Introduction
Colorectal cancer is the third most common type of
cancer worldwide, with one million new cases diagnosed annually
(1). In Japan, colorectal cancer
is the second most common type of cancer and the third most common
cause of mortality (2).
FOLFOX4, a bi-weekly schedule of intravenous bolus
and infusion of 5-fluorouracil/folinic acid (5-FU/FA) plus
oxaliplatin (Elplat®), is a widely used regimen for the
first-line treatment of metastatic colorectal cancer (mCRC)
(3,4). However, oral fluoropyrimidines can
replace the intravenous fluoropyrimidine component of combination
regimens. Capecitabine (Xeloda®) is an oral
fluoropyrimidine with similar efficacy to bolus 5-FU/FA in the
first-line treatment of mCRC and as adjuvant therapy for stage III
colon cancer (5–7). The efficacy of capecitabine and a
3-week dose of oxaliplatin (XELOX regimen) has also been
demonstrated to be inferior to 5-FU/FA plus oxaliplatin (FOLFOX4 or
FOLFOX6) in the first- and second-line treatment of patients with
mCRC (8–10). The addition of bevacizumab
(Avastin®) to oxaliplatin-based chemotherapy
significantly improved progression-free survival (PFS) by 20% in
the first-line treatment of mCRC (11). XELOX plus bevacizumab is an
effective treatment strategy and has a manageable tolerability
profile when administered to Japanese patients with mCRC (12). In this study, we retrospectively
reviewed cases in which XELOX plus bevacizumab was administered to
evaluate its efficacy and safety in clinical practice.
Patients and methods
Patients
In total, 40 patients, 22 males and 18 females with
a median age of 62.5 years, with mCRC presented at the Fuchu
Hospital. These patients were administered XELOX plus bevacizumab
as a first-line treatment between September, 2009 and April, 2012.
Eligible patients had histologically confirmed mCRC. Other
inclusion criteria were an Eastern Cooperative Oncology Group
(ECOG) performance status of 0–1 and adequate hematological, liver
and renal functions. Other assessments were carried out at the
investigator’s discretion.
Treatment
Oxaliplatin was purchased from Yakult Honsha Co.,
Ltd. (Tokyo, Japan) and capecitabine and bevacizumab were purchased
from Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). XELOX
consisted of a 2-h intravenous infusion of oxaliplatin 130
mg/m2 on day 1 plus oral capecitabine 1,000
mg/m2 twice daily for 2 weeks of a 3-week cycle. The
first dose of capecitabine was administered on the evening of day 1
and the last dose was administered on the morning of day 15.
Bevacizumab at a dose of 7.5 mg/kg was administered as a 30- to
90-min intravenous infusion prior to oxaliplatin on day 1 of the
3-week cycle. Treatment was continued until disease progression,
intolerable adverse events or the withdrawal of consent.
Treatment was interrupted if grade 2–4 adverse
events occurred. No dose modification of bevacizumab was performed.
The dose of capecitabine was adjusted for grade 3 or 4
thrombocytopenia or neutropenia, febrile neutropenia or
non-hematological toxicities of grade ≥2, according to the standard
scheme described in detail by Doi et al (12). The dose of oxaliplatin was reduced
to 100 or 85 mg/m2 when patients experienced grade 3 or
4 thrombocytopenia or neutropenia, febrile neutropenia or a grade 3
non-hematological toxicity, grade 3 neurosensory toxicity lasting
>7 days, or grade 2 neurosensory toxicity persisting between
cycles. If grade 3 neurosensory toxicity persisted between cycles,
oxaliplatin was discontinued. This treatment plan was almost
identical to that of the NO16966 study (11).
If oxaliplatin and/or bevacizumab were discontinued,
treatment with the remaining components were continued, such as
capecitabine with or without bevacizumab subsequent to the
discontinuation of oxaliplatin and XELOX or capecitabine after the
discontinuation of bevacizumab. The continuation of oxaliplatin or
bevacizumab without capecitabine was not permitted.
Evaluation of the methods
Objective tumor responses were evaluated according
to the Response Evaluation Criteria in Solid Tumors version 1.0
(RECIST v 1.0) by each attending phycisian. Adverse events were
assessed according to the Common Terminology Criteria for Adverse
Events version 3.0 (CTCAE v 3.0).
Statistical analysis
Statistical analyses were performed using the
Statcel 2 software program (OMS, Saitama, Japan). The evaluation of
response and progression was based on measurements reported by the
radiologist. Complete and partial response required subsequent
confirmation of response following an interval of at least 4 weeks.
All clinical courses including subsequent chemotherapy were
followed-up until death or last contact.The Kaplan-Meier method was
used to evaluate the median duration of treatment, progression-free
survival, and overall survival (OS). The median duration of
treatment was calculated from the date that treatment was initiated
to the date of disease progression or the cessation of treatment
for any reason, whichever date occurred first. Progression-free
survival was calculated from the date at which the treatement was
started to the earlier date of disease progression or death.
Without contradictory dates, patients who were lost to follow-up
were assumed to have progressed at the last date of confirmation
and to be progression-free. For patients whose treatments were
ceased without progression and who had received subsequent surgery
or an alternative treatment, progression-free survival was defined
as the time from the initiation of treatment to the date of its
cessation. OS was calculated from the date at which treatment was
started to death or that of the last contact. Patients who were
lost to follow-up were assumed to have succumbed to their disease
at the last contact. The cutoff date was April 30, 2013 for
progression-free survival and OS.
Results
Table I shows the
characteristics of the 40 enrolled patients. The median age of the
patients was 65.2 (range, 51–79 years). Of the 40 patients, 22 were
male and 18 were female. The ECOG performance status was 0 in all
40 patients. The most common sites of metastasis were the liver,
lungs, lymph nodes and peritoneum.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients |
|---|
| Age (years) | |
| Median (range) | 65.2 (51–79) |
| Gender (%) | |
| Male | 22 (55) |
| Female | 18 (45) |
| Eastern Cooperative
Oncology | |
| Group performance
status | |
| 0 (%) | 40 (100) |
| Primary tumor site
(%) | |
| Colon | 24 (60) |
| Rectum | 16 (40) |
| Stage at first
diagnosis (%) | |
| Local regional | 24 (60) |
| Metastatic | 16 (40) |
| Site of metastasis
(%) | |
| Liver | 20 (50) |
| Lung | 9 (22.5) |
| Lymph node | 17 (42.5) |
| Peritoneum | 15 (37.5) |
| Local
recurrence | 3 (7.5) |
| Line of treatment
(%) | |
| First | 40 (100) |
| Prior adjuvant
therapy (%) | |
| No | 27 (67.5) |
| Yes | 13 (32.5) |
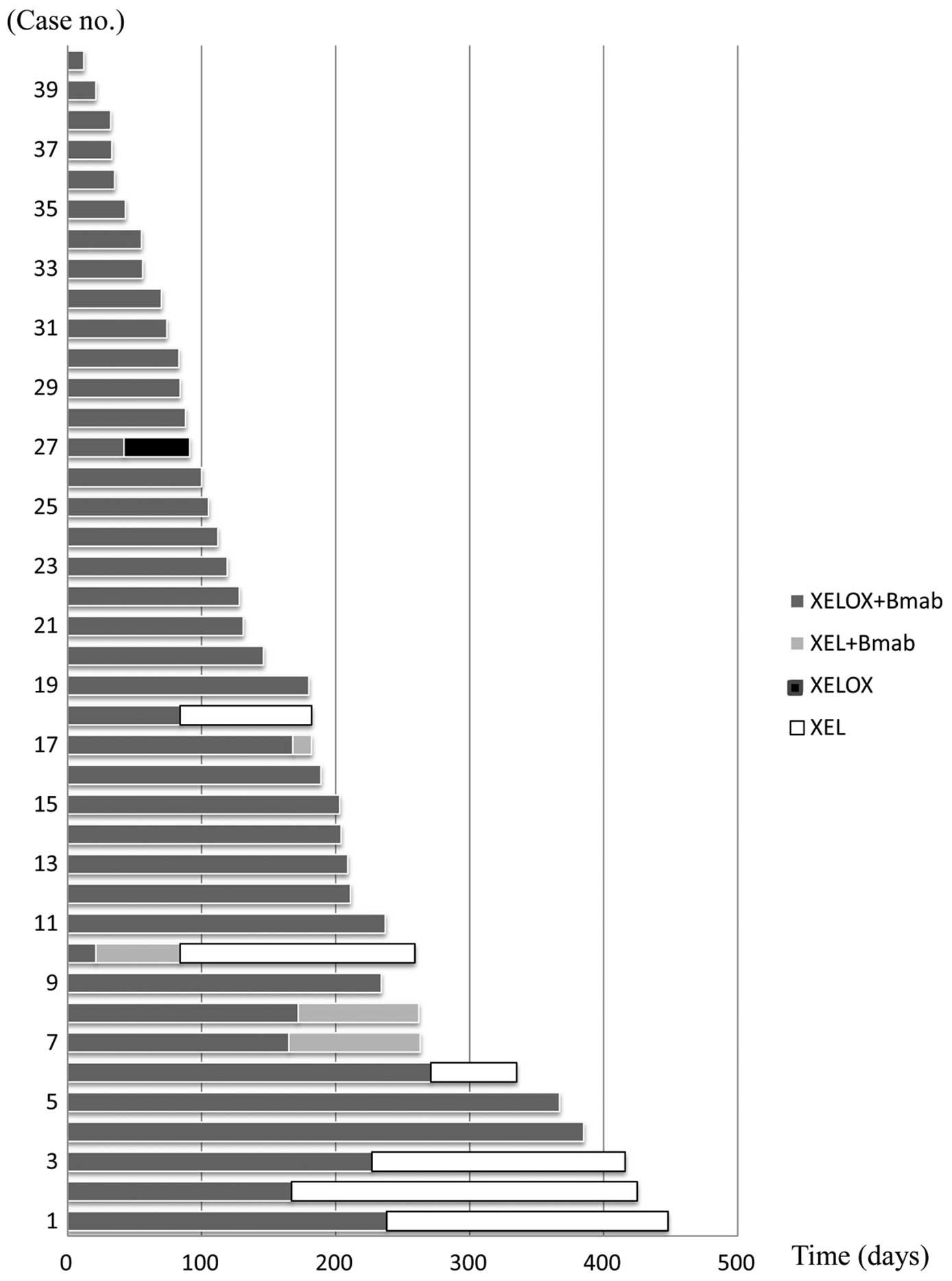
The median duration of treatment was 170.2 (range,
12–448 days) with a median of 8.0 (range, 1–21 treatment cycles).
XELOX plus bevacizumab combination therapy was administered for a
median of 6.5 (range, 1–18 cycles). Following the discontinuation
of oxaliplatin, 4 patients (10%) continued with capecitabine and
bevacizumab combination therapy and received a median of 3.2
(range, 1–5 cycles). Five patients (12.5%) received capecitabine
monotherapy for a median of 7.7 (range, 4–12 cycles). One patient
received XELOX therapy for 2 cycles during the permanent
discontinuation of bevacizumab (Fig.
1).
At the final data cut-off date of August 31, 2012,
the median duration of follow-up was 500.5 days. Seventeen patients
(42.5%) succumbed to disease progression and two patients were
still receiving medication.
The analysis of efficacy is presented in Table II. The median PFS was 290 [95%
confidence interval (CI): 222–409 days] and the median OS was 816
(95% CI: 490 days-not calculated). The response rate (RR; complete
plus partial response) was 67.5% and the disease control rate (RR
plus stable disease) was 90%.
 | Table II.Analysis of efficacy. |
Table II.
Analysis of efficacy.
A, Endpoint
|
| Survival | Days | 95% CI |
|
| Median
progressive-free survival | 290 | 222–409 |
| Median overall
survival | 816 | 490–NC |
|
B, Patient response
|
| Response rate | No. of patients
(%) |
|
| Complete
response | 1 (2.5) |
| Partial response | 26 (65) |
| Stable disease | 9 (22.5) |
| Progressive
disease | 1 (2.5) |
| Not evaluable | 3 (7.5) |
| Complete
response | 1 (2.5) |
Table III shows the
second- and third-line regimens used for patients treated with
bevacizumab in the first-line regimen. Results revealed that 36.8%
of patients who were treated with bevacizumab in the second-line
regimen were administered bevacizumab continuously. A total of
42.1% of patients in the second-line regimen were administered
combination chemotherapy with cetuximab or panitumumab.
 | Table III.The second and third line regimens
used for patients receiving bevacizumab as the first-line
treatment. |
Table III.
The second and third line regimens
used for patients receiving bevacizumab as the first-line
treatment.
| Line of
treatment | Regimen | No. of patients
(%) |
|---|
| Second line | | n=19 |
| Combination with
bevacizumab | 7 (36.8) |
| Chemotherapy
only | 4 (21.1) |
| Combination with
cetuximab/panitumumab | 8 (42.1) |
| Third line | | n=7 |
| Combination with
bevacizumab | 1 (16.7) |
| Chemotherapy
only | 2 (33.3) |
| Combination with
cetuximab/panitumumab | 4 (50) |
The adverse events that occurred in all 40 patients
are provided in Table IV. The most
common adverse events with XELOX plus bevacizumab were neurosensory
toxicity (82.5%), anorexia (50%), hypertension (45%) and a decrease
in the platelet count (40%). The most common grade 3/4 adverse
events were neurosensory toxicity (15%) and fatigue (15%).
Regarding patients receiving XELOX plus bevacizumab, dose
reductions were required for capecitabine in 6 patients (15%) due
to hand-foot syndrome (n=5) and diarrhea (n=1). Capecitabine doses
were reduced to 75% of the starting dose in all 6 patients. Dose
reductions were required for oxaliplatin in 10 patients (25%) due
to neurosensory toxicity and the oxaliplatin dose was reduced to
100 mg/m2 in all of these patients.
 | Table IV.Incidence of common adverse
events. |
Table IV.
Incidence of common adverse
events.
| Adverse event | Grade 1–4
| Grade 3–4
|
|---|
| No. of patients
(%) | No. of patients
(%) |
|---|
| Hypertension | 18 (45) | 0 (0) |
| Neurosensory
toxicity | 33 (82.5) | 6 (15) |
| Anorexia | 20 (50) | 3 (7.5) |
| Fatigue | 12 (30) | 6 (15) |
| Hand-foot
syndrome | 15 (37.5) | 0 (0) |
| Nausea/vomitting | 7 (17.5) | 0 (0) |
| Diarrhea | 2 (5) | 0 (0) |
| Oral ulcer | 7 (17.5) | 0 (0) |
| Allergic
reaction | 2 (5) | 2 (5) |
| Hiccups | 1 (2.5) | 1 (2.5) |
| Neutrophil
count | 12 (30) | 0 (0) |
| decreased | | |
| Platelet count | 16 (40) | 0 (0) |
| decreased | | |
Discussion
Results of this study have demonstrated the safety
and efficacy of XELOX with bevacizumab in combination with
oxaliplatin 130 mg/m2 plus oral capecitabine 1,000
mg/m2 in Japanese patients. Of particular significance
are our novel results demonstrating the safety and efficacy of the
international standard-dose XELOX with bevacizumab in Japanese
patients. No fatal adverse events occurred and any complications
arising were managed successfully using appropriate support care
and drug cessation/dose reductions.
The results of randomized controlled trials in
patients with advanced colorectal cancer demonstrated that the
median OS was 16–23 months in patients who received bevacizumab
with fluoropyrimidine-based chemotherapy, including 5-FU/FA,
irinotecan plus 5-FU/leucovorin (IFL), 5-FU/IFL plus oxaliplatin
(FOLFOX) and capecitabine plus oxaliplatin (XELOX), as first-line
chemotherapy (11,13,14).
Previous randomized or observation trials that
included the XELOX plus bevacizumab regimen as first-line therapy
have been conducted mainly in North America and Europe (11,15,16).
The NO16966 study (10,15) demonstrated a longer PFS and OS in
the XELOX plus bevacizumab arm than that with the XELOX plus
placebo arm in the subgroup analysis, which reported a median PFS
of 9.3 vs. 7.4 months, hazard ratio (HR)=0.77 (95% CI: 0.63–0.94,
P=0.0026) and a median OS of 21.6 vs. 18.8 months (HR was not
shown) (15). Furthermore, another
phase III trial (CAIRO2) reported a RR of 50.0%, a median PFS of
10.7 months and a median OS of 20.3 months in the XELOX plus
bevacizumab arm (16). A Japanese
clinical trial of XELOX plus bevacizumab in patients with mCRC
reported that the median OS was 27.4 months and that the median
progression-survival was 11.0 months (12). In this study, the median OS was 816
days, the median PFS was 290 days and the RR was 67.5%. These
efficacy results are similar to those obtained in first-line
therapy with XELOX plus bevacizumab.
The safety profile observed in the present study was
similar to that observed in previous clinical trials with Western
patients, including the NO16966 study (11,16,17).
Notably, the incidence of grade 3/4 diarrhea and nausea/vomiting
was 0%, which is markedly lower than that reported with XELOX plus
bevacizumab in previous phase II and III studies (19–21%) (11,16,17).
A lower incidence of diarrhea and nausea/vomiting has been reported
in other studies of Japanese or Asian patients treated with
fluoropyrimidine-based chemotherapy (18,19).
The reason for this regional variation remains unclear; however, it
is hypothesized that differences in dietary folate intake is a
potential explanation (20). The
incidence of all grades of hand-foot syndrome (37.5%) was similar
to that in the XELOX plus bevacizumab arm of the NO16966 study
(39%) (15).
Approximately 90% of patients with severe peripheral
neuropathy reportedly improve 20 weeks following the
discontinuation of oxaliplatin (3). The results of the OPTIMOX-1 study
demonstrated that a ‘stop-and-go’ approach employing an
oxaliplatin-free interval resulted in decreased neurotoxicity, but
did not affect OS in patients receiving FOLFOX as initial therapy
for mCRC (21). The antitumor
effects of capecitabine used alone on mCRC have been reported to be
similar to those of intravenous 5-FU/IFL therapy (22). Capecitabine and bevacizumab therapy
showed a RR of 34% and median PFS and OS were 10.8 and 18 months,
respectively (23). Regarding peripheral neuropathy, the overall
incidence of peripheral neuropathy in our study (82.5%) was similar
to that in the XELOX plus bevacizumab arm of previous studies
(84–93%) (12,15). In the present study, following the
discontinuation of oxaliplatin, 4 patients (10%) continued with
capecitabine and bevacizumab combination therapy and received a
median of 3.2 (range, 1–5 cycles). Five patients (12.5%) received
capecitabine monotherapy for a median of 7.7 (range, 4–12 cycles).
One patient received XELOX therapy for 2 cycles during the
permanent discontinuation of bevacizumab. Peripheral neuropathy
disappeared and successful tumor control was achieved (partial
response continued).
In conclusion, findings of this study have
demonstrated that the survival benefit of XELOX plus bevacizumab in
Japanese patients with mCRC was similar to that observed in
previous clinical trials from Western countries. Therefore, XELOX
plus bevacizumab may be considered a routine first-line treatment
option for patients with mCRC. Additionally, the combination of
capecitabine and bevacizumab was found to be safe with an
acceptable toxicity profile, and induced a significant rate of
disease control.
References
|
1.
|
Parkin DM, Bray F, Ferlay J, et al: Global
cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Kotake K, Honjo S, Sugihara K, et al:
Changes in colorectal cancer during a 20-year period: an extended
report from the multi-institutional registry of large bowel cancer,
Japan. Dis Colon Rectum. 46(Suppl 10): S32–S43. 2003.PubMed/NCBI
|
|
3.
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
4.
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
5.
|
Hoff PM, Ansari R, Batist G, et al:
Comparison of oral capecitabine versus intravenous fluorouracil
plus leucovorin as first-line treatment in 605 patients with
metastatic colorectal cancer: results of a randomized phase III
study. J Clin Oncol. 19:2282–2292. 2001.
|
|
6.
|
Van Cutsem E, Twelves C, Cassidy J, et al:
Oral capecitabine compared with intravenous fluorouracil plus
leucovorin in patients with metastatic colorectal cancer: results
of a large phase III study. J Clin Oncol. 19:4097–4106. 2001.
|
|
7.
|
Twelves C, Wong A, Nowacki MP, et al:
Capecitabine as adjuvant treatment for stage III colon cancer. N
Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cassidy J, Clarke S, Diaz-Rubio E, et al:
Randomized phase III study of capecitabine plus oxaliplatin
compared with fluorouracil/folinic acid plus oxaliplatin as
first-line therapy for metastatic colorectal cancer. J Clin Oncol.
26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ducreux M, Bennouna J, Hebbar M, et al:
Capecitabine plus oxaliplatin (XELOX) versus
5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line
treatment for metastaic colorectal cancer. Int J Cancer.
128:682–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Rothenberg ML, Cox JV, Butts C, et al:
Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic
acid plus oxaliplatin (FOLFOX-4) as second-line therapy in
metastatic colorectal cancer: a randomized phase III noninferiority
study. Ann Oncol. 19:1720–1726. 2008. View Article : Google Scholar
|
|
11.
|
Saltz LB, Clarke S, Diaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Doi T, Boku N, Kato K, et al: Phase I/II
study of capecitabine plus oxaliplatin (XELOX) plus bevacizumab as
first-line therapy in Japanese patients with metastatic colorectal
cancer. Jpn J Clin Oncol. 40:913–920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kabbinavar FF, Schulz J, McCleod M, et al:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cassidy J, Clarke S, Diaz-Rubio E, et al:
XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal
cancer: NO16966 updated results. Br J Cancer. 105:58–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tol J, Koopman M, Cats A, et al:
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal
cancer. N Engl J Med. 360:563–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hochster HS, Hart LL, Ramanathan RK, et
al: Safety and efficacy of oxaliplatin and fluoropyrimidine
regimens with or without bevacizumab as first-line treatment of
metastatic colorectal cancer: results of the TREE Study. J Clin
Oncol. 26:3523–3539. 2008. View Article : Google Scholar
|
|
18.
|
Hyodo I, Shirao K, Doi T, et al: A phase
II study of the global dose and schedule of capecitabine in
Japanese patients with metastatic colorectal cancer. Jpn J Clin
Oncol. 36:410–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yoshino T, Boku N, Onozawa Y, et al:
Efficacy and safety of an irinotecan plus bolus 5-fluorouracil and
L-leucovorin regimen for metastatic colorectal cancer in Japanese
patients: experience in a single institution in Japan. Jpn J Clin
Oncol. 37:686–691. 2007. View Article : Google Scholar
|
|
20.
|
Haller DG, Cassidy J, Clarke SJ, et al:
Potential regional differences for the tolerability profiles of
fluoropyrimidines. J Clin Oncol. 26:2118–2123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tournigand C, Cervantes A, Figer A, et al:
OPTIMOX-1: a randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-go fashion in advanced colorectal cancer
- a GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Feliu J, Safont MJ, Salud A, et al:
Capecitabine and bevacizumab as first-line treatment in elderly
patients with metastatic colorectal cancer. Br J Cancer.
102:1468–1473. 2010. View Article : Google Scholar : PubMed/NCBI
|