Introduction
Malignant mesothelioma (MM) is a tumor that develops
from the serous membranes that line the body cavities and it may
arise in the pleura, peritoneum and pericardium; in addition,
although extremely rare, it may also develop in the tunica
vaginalis testis. The most common form of this disease is the
malignant pleural mesothelioma (MPM). MM was previously considered
as being extremely rare; however, its incidence and associated
mortality rate exhibited a sharp increase worldwide over the last
50 years, due to the close association of MM with asbestos
exposure. The prognosis of MPM is poor, with a median survival of
∼9–17 months (1). However, in
selected patients with epithelioid tumor histology, early-stage
disease, who undergo trimodality treatment (combination of
chemotherapy, postoperative radiotherapy and extrapleural
pneumonectomy), median overall survival of 51 months and 5-year
survival rates of 46% have been reported (2). Recent phase II trials reported a
median survival of ∼30 months for the patients who completed the
trimodality treatment (3,4). Therefore, early diagnosis may play a
vital role in the improvement of therapeutic outcomes. Together
with the advances in imaging studies and endoscopic examinations,
the development of biomarkers useful for serum or effusion
diagnosis is crucial for the early diagnosis of MM. Currently known
biomarkers for diagnosing MM include cytokeratin 19 fragment
(CYFRA) (5–7), tissue polypeptide antigen (TPA)
(5,6,8),
hyaluronic acid (8), carbohydrate
antigen (CA125) (8,9) and osteopontin (10–15).
However, these markers have low specificity for MM.
Mesothelin is a 40-kDa cell surface glycoprotein
that is overexpressed in cells of pancreatic and ovarian cancer,
mesothelioma and other malignancies. The mesothelin gene encodes a
69-kDa glycoprotein, the mesothelin precursor protein, which is
cleaved by a furin-like protease and its N-terminal region is
released in the blood as a 31-kDa protein, the megakaryocyte
potentiating factor (MPF). The 40-kDa C-terminal region of this
glycoprotein binds to the cell membrane as mesothelin. Three
distinct variants of mesothelin have been identified, one of which
has a modified C-terminus and becomes detached from the cell
membrane since it lacks a glycosylphosphatidylinositol (GPI)
anchor. This soluble isoform corresponds to the soluble
mesothelin-related peptide (SMRP) (16). SMRP and MPF may be highly specific
biomarkers for MM and have an equivalent diagnostic performance
(17–19). SMRP is currently the most
extensively investigated and is considered to be the best available
blood protein biomarker of MM (20).
However, the diagnostic performance of SMRP alone is
not considered to be sufficiently high, as it appears to exhibit
insufficient sensitivity for MM (20,21).
In diagnosing malignant tumors, such as ovarian or prostate cancer,
the diagnostic performance of individual serum biomarkers was
improved by combining data obtained using multiple biomarkers
(22,23).
In the present study, we evaluated the performance
of serum SMRP levels in the diagnosis of MM and investigated
whether its diagnostic value could be improved through its
combination with other biomarkers.
Materials and methods
Study design
The subjects of this study were patients who
satisfied the following inclusion criteria: i) age ≥20 years; ii)
pathologically proven MM or lung cancer; and iii) except for ii),
individuals with asbestos exposure proven on the basis of their
history or from the medical viewpoint. Only patients who personally
provided written informed consent for the measurement of their
serum biomarkers were enrolled in this study. Subjects who
satisfied the above inclusion criteria during the study period were
retrospectively enrolled. The pathological diagnosis was based on
standard histological and immunohistochemical criteria (24,25).
The subjects were classified into three groups: individuals with a
history of asbestos exposure, patients with lung cancer and
patients with MM. This study was approved by the Institutional
Review Board of the Hyogo College of Medicine.
Measurement of serum biomarker
levels
At the time of confirmation of the diagnosis, blood
samples were collected from the subjects and, following prompt
separation of the serum, the samples were stored at −80°C. The
serum SMRP levels were measured using an ELISA kit (Mesomark™;
Fujirebio Diagnostics Inc., Malvern, PA, USA) according to the
manufacturer’s instructions. The serum levels of CYFRA and
carcinoembryonic antigen (CEA) were measured using commercially
available immunoassay systems according to the manufacturer’s
instructions: the serum CEA levels were determined using a
chemiluminescent immunoassay (Abbott Japan Co., Ltd., Tokyo, Japan)
and the serum levels of CYFRA were determined using a solid-phase
sandwich immunoradiometric assay (CIS Bio International,
Gif-sur-Yvette, France). The manufacturer suggests 3.5 ng/ml for
CYFRA and 5.0 ng/ml for CEA as the cut-off values to differentiate
between non-malignant disease and malignant tumors.
Statistical analysis
Summary statistics were used (median and 25th and
75th percentiles) to evaluate the distribution of serum SMRP
levels. The Steel’s test, a non-parametric form of the Dunnett’s
test, was used for comparing MM to the other groups. The
sensitivity and specificity of SMRP for diagnosing MM were
calculated, along with the corresponding 95% exact confidence
intervals (CIs). The above analyses were also performed for CYFRA
and its performance was compared to that of SMRP by using the
McNemar’s test. To compare the serum SMRP levels between each
histological subtype of MM, the Steel-Dwass test, a non-parametric
form of the Tukey’s test, was performed. Subsequently, a stepwise
logistic regression analysis was used to select marker combinations
(MCs) that were more effective for diagnosing MM. The criterion for
assessing whether a difference was significant in the variable
selection was 5%. The diagnostic performance of SMRP and the MC was
assessed by constructing a receiver operating characteristic (ROC)
curve and calculating the area under the curve (AUC). The AUC for
SMRP and that for the MC were compared using the theory on
generalized U-statistics to generate an estimated covariance matrix
and the χ2 test (26).
For each test, two-sided P<0.05 was considered to indicate a
statistically significant difference. Data were analyzed using the
statistical software SAS, version 9.1.3 (SAS Institute Inc., Cary,
NC, USA) and Stata, version 11.0 (StataCorp College Station, TX,
USA). The GraphPad Prism software, version 4.00 for Windows
(GraphPad Software, San Diego, CA, USA) was used to prepare the
figures.
Results
Patient characteristics
A total of 190 subjects were enrolled in this study.
A summary of the clinical characteristics of these subjects,
together with a breakdown of each group by age, gender, history of
asbestos exposure and presence of effusion (pleural or peritoneal)
is presented in Table I. Among the
39 individuals with asbestos exposure, pleural plaque was present
in 16, benign asbestos pleurisy in 7, asbestosis in 3 patients,
asbestosis plus benign asbestos pleurisy in 5, round atelectasis in
2 and no imaging abnormalities in 6 patients. The histological
subtype in the 55 patients with lung cancer was adenocarcinoma in
24, squamous cell carcinoma in 14 and small-cell carcinoma in 17
patients. Among the 96 patients with MM, the primary tumor site was
the pleura in 91 and the peritoneum in 5 patients (Table II). The histological subtype was
epithelioid in 57 patients, sarcomatoid in 12, biphasic in 6,
desmoplastic in 4 and unspecified in the remaining 7 patients
(Table II). Of the 91 patients
with MPM, 74 were diagnosed with clinical stage IV disease
according to the staging classification proposed by the
International Mesothelioma Interest Group (IMIG). Only 5 patients
had either stage I or II disease (Table II).
 | Table I.Characteristics of the study
subjects. |
Table I.
Characteristics of the study
subjects.
| Characteristics | AE (n=39) | LC (n=55) | MM (n=96) |
|---|
| Age (years) | | | |
| Mean ± SD | 68.1±8.1 | 64.7±10.6 | 61.2±9.5 |
| Range | 44–90 | 39–84 | 33–83 |
| Gender | | | |
| Male | 36 | 45 | 75 |
| Female | 3 | 10 | 21 |
| Asbestos
exposure | | | |
| Occupational | 26 | 1 | 55 |
|
Environmental | 13 | 1 | 27 |
| None | 0 | 53 | 14 |
| Presence of
effusion | 12 | 16 | 78 |
 | Table II.Demographic data of MM patients. |
Table II.
Demographic data of MM patients.
|
Characteristics | Patient no.
(%) |
|---|
| Primary site | |
| Pleura | 91 (94.8) |
| Peritoneum | 5 (5.2) |
| Histological
subtype | |
| Epithelioid | 57 (59.4) |
| Sarcomatoid | 12 (17.4) |
| Biphasic | 16 (16.7) |
| Desmoplastic | 4 (5.8) |
| NOS | 7 (7.3) |
| Staging
classificationa | |
| I | 3 (3.3) |
| II | 2 (2.2) |
| III | 12 (13.2) |
| IV | 74 (81.3) |
Performance of serum SMRP in diagnosing
MM
Fujirebio Diagnostics, Inc., the developer of the
Mesomark assay, recommends a cut-off value of 1.5 nM, which was the
99th percentile of the normal serum SMRP concentration in a
population of 409 healthy Americans (27). An investigation in a population of
healthy Germans revealed a cut-off value of 1.5–1.6 nM, which was
the 95th percentile of the serum SMRP concentration (28). In our study, we performed a
preliminary investigation of the distribution of serum SMRP levels
among 72 healthy individuals without a history of asbestos
exposure. Since this investigation revealed that 69 individuals
(96%) had serum SMRP levels of <1.5 nM, we selected 1.5 nM, the
96th percentile, as the cut-off value.
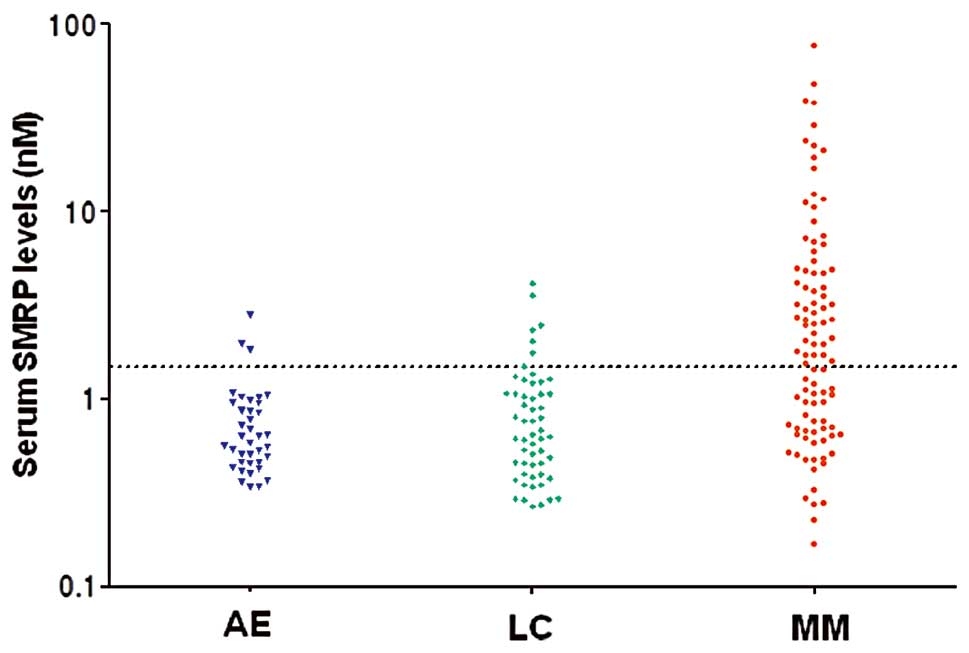
The distributions of serum SMRP levels in each group
are shown in Fig. 1. The serum
SMRP levels in MM patients were significantly higher compared to
those in the other groups (P<0.001) (Table III). The sensitivity of SMRP for
diagnosing MM was 56% (95% CI: 46–66%) and its specificity for MM
vs. lung cancer and individuals with asbestos exposure was 87% (95%
CI: 76–95%) and 92% (95% CI: 79–98%), respectively (Table IV). By contrast, the sensitivity of
CYFRA for diagnosing MM was 63% (95% CI: 52–72%) and its
specificity for MM vs. lung cancer was 49% (95% CI: 35–63%)
(Table IV). The sensitivity of
SMRP and CYFRA did not differ significantly (P= 0.157), although
the specificity of SMRP for MM vs. lung cancer was significantly
higher compared to that of CYFRA (P<0.001). The serum SMRP
levels in epithelioid disease [median, 2.47 nM; interquartile range
(IQR): 0.97–4.86] were significantly higher compared to those in
sarcomatoid disease (median, 0.8 nM; IQR: 0.38–1.15) (P=0.04).
However, there were no significant differences when compared to the
other histological subtypes. There was no significant association
between the serum SMRP levels and MPM stages (data not shown).
 | Table III.Diagnostic findings based on the
serum SMRP levels. |
Table III.
Diagnostic findings based on the
serum SMRP levels.
| Serum SMRP levels
(nM) | AE (n=39) | LC (n=55) | MM (n=96) |
|---|
| Mean ± SD | 0.78±0.50 | 0.93±0.77 | 5.77±11.1 |
| Median | 0.64 | 0.65 | 1.88a |
| QR25-QR75 | 0.49–0.96 | 0.40–1.08 | 0.71–4.79 |
| Min-max | 0.30–2.80 | 0.30–4.10 | 0.30–75.4 |
 | Table IV.Sensitivity and specificity of
biomarkers for diagnosing MM. |
Table IV.
Sensitivity and specificity of
biomarkers for diagnosing MM.
| Biomarkers | AE (n=39) | LC (n=55) | MM (n=96) |
|---|
| SMRP (%) | | | |
| Sensitivity | 8 | 13 | 56 |
| 95% CI | 2–21 | 5–24 | 46–66 |
| Specificity | 92 | 87 | |
| 95% CI | 79–98 | 76–95 | |
| CYFRA (%) | | | |
| Sensitivity | 8 | 51 | 63 |
| 95% CI | 2–21 | 37–65 | 52–72 |
| Specificity | 92 | 49 | |
| 95% CI | 79–98 | 35–63 | |
| CEA (%) | | | |
| Sensitivity | 64 | 57 | 9 |
| 95% CI | 41–83 | 41–72 | 4–17 |
| Specificity | 36 | 43 | |
| 95% CI | 17–59 | 28–59 | |
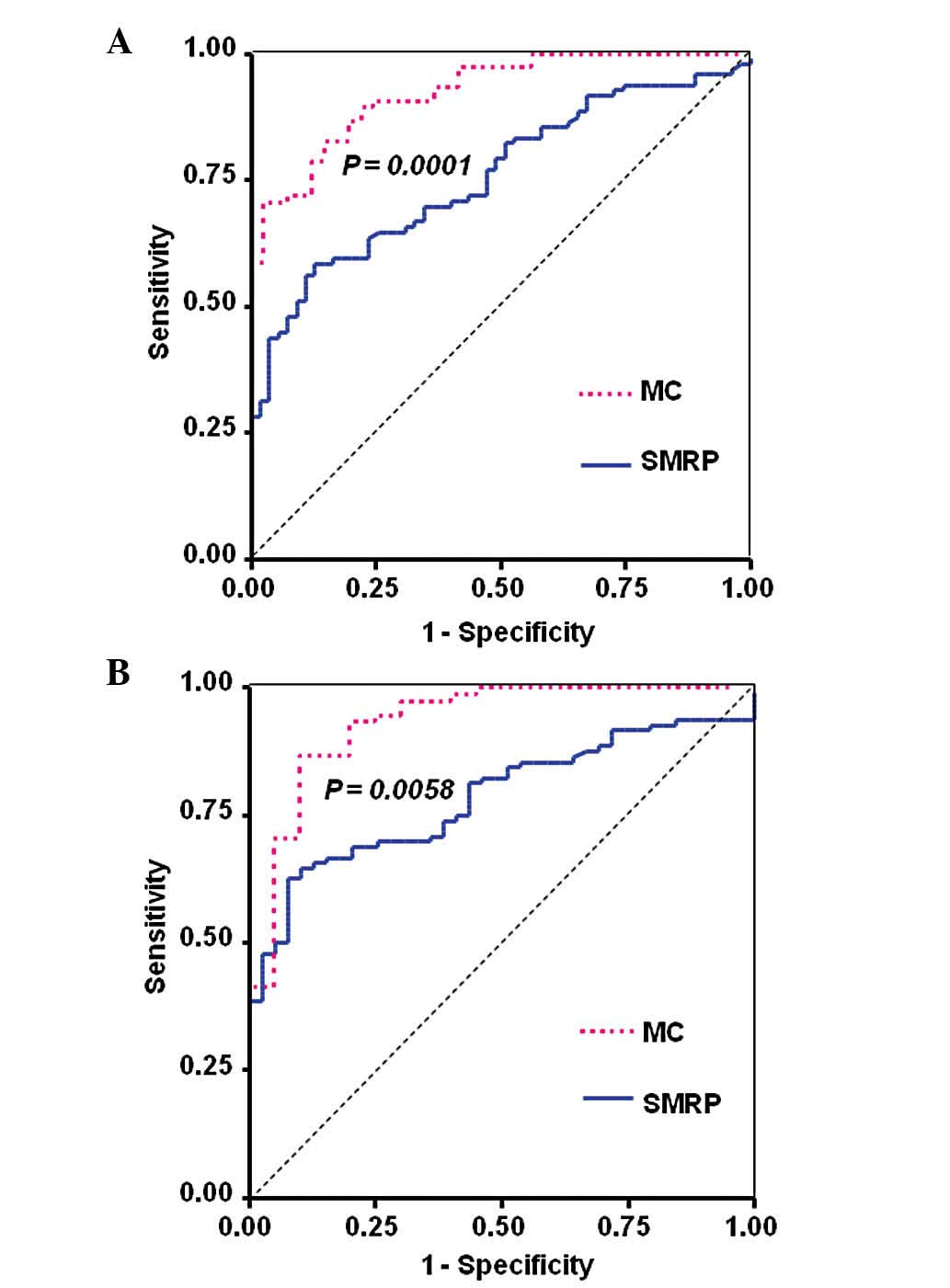
The diagnostic performance of SMRP was evaluated
using ROC curves (Fig. 2). For the
differentiation between MM and lung cancer, the AUC was 0.76 (95%
CI: 0.68–0.83) (Fig. 2A) and for
the differentiation between MM and individuals with asbestos
exposure, the AUC was 0.78 (95% CI: 0.71–0.86) (Fig. 2B). For CYFRA, the AUC for the
differentiation between MM and lung cancer was 0.55 (data not
shown). Therefore, the diagnostic performance of SMRP for
differentiating between MM and lung cancer was superior to that of
CYFRA.
Investigation of MCs and their
performance in diagnosing MM
To improve the performance of serum biomarkers in
diagnosing MM, we investigated the optimal MCs. The measured
variables common to patients with MM and lung cancer were age,
gender, presence of effusion, clinical stage and the levels of
SMRP, CYFRA and CEA. The measured variables common to patients with
MM and individuals with a history of asbestos exposure were age,
presence of effusion and the levels of SMRP, CYFRA and CEA. Since
the distributions of all the biomarkers were significantly skewed
to the right, the variables were logarithmically transformed using
common logarithms. A stepwise logistic regression analysis was used
to select the variables. To differentiate between MM and lung
cancer, SMRP levels, presence of effusion and CEA levels were
selected (Table V). From the signs
of the estimates, we determined that the probability of a diagnosis
of MM was higher for elevated SMRP levels, presence of pleural
effusion and lower CEA levels. It was concluded that the selected
markers were reasonable from the clinical standpoint. Subsequently,
the markers selected to differentiate between MM and individuals
with a history of asbestos exposure were age and CYFRA (data not
shown). However, this model was composed of a single marker rather
than multiple markers. Therefore, it was excluded from further
investigation.
 | Table V.Results of stepwise logistic
regression analysis (MM vs. LC). |
Table V.
Results of stepwise logistic
regression analysis (MM vs. LC).
| Parameter | DF | Estimate | SE | Wald
χ2 | P-value |
|---|
| Intercept | 1 | 3.08 | 0.79 | 15.45 | <0.001 |
| SMRPa | 1 | 2.83 | 0.92 | 9.48 | 0.002 |
| Presence of
effusion | 1 | 1.28 | 0.42 | 9.15 | 0.003 |
| CEAa | 1 | −5.52 | 1.46 | 14.20 | <0.001 |
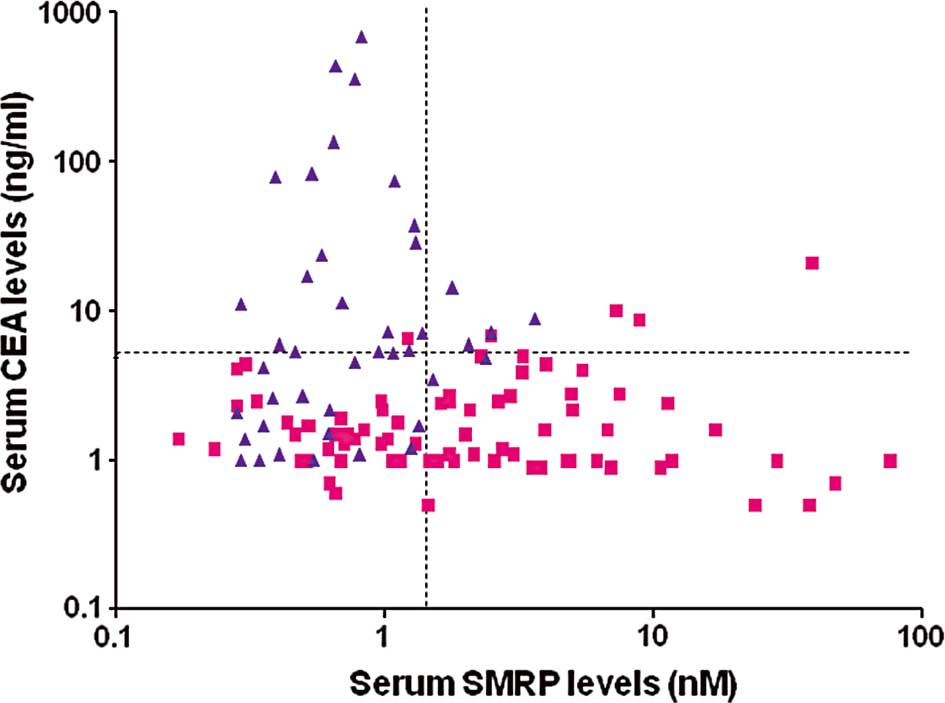
To further evaluate the models in Table V, the association between SMRP and
CEA was analyzed using scatter diagrams (Fig. 3). The scatter diagrams demonstrated
that the majority of patients with high CEA levels were those with
lung cancer. In addition, the majority of patients with high SMRP
levels were those with MM. Therefore, the combination of SMRP and
CEA resulted in only a minor overlap of the diagnostic findings of
MM and lung cancer, suggesting that the diagnostic performance for
MM was improved. By contrast, since the combination of SMRP and
CYFRA resulted in a significant overlap of the diagnostic findings
of MM and lung cancer, it was inferred that the diagnostic
performance was scarcely improved (data not shown).
The MC was composed using the results of Table V. Since the ratio of the estimates
for SMRP, presence of effusion and CEA was ∼3:1:5, the following MC
was selected: MC=1×I(presence of effusion) + 3 ×
log10(SMRP) − 5 × log10(CEA), where I
(presence of effusion) was defined as an indicator function with a
value of 1 when effusion was present and 0 when effusion was
absent. Wherein -1 was selected as the cut-off value to maximize
the sum of the sensitivity and specificity, the sensitivity of MC
for diagnosing MM was 76% (95% CI: 64–85%) and its specificity for
MM vs. lung cancer and individuals with asbestos exposure was 88%
(95% CI: 74–96%) and 90% (95% CI: 68–99%), respectively. While the
specificity of MC was comparable to SMRP alone, its sensitivity was
∼20% higher compared to that of SMRP alone. In addition, three of
the five MPM patients with stage I–II disease were above the
cut-off value, although none exhibited elevated serum levels of
SMRP alone. The ROC curves for MC are shown in Fig. 2. The AUC for the differentiation
between MM and lung cancer was 0.92 (95% CI: 0.88–0.97), which was
significantly higher compared to that for SMRP alone (P=0.0001)
(Fig. 2A). The AUC for the
differentiation between MM and individuals with a history of
asbestos exposure was 0.93 (95%CI: 0.87–1.0), which was also
significantly higher compared to that for SMRP alone (P= 0.0058)
(Fig. 2B). These results indicate
that combining CEA with SMRP improves the performance of SMRP alone
in diagnosing MM and may facilitate early detection of MPM.
Discussion
The recent development of Mesomark, a quantitative
ELISA kit using two monoclonal antibodies (OV569 and 4H3) that
recognize SMRP, has enabled the measurement of serum SMRP levels.
The findings of key studies on the performance of SMRP in
diagnosing MM by using the Mesomark kit demonstrated that serum
SMRP levels were significantly higher in MM patients compared to
those in controls, such as healthy individuals, subjects with a
history of asbestos exposure, or patients with asbestos-related
benign pleural disease or lung cancer (9,11–21,27–35).
In the present study, also undertaken using the Mesomark kit, the
serum SMRP levels were found to be significantly higher in MM
patients compared to those in lung cancer patients and individuals
with asbestos exposure. These findings are consistent with those
first reported by Robinson et al (36), suggesting that the use of serum
SMRP levels for diagnosing MM has excellent universality and
reproducibility. Based on previous studies, including our own, SMRP
is considered to be a highly specific biomarker for MM; however,
its sensitivity, ranging from 48–80%, is moderate (9,11–21,27–35).
To improve the performance of SMRP in diagnosing MM, there is a
need to increase the sensitivity while maintaining a high degree of
specificity.
One way of improving the sensitivity may be by
lowering the cut-off value; however, this is not recommended, since
it may result in a simultaneous reduction of specificity (26,28).
Another approach may be to improve the diagnostic performance by
combining data obtained using multiple biomarkers. The accuracy of
the histopathological diagnosis of MM has markedly improved. One
reason for this improvement has been the introduction of
immunohistochemical analysis involving the combination of a
positive marker that is highly expressed in MM and a negative
marker that has a low frequency of expression in MM (37,38).
A systemic review of markers for diagnosis of MM demonstrated that
positive staining for CEA and epithelial antigen (clone Ber-EP4)
and negative staining for epithelial membrane antigens and
calretinin may confirm that a patient does not have MM (21). In addition, based on biomarker
measurements in the pleural effusion, algorithms for the diagnosis
of malignant pleural diseases were established. The CEA level
achieved a greater accuracy in the differential diagnosis of MPM
through its combination with other markers. For example, an
elevated CYFRA level with a low CEA level in pleural effusion was
shown to be highly suggestive of MPM (7).
To date, whether the combination of blood
biomarkers, including SMRP, is able to improve the performance of
SMRP alone in diagnosing MM remains controversial. A previous study
by van den Heuvel et al (34) reported that the combination of two
serum markers (CEA and SMRP) was the most accurate in
differentiating MPM from non-small-cell lung cancer. The AUC of
this marker combination demonstrated a significant improvement
compared to the inverse levels of CEA alone. However, in that
study, a direct comparison of diagnostic performance between this
combination and SMRP alone was not performed.
Amati et al (31) evaluated the combination of two
hematological biomarkers: 8-hydroxy-2′-deoxyguanosine (8-OHdG), an
indicator of oxidative DNA damage and vascular endothelial growth
factor β (VEGFβ), an angiogenic molecule. The results of that study
indicated that the diagnostic performance of this combination in
differentiating between healthy individuals and those with a
history of asbestos exposure was superior to that of each biomarker
alone. Although it was also mentioned that a combination of SMRP,
8-OhdG and VEGFβ was optimal for distinguishing between individual
groups, including the MM group, that study provided no specific
measures of diagnostic performance or any further details.
Several previous studies evaluated the diagnostic
performance of combined SMRP and osteopontin measurements in MM.
Creaney et al (12)
demonstrated that the combination of SMRP, serum osteopontin and
MPF did not exhibit increased sensitivity for detecting MM compared
to that of SMRP alone. A recent study investigated serum SMRP and
plasma osteopontin levels in 66 patients with MPM, 47 patients with
non-malignant asbestos-related lung or pleural diseases, 42
patients with other benign pleural and lung diseases and 21
patients with lung cancer, as plasma osteopontin was proven to be
more stable compared to serum osteopontin (14). A logistic regression analysis
revealed that the combined marker model had an AUC of 0.912 and a
sensitivity of 76%, with a 95% specificity (14). The AUC for this marker combination
did not differ from that for serum SMRP alone. In previous studies,
the majority of osteopontin-positive MM patients were also found to
be positive for SMRP. This high degree of concordance may result in
the finding that a combination of these two markers does not
improve the performance of SMRP alone in diagnosing MM (12,14).
Cristaudo et al (15) also
measured serum SMRP and plasma osteopontin levels in 93 healthy
subjects, 111 individuals with benign respiratory disease and 31
patients with MPM. That study was the first to demonstrate that a
combination of these two markers was more efficient in MPM
diagnosis compared to each marker used alone by means of the
combined risk index, a new statistical approach of a logistic
regression analysis. In that study, however, a small number of
patients with MPM were enrolled and its histological subtype was
limited to the epithelioid type. To confirm those findings,
larger-scale studies are required. The combination of SMRP with
CA125 (9), or MPF (12,18)
has also been investigated. However, none of those studies
demonstrated that the diagnostic performance of SMRP in combination
with other markers outperformed that of SMRP alone.
The present study demonstrated that combining SMRP
and CEA improved the diagnostic performance of SMRP alone, since
these two markers act in a complementary manner. However, since we
used the same data for selecting and assessing the performance of
MC, it is possible that our evaluation of the MC may have been
optimistic. Furthermore, in our study, data were collected from a
single center; validation of the diagnostic performance of this
particular MC by a multicenter study is recommended in the
future.
It is difficult to determine whether pleural
effusion developing in individuals with a history of asbestos
exposure represents benign asbestos pleurisy or is an initial
symptom of MPM and misdiagnosis at this stage may hinder the early
detection of MPM. Future prospective research is required to
confirm whether a combination of serum biomarkers, including SMRP,
may be useful in diagnosing early-stage MPM.
Acknowledgements
This study was supported in part by
the Special Coordination Funds for Science and Technology from
Japan Science and Technology Agency and the Japanese Ministry of
Education, Culture, Sports, Science and Technology.
References
|
1.
|
Tsao AS, Wistuba I, Roth JA and Kindler
HL: Malignant pleural mesothelioma. J Clin Oncol. 27:2081–2090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sugarbaker DJ, Flores RM, Jaklitsch MT, et
al: Resection margins, extrapleural nodal status, and cell type
determine postoperative long-term survival in trimodality therapy
of malignant pleural mesothelioma: results in 183 patients. J
Thorac Cardiovasc Surg. 117:54–63. 1999. View Article : Google Scholar
|
|
3.
|
Krug LM, Pass HI, Rusch VW, et al:
Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin
followed by extrapleural pneumonectomy and radiation for malignant
pleural mesothelioma. J Clin Oncol. 27:3007–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Van Schil PE, Baas P, Gaafar R, et al:
Trimodality therapy for malignant pleural mesothelioma: results
from an EORTC phase II multicentre trial. Eur Respir J.
36:1362–1369. 2010.PubMed/NCBI
|
|
5.
|
Bonfrer JM, Schouwink JH, Korse CM and
Baas P: Cyfra 21-1 and TPA as markers in malignant mesothelioma.
Anticancer Res. 17:2971–2973. 1997.PubMed/NCBI
|
|
6.
|
Schouwink J, Korse CM, Bonfrer JM, Hart AA
and Baas P: Prognostic value of the serum tumour markers Cyfra 21-1
and tissue polypeptide antigen in malignant mesothelioma. Lung
Cancer. 25:25–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Paganuzzi M, Onetto M, Marroni P, et al:
Diagnostic value of CYFRA 21-1 tumor marker and CEA in pleural
effusion due to mesothelioma. Chest. 119:1138–1142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hedman M, Arnberg H, Wernlund J, Riska H
and Brodin O: Tissue polypeptide antigen (TPA), hyaluronan and CA
125 as serum markers in malignant mesothelioma. Anticancer Res.
23:531–536. 2003.PubMed/NCBI
|
|
9.
|
Creaney J, van Bruggen I, Hof M, et al:
Combined CA125 and mesothelin levels for the diagnosis of malignant
mesothelioma. Chest. 132:1239–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Pass HI, Lott D, Lonardo F, et al:
Asbestos exposure, pleural mesothelioma, and serum osteopontin
levels. N Engl J Med. 353:1564–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Grigoriu BD, Scherpereel A, Devos P, et
al: Utility of osteopontin and serum mesothelin in malignant
pleural mesothelioma diagnosis and prognosis assessment. Clin
Cancer Res. 13:2928–2935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Creaney J, Yeoman D, Demelker Y, et al:
Comparison of osteopontin, megakaryocyte potentiating factor, and
mesothelin proteins as markers in the serum of patients with
malignant mesothelioma. J Thorac Oncol. 3:851–857. 2008. View Article : Google Scholar
|
|
13.
|
Rai AJ, Flores RM, Mathew A, et al:
Soluble mesothelin related peptides (SMRP) and osteopontin as
protein biomarkers for malignant mesothelioma: analytical
validation of ELISA based assays and characterization at mRNA and
protein levels. Clin Chem Lab Med. 48:271–278. 2010.
|
|
14.
|
Creaney J, Yeoman D, Musk AW, de Klerk N,
Skates SJ and Robinson BW: Plasma versus serum levels of
osteopontin and mesothelin in patients with malignant mesothelioma
- which is best? Lung Cancer. 74:55–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cristaudo A, Bonotti A, Simonini S, et al:
Combined serum mesothelin and plasma osteopontin measurements in
malignant pleural mesothelioma. J Thorac Oncol. 6:1587–1593. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hassan R, Bera T and Pasten I: Mesothelin:
a new target for immunotherapy. Clin Cancer Res. 10:3937–3942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ray M and Kindler HL: Malignant pleural
mesothelioma: an update on biomarkers and treatment. Chest.
136:888–896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hollevoet K, Nackaerts K, Thimpont J, et
al: Diagnostic performance of soluble mesothelin and megakaryocyte
potentiating factor in mesothelioma. Am J Respir Crit Care Med.
181:620–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cristaudo A, Bonotti A, Simonini S, Bruno
R and Foddis R: Soluble markers for diagnosis of malignant pleural
mesothelioma. Biomark Med. 5:261–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hollevoet K, Reitsma JB, Creaney J, et al:
Serum mesothelin for diagnosing malignant pleural mesothelioma: an
individual patient data meta-analysis. J Clin Oncol. 30:1541–1549.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
van der Bij S, Schaake E, Koffijberg H,
Burgers JA, de Mol BA and Moons KG: Markers for the non-invasive
diagnosis of mesothelioma: a systematic review. Br J Cancer.
104:1325–1333. 2011.PubMed/NCBI
|
|
22.
|
Schorge JO, Drake RD, Lee H, et al:
Osteopontin as an adjunct to CA125 in determining recurrent ovarian
cancer. Clin Cancer Res. 10:3474–3478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Etzioni R, Falcon S, Gann PH, Kooperberg
CL, Penson DF and Stampfer MJ: Prostate-specific antigen and free
prostate-specific antigen in the early detection of prostate
cancer: do combination tests improve detection? Cancer Epidemiol
Biomarkers Prev. 13:1640–1645. 2004.PubMed/NCBI
|
|
24.
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumors, Pathology and Genetics. Tumours of the Lung, Pleura,
Thymus, and Heart. IARC Press; Lyon: 2004
|
|
25.
|
Husain A, Colby T, Ordonez N, et al:
Guidelines for pathologic diagnosis of malignant mesothelioma: a
consensus statement from the International Mesothelioma Interest
Group. Arch Pathol Lab Med. 133:1317–1331. 2009.PubMed/NCBI
|
|
26.
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristics curves: a nonparametric approach.
Biometrics. 44:837–845. 1988. View
Article : Google Scholar
|
|
27.
|
Beyer HL, Geschwindt RD, Glover CL, et al:
Mesomark: a potential test for malignant pleural mesothelioma. Clin
Chem. 53:666–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Weber DG, Taeger D, Pesch B, Kraus T,
Brüning T and Johnen G: Soluble mesothelin-related peptides
(SMRP)-high stability of a potential tumor marker for mesothelioma.
Cancer Biomark. 3:287–292. 2007.PubMed/NCBI
|
|
29.
|
Scherpereel A, Grigoriu B, Conti M, et al:
Soluble mesothelin-related peptides in the diagnosis of malignant
pleural mesothelioma. Am J Respir Crit Care Med. 173:1155–1160.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Cristaudo A, Foddis R, Vivaldi A, et al:
Clinical significance of serum mesothelin in patients with
mesothelioma and lung cancer. Clin Cancer Res. 13:5076–5081. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Amati M, Tomasetti M, Scartozzi M, et al:
Profiling tumor-associated markers for early detection of malignant
mesothelioma: an epidemiologic study. Cancer Epidemiol Biomarkers
Prev. 17:163–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Pass HI, Wali A, Tang N, et al: Soluble
mesothelin-related peptide level elevation in mesothelioma serum
and pleural effusions. Ann Thorac Surg. 85:265–272. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Schneider J, Hoffmann H, Dienemann H,
Herth FJ, Meister M and Muley T: Diagnostic and prognostic value of
soluble mesothelin-related proteins in patients with malignant
pleural mesothelioma in comparison with benign asbestosis and lung
cancer. J Thorac Oncol. 3:1317–1324. 2008. View Article : Google Scholar
|
|
34.
|
van den Heuvel MM, Korse CM, Bonfrer JM
and Baas P: Non-invasive diagnosis of pleural malignancies: the
role of tumour markers. Lung Cancer. 59:350–354. 2008.PubMed/NCBI
|
|
35.
|
Rodriguez Portal JA, Rodriguez Becerra E,
Rodriguez Rodriguez D, et al: Serum levels of soluble
mesothelin-related peptides in malignant and nonmalignant
asbestos-related pleural disease: relation with past asbestos
exposure. Cancer Epidemiol Biomarkers Prev. 18:646–650. 2009.
|
|
36.
|
Robinson BW, Creaney J, Lake R, et al:
Mesothelin-family proteins and diagnosis of mesothelioma. Lancet.
362:1612–1616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ordonez NG: The immunohistochemical
diagnosis of mesothelioma: a comparative study of epithelioid
mesothelioma and lung adenocarcinoma. Am J Surg Pathol.
27:1031–1051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ordonez NG: Application of mesothelin
immunostaining in tumor diagnosis. Am J Surg Pathol. 27:1418–1428.
2003. View Article : Google Scholar : PubMed/NCBI
|