2, p53 and
Ki-67 biological markers between primary and recurrent breast
cancer: discordance rates and prognosis. World J Surg Oncol.
9:1312011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yonemori K, Tsuta K, Shimizu C, Hatanaka
Y, Hashizume K, Ono M, Nakanishi Y, Hasegawa T, Miyakita Y, Narita
Y, Shibui S and Fujiwara Y: Immunohistochemical profiles of brain
metastases from breast cancer. J Neurooncol. 90:223–228. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Johnston SR, Saccani-Jotti G, Smith IE,
Salter J, Newby J, Coppen M, Ebbs SR and Dowsett M: Changes in
estrogen receptor, progesterone receptor, and pS2 expression in
tamoxifen-resistant human breast cancer. Cancer Res. 55:3331–3338.
1995.PubMed/NCBI
|
|
7.
|
Edgerton SM, Moore D II, Merkel D and Thor
AD: erbB-2 (HER-2) and breast cancer progression. Appl
Immunohistochem Mol Morphol. 11:214–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kerbel RS: Growth dominance of the
metastatic cancer cell: cellular and molecular aspects. Adv Cancer
Res. 55:87–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pertschuk LP, Axiotis CA, Feldman JG, Kim
YD, Karavattayhayyil SJ and Braithwaite L: Marked intratumoral
heterogeneity of the proto-oncogene Her-2/neu determined by three
different detection systems. Breast J. 5:369–374. 1999. View Article : Google Scholar
|
|
10.
|
Gancberg D, Di Leo A, Cardoso F, Rouas G,
Pedrocchi M, Paesmans M, Verhest A, Bernard-Marty C, Piccart MJ and
Larsimont D: Comparison of HER-2 status between primary breast
cancer and corresponding distant metastatic sites. Ann Oncol.
13:1036–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sari E, Guler G, Hayran M, Gullu I,
Altundag K and Ozisik Y: Comparative study of the
immunohistochemical detection of hormone receptor status and HER-2
expression in primary and paired recurrent/metastatic lesions of
patients with breast cancer. Med Oncol. 28:57–63. 2011. View Article : Google Scholar
|
|
12.
|
Baselga J, Tripathy D, Mendelsohn J, et
al: Phase II study of weekly intravenous recombinant humanized
anti-p185HER2 monoclonal antibody in patients with
HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol.
14:737–744. 1996.
|
|
13.
|
Harvey JM, Clark GM, Osborne CK and Allred
DC: Estrogen receptor status by immunohistochemistry is superior to
the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481.
1999.
|
|
14.
|
Perren A, Weng LP, Boag AH, Ziebold U,
Thakore K, Dahia PL, Komminoth P, Lees JA, Mulligan LM, Mutter GL
and Eng C: Immunohistochemical evidence of loss of PTEN expression
in primary ductal adenocarcinomas of the breast. Am J Pathol.
155:1253–1260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
Radiation Therapy Oncology Group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar
|
|
16.
|
Flowers A and Levin VA: Management of
brain metastases from breast carcinoma. Oncology (Williston Park).
7:21–26; discussion 31–34, 1993.
|
|
17.
|
Bendell JC, Domchek SM, Burstein HJ,
Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R and Winer
E: Central nervous system metastases in women who receive
trastuzumab-based therapy for metastatic breast carcinoma. Cancer.
97:2972–2977. 2003. View Article : Google Scholar
|
|
18.
|
Kirsch DG, Ledezma CJ, Mathews CS, Bhan
AK, Ancukiewicz M, Hochberg FH and Loeffler JS: Survival after
brain metastases from breast cancer in the trastuzumab era. J Clin
Oncol. 23:2114–2117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Le Scodan R, Jouanneau L, Massard C,
Gutierrez M, Kirova Y, Cherel P, Gachet J, Labib A and
Mouret-Fourme E: Brain metastases from breast cancer: prognostic
significance of HER-2 overexpression, effect of trastuzumab and
cause of death. BMC Cancer. 11:3952011.PubMed/NCBI
|
|
20.
|
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS,
Kim TH and Ro J: Breast cancer subtypes and survival in patients
with brain metastases. Breast Cancer Res. 10:R202008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Park IH, Ro J, Lee KS, Nam BH, Kwon Y and
Shin KH: Trastuzumab treatment beyond brain progression in
HER2-positive metastatic breast cancer. Ann Oncol. 20:56–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Duchnowska R, Dziadziuszko R,
Czartoryska-Arlukowicz B, Radecka B, Szostakiewicz B,
Sosinska-Mielcarek K, Karpinska A, Staroslawska E, Kubiatowski T
and Szczylik C: Risk factors for brain relapse in HER2-positive
metastatic breast cancer patients. Breast Cancer Res Treat.
117:297–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Smith I, Procter M, Gelber RD, et al: HERA
study team: 2-year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: a randomised
controlled trial. Lancet. 369:29–36. 2007.
|
|
25.
|
van Vulpen M, Kal HB, Taphoorn MJ and
El-Sharouni SY: Changes in blood-brain barrier permeability induced
by radiotherapy: Implications for timing of chemotherapy? (Review).
Oncol Rep. 9:683–688. 2002.PubMed/NCBI
|
|
26.
|
Maki DD and Grossman RI: Patterns of
disease spread in meta-static breast carcinoma: influence of
estrogen and progesterone receptor status. AJNR Am J Neuroradiol.
21:1064–1066. 2000.PubMed/NCBI
|
|
27.
|
Samaan NA, Buzdar AU, Aldinger KA, Schultz
PN, Yang KP, Romsdahl MM and Martin R: Estrogen receptor: a
prognostic factor in breast cancer. Cancer. 47:554–560. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tham YL, Sexton K, Kramer R, Hilsenbeck S
and Elledge R: Primary breast cancer phenotypes associated with
propensity for central nervous system metastases. Cancer.
107:696–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Leyland-Jones B: Human epidermal growth
factor receptor 2-positive breast cancer and central nervous system
metastases. J Clin Oncol. 27:5278–5286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
No authors listed. Tamoxifen for early
breast cancer: an overview of the randomised trials. Early Breast
Cancer Trialists’ Collaborative Group. Lancet. 351:1451–1467.
1998.
|
|
31.
|
Schneeweiss A, Marme F, Ruiz A, Manikhas
AG, Bottini A, Wolf M, Sinn HP, Mansouri K, Kennedy L and Bauknecht
T: A randomized phase II trial of doxorubicin plus pemetrexed
followed by docetaxel versus doxorubicin plus cyclophosphamide
followed by docetaxel as neoadjuvant treatment of early breast
cancer. Ann Oncol. 22:609–617. 2011. View Article : Google Scholar
|
|
32.
|
Yonemori K, Tsuta K, Ono M, Shimizu C,
Hirakawa A, Hasegawa T, Hatanaka Y, Narita Y, Shibui S and Fujiwara
Y: Disruption of the blood brain barrier by brain metastases of
triple-negative and basal-type breast cancer but not
HER2/neu-positive breast cancer. Cancer. 116:302–308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Konecny G, Pauletti G, Pegram M, et al:
Quantitative association between HER-2/neu and steroid hormone
receptors in hormone receptor-positive primary breast cancer. J
Natl Cancer Inst. 95:142–153. 2003. View Article : Google Scholar
|
|
34.
|
Gong Y, Booser DJ and Sneige N: Comparison
of HER-2 status determined by fluorescence in situ hybridization in
primary and metastatic breast carcinoma. Cancer. 103:1763–1769.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lower EE, Glass E, Blau R and Harman S:
HER-2/neu expression in primary and metastatic breast cancer.
Breast Cancer Res Treat. 113:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Niehans GA, Singleton TP, Dykoski D and
Kiang DT: Stability of HER-2/neu expression over time and at
multiple metastatic sites. J Natl Cancer Inst. 85:1230–1235. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Pectasides D, Gaglia A, Arapantoni-Dadioti
P, Bobota A, Valavanis C, Kostopoulou V, Mylonakis N, Karabelis A,
Pectasides M and Economopoulos T: HER-2/neu status of primary
breast cancer and corresponding metastatic sites in patients with
advanced breast cancer treated with trastuzumab-based therapy.
Anticancer Res. 26:647–653. 2006.PubMed/NCBI
|
|
38.
|
Regitnig P, Schippinger W, Lindbauer M,
Samonigg H and Lax SF: Change of HER-2/neu status in a subset of
distant metastases from breast carcinomas. J Pathol. 203:918–926.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Shimizu C, Fukutomi T, Tsuda H,
Akashi-Tanaka S, Watanabe T, Nanasawa T and Sugihara K: c-erbB-2
protein overexpression and p53 immunoreaction in primary and
recurrent breast cancer tissues. J Surg Oncol. 73:17–20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Zidan J, Dashkovsky I, Stayerman C, Basher
W, Cozacov C and Hadary A: Comparison of HER-2 overexpression in
primary breast cancer and metastatic sites and its effect on
biological targeting therapy of metastatic disease. Br J Cancer.
93:552–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Fuchs IB, Loebbecke M, Buhler H,
Stoltenburg-Didinger G, Heine B, Lichtenegger W and Schaller G:
HER2 in brain metastases: issues of concordance, survival, and
treatment. J Clin Oncol. 20:4130–4133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Liedtke C, Broglio K, Moulder S, et al:
Prognostic impact of discordance between triple-receptor
measurements in primary and recurrent breast cancer. Ann Oncol.
20:1953–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Geyer CE, Forster J, Lindquist D, et al:
Lapatinib plus capecitabine for HER2-positive advanced breast
cancer. N Engl J Med. 355:2733–2743. 2006. View Article : Google Scholar : PubMed/NCBI
|