Introduction
Squamous cell carcinoma developing from the oral
cavity is a common malignant neoplasm of the head and neck, with
tongue carcinomas accounting for 25–40% of all oral carcinomas
(1,2). Over 50% of patients experience a
relapse and the incidence is expected to increase in the next few
decades (3). Carcinoma cells
exhibit marked changes in cellular composition, signaling and
energy metabolism, and these changes lead to advanced stages of
progression (4,5). Identification of molecular aspects of
carcinoma cells contributes to the development of novel therapeutic
approaches and improves patient prognosis.
Carcinoma cells change the lipid composition of cell
membranes (6) and stimulate lipid
metabolism during tumor progression (7). Fatty acid-binding proteins (FABPs)
are the lipid chaperones that transport long chain fatty-acids
(LCFAs) to specific cell compartments, such as lipid droplets for
storage; the endoplasmic reticulum for signaling, trafficking and
membrane synthesis; mitochondria or peroxisomes for oxidation;
cytosoles or other enzymes for activity regulation; the nucleus for
gene transcription; or even outside of the cells in order to signal
in an autocrine or paracrine manner (8,9). The
FABP family consists of at least nine members that were originally
identified in different cell or tissue types, such as FABP4 in
adipocytes and FABP5 in the epidermis (10,11).
Studies have revealed the involvement of aberrant FABP expression
in the pathology of various diseases including malignant neoplasms
(8,9). Although FABP5 is reported to be
upregulated in oral carcinomas, its involvement in disease
progression remains controversial (11–13).
The ectopic expression of FABP4 in carcinomas of the
stomach (14) and ovary (15) facilitates disease progression,
whereas it is downregulated in aggressive subsets of bladder and
breast carcinomas (16–18). These data suggest the differential
role of FABP4 in carcinomas depending on the tissue origin. FABP4
expression in oral carcinomas requires elucidation. Therefore, in
this study, we examined FABP4 and FABP5 expression in tongue
carcinomas by immunohistochemistry, and analysed their involvement
in disease progression.
Materials and methods
Patient population
A total of 58 cases of tongue carcinomas obtained at
incisional biopsy or surgery at Meikai University Hospital (Sakado,
Japan) from 1990 to 2010 were examined. The patients were comprised
of 34 males and 24 females, with an age range of 29–92 years (mean
± SD, 62.8±14.9 years) at the time of diagnosis. The details of
pretreatment clinical and pathological characteristics are provided
in Table I. Histologic grading and
staging were assessed according to the International Union Against
Cancer (UICC) tumor-node-metastasis classification. Tissues were
obtained subsequent to the written consent of the patient and with
the approval of the Ethics Committee of the Institutional Review
Boards of Meikai University.
 | Table I.Fatty acid-binding protein 5 staining
score and clinicopathological parameters. |
Table I.
Fatty acid-binding protein 5 staining
score and clinicopathological parameters.
| Parameters | No. | Nucleus
| Cytoplasm
|
|---|
| Mean ± SD | P-valuea | Mean ± SD | P-valuea |
|---|
| Age (years) | | | 0.220 | | 0.553 |
| <65 | 33 | 4.969±2.495 | | 8.188±2.571 | |
| ≥65 | 25 | 5.120±3.180 | | 8.120±3.206 | |
| Gender | | | 0.491 | | 0.411 |
| Female | 24 | 4.125±2.153 | | 7.542±3.036 | |
| Male | 34 | 5.697±3.036 | | 8.606±2.645 | |
| T-stageb | | | 0.137 | | 0.125 |
| T1 | 38 | 4.842±2.666 | | 7.553±2.617 | |
| T2 | 18 | 4.824±2.580 | | 9.059±2.968 | |
| T4 | 2 | 10.500±2.121 | | 12.000±0.000 | |
| N-stageb | | | 0.482 | | 0.764 |
| N0 | 49 | 5.021±2.809 | | 7.979±2.779 | |
| N1 | 6 | 4.000±1.265 | | 9.167±3.488 | |
| N2 | 3 | 7.333±4.163 | | 9.000±3.000 | |
| Clinical
stageb | | | 0.720 | | 0.619 |
| 1 | 38 | 4.842±2.666 | | 7.553±2.617 | |
| 2 | 10 | 5.333±3.354 | | 9.333±2.958 | |
| 3 | 6 | 4.000±1.265 | | 9.167±3.488 | |
| 4 | 4 | 7.750±3.500 | | 9.750±2.872 | |
| Histological
differentiation | | | 0.463 | | 0.161 |
| Well | 32 | 5.469±2.771 | | 8.189±2.856 | |
| Moderate | 20 | 4.450±2.395 | | 7.750±2.899 | |
| Poor | 6 | 4.600±4.334 | | 9.600±2.510 | |
Mouse skin wounds
Mouse skin wound tissues were obtained as previously
described (19). Briefly, 2 cm
long full-thickness skin incisions were created on the dorsum of
C57BL/6 female mice. The mice were sacrificed at 1, 3, 5, 7, 14 and
21 days after wounding and tissue samples were obtained from three
different wounds at each time point. The animals were housed and
used according to the Rules for the Care and Use of Laboratory
Animal Guidelines of the Nippon Dental University under a protocol
approved by the Institutional Review Board.
Immunostaining
Unstained formalin-fixed, paraffin-embedded
carcinoma and mouse wound sections were incubated with rabbit
anti-FABP4 (ab13979; Abcam, Tokyo, Japan) or rat anti-FABP5
(MAB3077; R&D Systems, Minneapolis, MN, USA) antibodies
followed by biotinylated anti-rabbit or -rat IgG. Following
treatment with avidin-biotin complexes, the color was developed
with 3,3’-diaminobenzidine hydrochloride. The immunostaining of
FABPs was evaluated as described previously (20). Briefly, the extent of staining was
scored on a scale of 0–4: 0, totally negative; 1, <10%; 2,
10–40%; 3, 41–60%; or 4, >61%. Positive nuclear and/or
cytoplasmic staining was subjectively classified as: 1, weak; 2,
moderate; or 3, strong at the area of strongest staining due to the
variable intensity of the staining. Weak staining was barely
discernible and only clearly visible on high-power examination;
moderate staining was easily seen at low power and was light brown
in color; and strong staining was intense and dark brown with a
painted-on appearance. An immunohistochemical composite score was
calculated by multiplying the extent and intensity scores to give a
value of between 0–12.
Statistical analysis
Pearson’s Chi-square test was used to analyze the
immunostaining score for clinicopathological parameters. The
Wilcoxon signed-rank test was used to compare between the scores.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression and distribution of FABPs in a
normal tongue
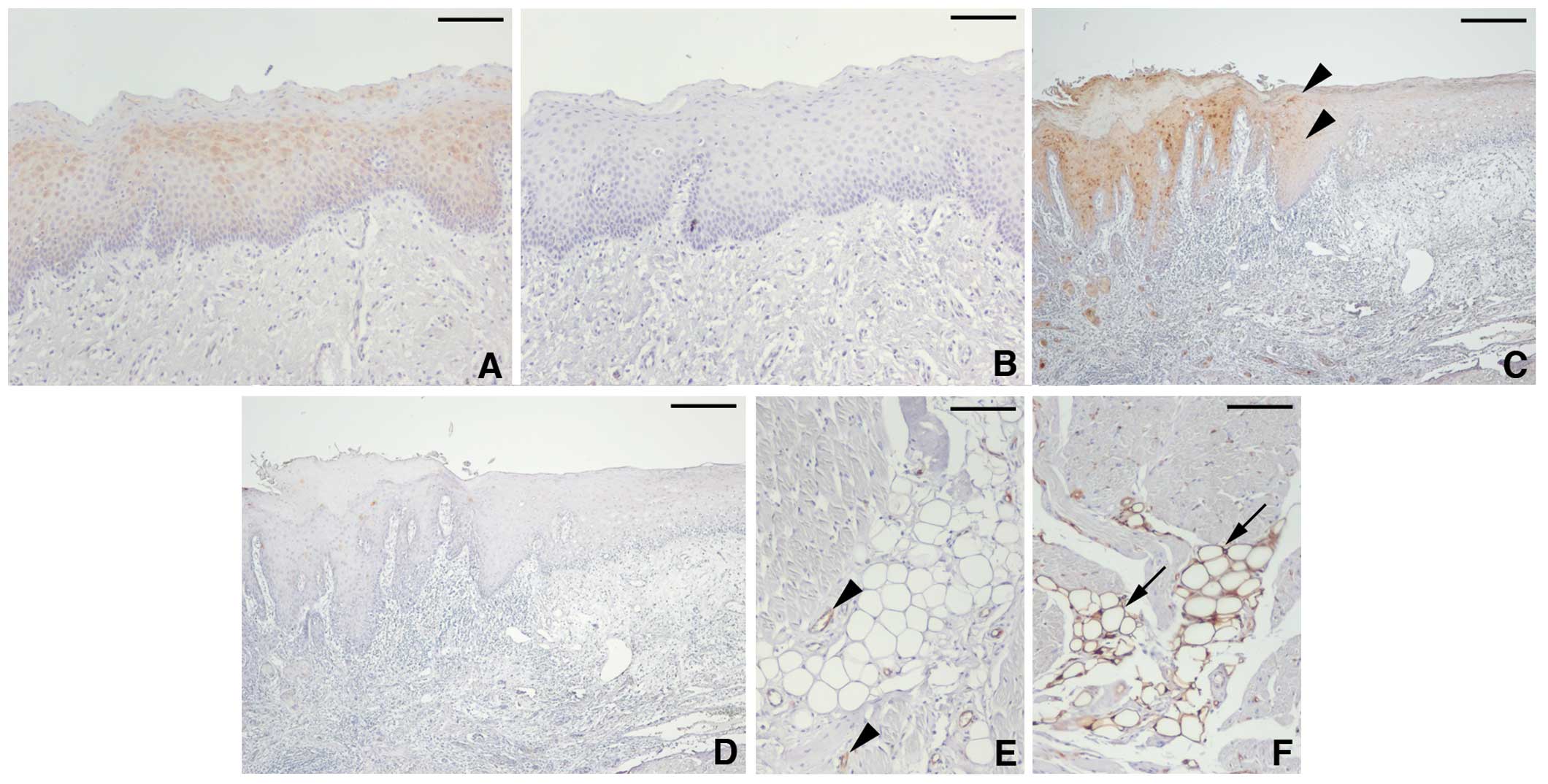
FABP5 expression was weakly detected at the
cytoplasm of upper-suprabasal cells beneath the keratinized layer
(Fig. 1A). FABP4 was not expressed
in the normal epithelial cells of the tongue (Fig. 1B). At the epithelium, adjacent to
carcinoma cells, FABP5-positive cells were extended to the
suprabasal layer with a moderate-to-strong staining intensity
(Fig. 1C), whereas FABP4
expression was negligible (Fig.
1D). The specificity of anti-FABP5 and -FABP4 antibodies was
confirmed by the staining of endothelial cells (Fig. 1E) (21) and adipocytes (Fig. 1F) (8), respectively.
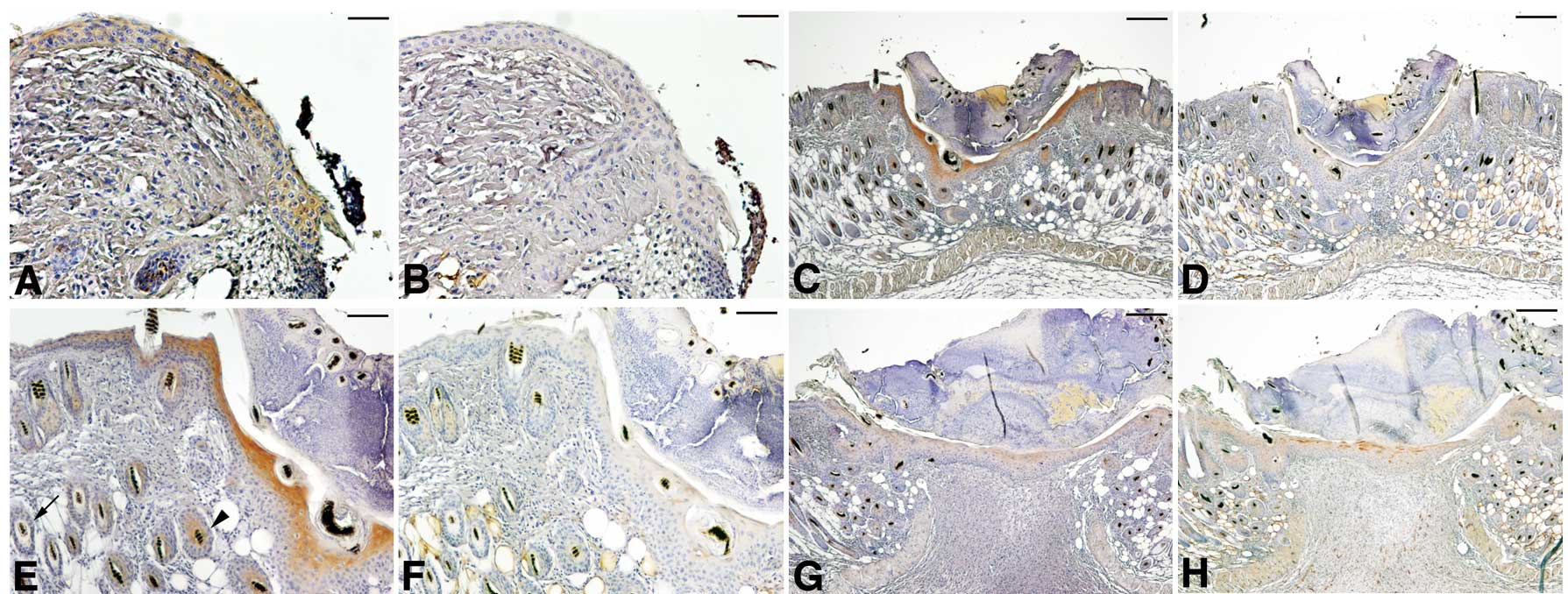
FABP expression in mouse skin wounds
The enhanced expression of FABP5 in the
carcinoma-adjacent epithelium is suggestive of the fact that
factors from the surrounding tissues or genetic alterations may
activate FABP5 expression. Subsequently, FABP expression at the
skin wounds of a genetically normal mouse was examined. As shown in
Fig. 2, FABP5 expression was
detected at the epithelial cells migrating on the wound surface at
day 1. It became prominent at the cytoplasm of suprabasal cells at
day 3 and gradually declined thereafter. FABP4 expression was
slightly detected in the epithelium at day 3 but was negatively
expressed at the earlier and later epithelium. The restricted
detection of FABP5 on the skin epithelium and the strong staining
of FABP4 on the subcutaneous adipocytes confirmed the specificity
of antibodies to mouse FABPs.
FABP5 expression in tongue
carcinomas
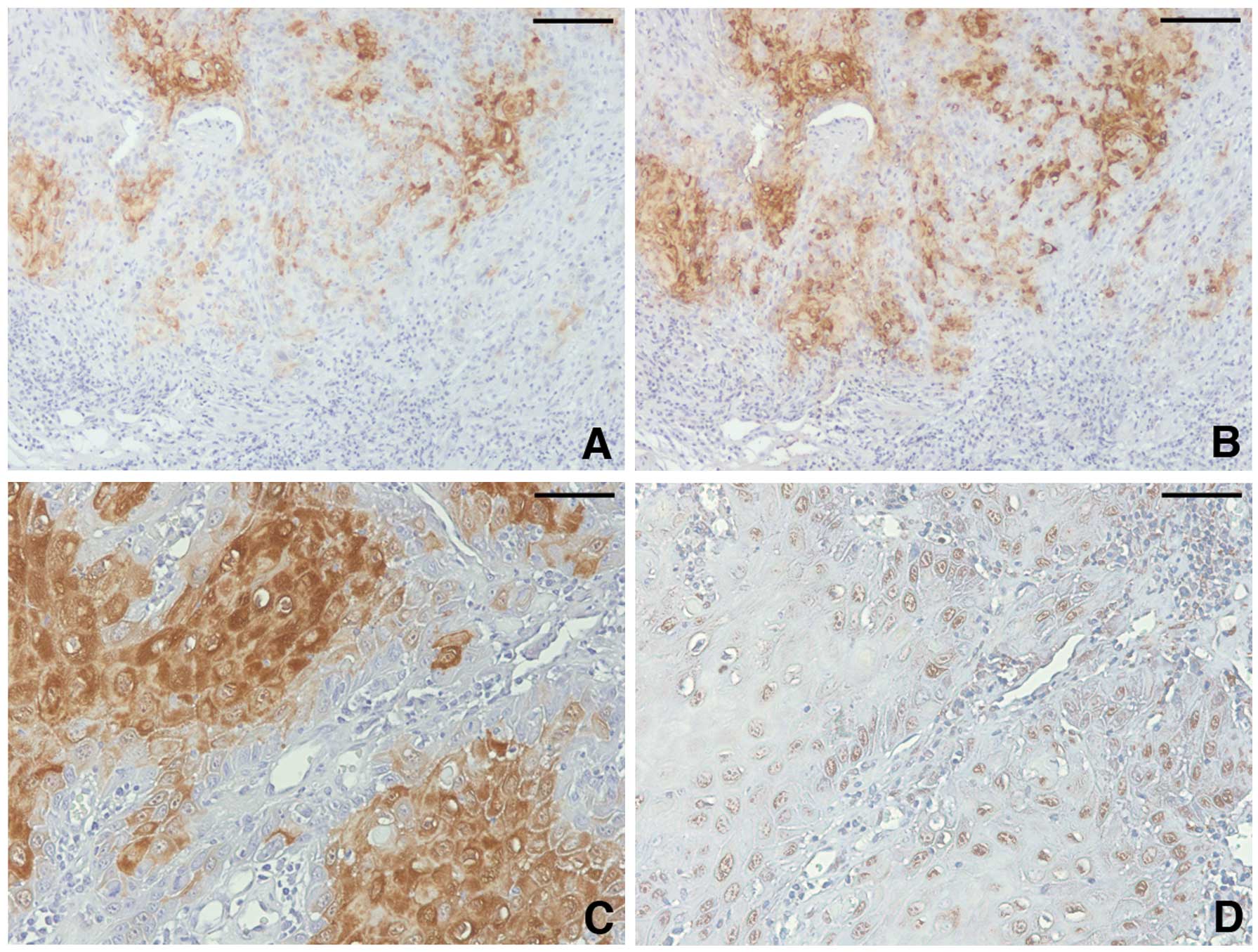
FABP5 expression was detected in all carcinoma
tissues. It was preferentially observed at the cytoplasm of
carcinoma cells, particularly at the center of tumor cell nests
(Fig. 3A and C). The nuclear
staining was weak and less frequent (mean ± SD, 5.035±2.790)
compared with the cytoplasmic staining (8.158±2.840, P<0.001).
The percentage (3.351±0.813) and staining intensity (2.439±0.598)
scores of the cytoplasm suggest that tongue carcinoma cells
frequently express FABP5 at a moderate-to-high level.
The correlation of the FABP5 staining score with the
clinicopathological parameters was statistically evaluated
(Table I). Although no significant
difference was observed between them, the score increased in the
advanced stages of the carcinomas. A number of observations at each
stage of the parameters were variable, thus we divided patients
into the advanced and non-advanced groups. The advanced group on
the T- and clinical stages exhibited a significantly higher score
compared with the non-advanced group (Table II).
 | Table II.Fatty acid-binding protein 5 staining
score in non-progressive/advanced stages of carcinomas. |
Table II.
Fatty acid-binding protein 5 staining
score in non-progressive/advanced stages of carcinomas.
| Parameters | No. | Nucleus
| Cytoplasm
|
|---|
| Mean ± SD | P-valuea | Mean ± SD | P-valuea |
|---|
| T-stageb | | | 0.357 | | 0.041 |
| T1 | 38 | 4.842±2.666 | | 7.552±2.617 | |
| T2–4 | 20 | 5.421±3.061 | | 9.368±2.948 | |
| N-stageb | | | 0.355 | | 0.383 |
| N0 | 49 | 5.021±2.809 | | 7.979±2.780 | |
| N1–2 | 9 | 5.111±2.848 | | 9.111±3.140 | |
| Clinical
stageb | | | 0.357 | | 0.041 |
| 1 | 38 | 4.842±2.666 | | 7.553±2.617 | |
| 2–4 | 20 | 5.421±3.061 | | 9.364±2.948 | |
| Histological
differentiationc | | | 0.277 | | 0.051 |
| Well | 32 | 5.469±2.771 | | 8.188±2.856 | |
| Less | 26 | 4.480±2.771 | | 8.120±2.877 | |
FABP4 expression in tongue
carcinomas
FABP4-positive cells are distributed randomly
through carcinoma tissues and frequently located at peripheries of
tumor cell nests where FABP5 staining was rapidly reduced (Fig. 3B). The percentage score of FABP4
cytoplasm-positive cells (2.368±1.057) and the staining intensity
score (1.776±0.750) were low compared wiht that of FABP5
(percentage score, 3.351±0.813, P<0.001; staining intensity
score, 2.439±0.598, P<0.001). Although the percentage and
intensity of FABP4 nuclear staining was comparable with FABP5 (data
not shown), FABP4 was frequently localized at the nucleus without
the cytoplasmic staining (Fig.
3D). This exclusive nuclear staining was not observed in the
FABP5 staining. No statistical difference was observed in the
nuclear and cytoplasmic score for any of the clinicopathological
parameters (Table III).
 | Table III.Fatty acid-binding protein 4 staining
score and clinicopathological parameters. |
Table III.
Fatty acid-binding protein 4 staining
score and clinicopathological parameters.
| Parameters | Nucleus
| Cytoplasm
|
|---|
| No. | Mean ± SD | P-valuea | Mean ± SD | P-valuea |
|---|
| Age (years) | | | 0.099 | | 0.194 |
| <65 | 33 | 5.212±3.798 | | 4.424±3.093 | |
| ≥65 | 25 | 4.400±2.972 | | 4.360±2.752 | |
| Gender | | | 0.266 | | 0.153 |
| Female | 24 | 4.208±3.134 | | 4.083±3.269 | |
| Male | 34 | 5.324±3.649 | | 4.618±2.686 | |
| T-stageb | | | 0.669 | | 0.852 |
| T1 | 38 | 4.737±3.629 | | 4.211±2.849 | |
| T2 | 18 | 4.833±3.222 | | 4.722±3.268 | |
| T4 | 2 | 7.500±2.121 | | 5.000±1.414 | |
| N-stageb | | | 0.915 | | 0.527 |
| N0 | 49 | 4.837±3.490 | | 4.347±2.803 | |
| N1 | 6 | 3.333±1.751 | | 4.000±4.336 | |
| N2 | 3 | 8.333±4.041 | | 6.000±2.000 | |
| Clinical
stageb | | | 0.979 | | 0.279 |
| 1 | 38 | 4.734±3.629 | | 4.211±2.849 | |
| 2 | 10 | 5.100±3.247 | | 4.900±2.846 | |
| 3 | 6 | 3.333±1.751 | | 4.000±4.336 | |
| 4 | 4 | 7.750±3.500 | | 5.500±1.915 | |
| Histological
differentiation | | | 0.695 | | 0.492 |
| Well | 32 | 5.094±3.762 | | 4.250±2.771 | |
| Moderate | 20 | 4.300±2.812 | | 4.850±3.281 | |
| Poor | 6 | 5.500±4.087 | | 3.667±2.733 | |
Discussion
FABPs transport LCFAs to the proper cell
compartments and play a multifaceted role, particularly for lipid
storage and β-oxidation in the cytoplasm and for transcription
factor activation in the nucleus (8,9).
Although FABP expression is restricted within the originally
identified tissues, findings of a previous study emphasized that
the aberrant expression is involved in carcinoma progression
(8). In this study, we examined
FABP4 and FABP5 expression in tongue carcinomas and identified a
correlation between cytoplasmic FABP5 staining and disease
progression.
The enhanced expression of FABP5 in epithelial cells
at the skin wound edge and near carcinoma cells confirmed the
findings of previous studies (22,23).
Epithelial cells at the wound edge stimulate metabolic pathways
(24) and its rapid proliferation
and migration are key features in the early phase of wound healing
(25,26). FABP5 is overexpressed in
proliferating keratinocytes (27)
and stimulates the proliferation and migration of oral carcinoma
cells (11). Although the staining
intensity was not strong, FABP5 expression was detected in wounds
at day 1, suggesting the involvement of FABP5 in the early phase of
wound coverage. However, FABP5 promotes keratinocyte
differentiation (28), as was
evident by strong staining in the keratinizing suprabasal cells of
wounded epithelium at day 3. These data confirmed the data of a
previous study which demonstrated that FABP5 is markedly expressed
in post-mitotic skin keratinocytes and weakly detectable in
proliferating keratinocytes (29).
Differentiating keratinocytes do not reside in the proliferation
cycle (30). These paradoxical
events suggest a multifaceted role and/or a biphasic action of
FABP5 in the definition of cells depending on the situation.
The majority of cells positioned at or near
carcinomas are associated with genetic alterations (31). The transient expression of FABP5 in
the wounded skin of a genetically normal mouse indicates that
epithelial cells upregulate FABP5 expression as a result of tissue
reaction. This finding was supported by the intense staining at
hair follicle cells near the wounds compared with the far distal
follicle cells. It seems likely that carcinoma cells and the
juxtaposed epithelial cells initiate the expression under the
tissue reaction. Epidermal growth factor, a representative tissue
factor, facilitates carcinoma progression (4) and skin wound healing (32) and overexpresses FABP5 (33). Other growth factors such as WNT and
transforming growth factor-β, which stimulate progression and
healing (34,35) regulate FABP5 expression (36,37).
Since proteolytic degradation of the extracellular matrix releases
growth factors (38), we should
consider the impact of carcinoma cell-tissue interactions on the
expression carefully. Furthermore, an intracellular signaling
molecule, mucosa-associated lymphoid tissue 1, which suppresses the
aggressive phenotype of oral carcinoma cells and is inactivated in
the patients with worse prognosis directly affects FABP expression
(39,40). Therefore both genetic and
environmental factors provoke carcinoma cells to express FABP5.
The cytoplasmic FABP5 staining score was increased
in carcinomas with the advanced T- and clinical stages in the
current study. FABP5 transports LCFAs to mitochondria for energy
production (8,9) and the increased production strongly
facilitates the aggressive properties of carcinoma cells (7). Carcinoma progression (clinical stage)
is a comprehensive issue evaluated by tumor expansion (T-stage) and
metastasis (N- and M-stage). Since carcinoma metastasis is a
consequence of various phenomena (4), the insignificance of expression in
N-stage is unlikely to negate the role of FABP5 in carcinoma
aggressiveness. Enhanced energy production by FABP5 may result in
the rapid proliferation of carcinoma cells and tumor expansion.
The pathological role of FABP4 is largely different
among carcinomas, as it is suppressive in bladder (17,41)
and breast carcinomas (18) and
stimulatory in gastric (14) and
ovarian carcinomas (15). FABP4
expression was identified in almost all the tongue carcinomas
examined. Although it was undetected in the normal epithelium, it
is expressed by the suprabasal cells of wounded skin epithelium at
day 5, although not the earlier and later wounds. This observation
suggests that the expression in keratinocytes is not largely
regulated by tissue factors. The FABP4 staining score did not
correlate with oral carcinoma progression in the current study.
FABP4, unlike FABP5, was frequently localized at the nucleus
without the cytoplasmic staining. FABP4 transports LCFAs to the
nucleus, which is required for stable binding with
peroxisome-proliferator-activated receptor (PPAR)-γ. PPAR-γ
expression in tongue carcinomas is higher in patients with an
improved prognosis (42) and the
administration of a PPAR-γ agonist inhibits the development of
tongue carcinoma (43). However,
oral carcinoma cells showing aggressive phenotypes in vitro
strongly upregulate FABP4 expression (40,44).
Detailed future studies are required in order to further clarify
the role of ectopic expression.
FABP5 was mainly detected at the cytoplasm that was
prominent in advanced tongue carcinomas. Enhanced expression may
contribute to the production of sufficient quantities of energy
that result in the progression of carcinomas to a more advanced
stage. Identification of the mechanism of FABP5 upregulation and
the pathological role in detail should therefore be analyzed for in
order to improve patient prognosis.
Acknowledgements
This study was supported by an
institutional grant from the Nippon Dental University (to K.I.) and
by grants from JSPS KAKENHI (no. 22592080, to T.C.) and (no.
22592103, to K.I.); and based on a partial fulfillment of doctoral
thesis submitted to the Graduate School of Dentistry, Meikai
University, in partial fulfillment of the requirements for the
Doctor of Dental Surgery degree.
References
|
1.
|
Regezi JA, Sciubba JJ and Jordan RCK: Oral
Pathology: Clinical Pathologic Correlations. 5th edition.
Saunders-Elsevier; Philadelphia, PA: pp. 552–556. 2008
|
|
2.
|
Ariyoshi Y, Shimahara M, Omura K, Yamamoto
E, Mizuki H, Chiba H, Imai Y, Fujita S, Shinohara M and Seto K;
Japanese Society of Oral and Maxillofacial Surgeons:
Epidemiological study of malignant tumors in the oral and
maxillofacial region: survey of member institutions of the Japanese
Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol.
13:220–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar
|
|
6.
|
Fuchs E: Epidermal differentiation: the
bare essentials. J Cell Biol. 111:2807–2814. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ward PS and Thompson CB: Metabolic
reprogramming: a cancer hallmark even Warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Furuhashi M and Hotamisligil GS: Fatty
acid-binding proteins: role in metabolic diseases and potential as
drug targets. Nat Rev Drug Discov. 7:489–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Smathers RL and Petersen DR: The human
fatty-acid binding protein family: evolutionary divergences and
functions. Hum Genomics. 5:170–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Siegenthaler G, Hotz R, Chatellard-Gruaz
D, Jaconi S and Saurat JH: Characterization and expression of a
novel human fatty acid-binding protein: the epidermal type
(E-FABP). Biochem Biophys Res Commun. 190:482–487. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fang LY, Wong TY, Chiang WF and Chen YL:
Fatty-acid-binding protein 5 promotes cell proliferation and
invasion in oral squamous cell carcinoma. J Oral Pathol Med.
39:342–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Melle C, Ernst G, Winkler R, Schimmel B,
Klussmann JP, Wittekindt C, Guntinas-Lichius O and von Eggeling F:
Proteomic analysis of human papillomavirus-related oral squamous
cell carcinoma: identification of thioredoxin and epidermal-fatty
acid binding protein as upregulated protein markers in
microdissected tumor tissue. Proteomics. 9:2193–2201. 2009.
View Article : Google Scholar
|
|
13.
|
Uma RS, Naresh KN, D’Cruz AK, Mulherkar R
and Borges AM: Metastasis of squamous cell carcinoma of the oral
tongue is associated with down-regulation of epidermal fatty acid
binding protein (E-FABP). Oral Oncol. 43:27–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hashimoto T, Kusakabe T, Sugino T, Fukuda
T, Watanabe K, Sato Y, Nashimoto A, Honma K, Kimura H, Fujii H and
Suzuki T: Expression of heart-type fatty acid-binding protein in
human gastric carcinoma and its association with tumor
aggressiveness, metastasis and poor prognosis. Pathobiology.
71:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Millis GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Celis JE, Ostergaard M, Basse B, Celis A,
Lauridsen JB, Ratz GP, Andersen I, Hein B, Wolf H, Orntoft TF and
Rasmussen HH: Loss of adipocyte-type fatty acid binding protein and
other protein biomarkers is associated with progression of human
bladder transitional cell carcinomas. Cancer Res. 56:4782–4790.
1996.
|
|
17.
|
Boiteux G, Lascombe I, Roche E,
Plissonnier ML, Clairotte A, Bittard H and Fauconnet S: A-FABP, a
candidate progression marker of human transitional cell carcinoma
of the bladder, is differentially regulated by PPAR in urothelial
cancer cells. Int J Cancer. 124:1820–1828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hammamieh R, Chakraborty N, Barmada M, Das
R and Jett M: Expression patterns of fatty acid binding proteins in
breast cancer cells. J Exp Ther Oncol. 5:133–143. 2005.PubMed/NCBI
|
|
19.
|
Okuse T, Chiba T, Katsuumi I and Imai K:
Differential expression and localization of WNTs in an animal model
of skin wound healing. Wound Repair Regen. 13:491–497. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
McCluggage WG, Connolly LE, McBride HA,
Kalloger S and Gilks CB: HMGA2 is commonly expressed in uterine
serous carcinomas and is a useful adjunct to diagnosis.
Histopathology. 60:547–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Masouye I, Hagens G, Van Kuppevelt TH,
Madsen P, Saurat JH, Veerkamp JH, Pepper MS and Siegenthaler G:
Endothelial cells of the human microvasculature express epidermal
fatty acid-binding protein. Circ Res. 81:297–303. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kusakari Y, Ogawa E, Owada Y, Kitanaka N,
Watanabe H, Kimura M, Tagami H, Kondo H, Aiba S and Okuyama R:
Decreased keratinocyte motility in skin wound on mice lacking the
epidermal fatty acid binding protein gene. Mol Cell Biochem.
284:183–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Han J, Kioi M, Chu WS, Kasperbauer JL,
Strome SE and Puri RK: Identification of potential therapeutic
targets in human head and neck squamous cell carcinoma. Head Neck
Oncol. 1:272009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chi C and Trinkaus-Randall V: New insights
in wound response and repair of epithelium. J Cell Physiol.
228:925–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ogawa E, Owada Y, Ikawa S, Adachi Y, Egawa
T, Nemoto K, Suzuki K, Hishinuma T, Kawashima H, Kondo H, et al:
Epidermal FABP (FABP5) regulates keratinocyte differentiation by
13(S)-HODE-mediated activation of the NF-κB signaling pathway. J
Invest Dermatol. 131:604–612. 2011.PubMed/NCBI
|
|
28.
|
Hagens G, Masouye I, Augsburger E, Hotz R,
Saurat JH and Siegenthaler G: Calcium-binding protein S100A7 and
epidermal-type fatty acid-binding protein are associated in the
cytosol of human keratinocytes. Biochem J. 339:419–427. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Dallaglio K, Marconi A, Truzzi F, Lotti R,
Palazzo E, Petrachi T, Saltari A, Coppini M and Pincelli C: E-FABP
induces differentiation in normal human keratinocytes and modulates
the differentiation process in psoriatic keratinocytes in vitro.
Exp Dermatol. 22:255–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Gandarillas A: The mysterious human
epidermal cell cycle, or an oncogene-induced differentiation
checkpoint. Cell Cycle. 11:4507–4516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ashida S, Orloff MS, Bebek G, Zhang L,
Zheng P, Peehl CM and Eng C: Integrated analysis reveals critical
genomic regions in prostate tumor microenvironment associated with
clinicopathologic phenotypes. Clin Cancer Res. 18:1578–1587. 2012.
View Article : Google Scholar
|
|
32.
|
Shirakata Y, Kimura R, Nanba D, Iwamoto R,
Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E,
et al: Heparin-binding EGF-like growth factor accelerates
keratinocyte migration and skin wound healing. J Cell Sci.
118:2363–2370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kannan-Thulasiraman P, Seachrist DD,
Mahabeleshwar GH, Jain MK and Noy N: Fatty acid-binding protein 5
and PPAR-β/δ are critical mediators of epidermal growth factor
receptor-induced carcinoma cell growth. J Biol Chem.
285:19106–19115. 2010.
|
|
34.
|
Uraguchi M, Morikawa M, Shirakawa M,
Sanada K and Imai K: Activation of WNT family expression and
signaling in squamous cell carcinomas of the oral cavity. J Dent
Res. 83:327–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bielefeld KA, Amini-Nik S and Alman BA:
Cutaneous wound healing: recruiting developmental pathways for
regeneration. Cell Mol Life Sci. 70:2059–2081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Collins CA and Watt FM: Dynamic regulation
of retinoic acid-binding proteins in developing, adult and
neoplastic skin reveals roles for beta-catenin and Notch
signalling. Dev Biol. 324:55–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Knoferle J, Ramljak S, Koch JC, Tonges L,
Asif AR, Michel U, Wouters FS, Heermann S, Krieglstein K, Zerr I,
et al: TGF-beta 1 enhances neurite outgrowth via regulation of
proteasome function and EFABP. Neurobiol Dis. 38:395–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Imai K, Hiramatsu A, Fukushima D,
Pierschbacher PD and Okada Y: Degradation of decorin by matrix
metalloproteinases: identification of the cleavage sites, kinetic
analyses and transforming growth factor-beta1 release. Biochem J.
322:809–814. 1997.PubMed/NCBI
|
|
39.
|
Chiba T, Maeda G, Kawashiri S, Kato K and
Imai K: Epigenetic loss of mucosa-associated lymphoid tissue 1
expression in patients with oral carcinomas. Cancer Res.
69:7216–7223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Ohyama Y, Kawamoto Y, Chiba T, Maeda G,
Sakashita H and Imai K: Inhibition of TGF-β and EGF pathway gene
expression and migration of oral carcinoma cells by
mucosa-associated lymphoid tissue 1. Br J Cancer. 109:207–214.
2013.
|
|
41.
|
Tuncman G, Erbay E, Hom X, De Vivo I,
Campos H, Rimm EB and Hotamisligil GS: A genetic variant at the
fatty acid-binding protein aP2 locus reduces the risk for
hypertriglyceridemia, type 2 diabetes, and cardiovascular disease.
Proc Natl Acad Sci USA. 103:6970–6975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Theocharis S, Klijanienko J, Giaginis C,
Rodriguez J, Jouffroy T, Girod A, Point D, Tsourouflis G and
Satre-Garau X: Peroxisome proliferator-activated receptor-γ in
mobile tongue squamous cell carcinoma: associations with
clinicopathological parameters and patients survival. J Cancer Res
Clin Oncol. 137:251–259. 2011.
|
|
43.
|
Yoshida K, Hirose Y, Tanaka T, Yamada Y,
Kuno T, Kohno H, Katayama H, Qiao Z, Sakata K, Sugie S, et al:
Inhibitory effects of troglitazone, a peroxisome
proliferator-activated receptor γ ligand, in rat tongue
carcinogenesis initiated with 4-nitroquinoline 1-oxide. Cancer Sci.
94:365–371. 2003.
|
|
44.
|
Kawamoto Y, Ohyama Y, Chiba T, Yagishita
H, Sakashita H and Imai K: Proteomic identification of keratin
alterations with enhanced proliferation of oral carcinoma cells by
loos of mucosa-associated lymphoid tissue 1 expression. Int J
Oncol. 43:729–736. 2013.
|