Introduction
Primary melanoma originating in the small intestine
is extremely rare. It is commonly believed that the vast majority
of cases are metastatic, originating from an occult primary
cutaneous or ocular lesion. The diagnostic criteria for primary
intestinal malignant melanoma have been determined. However, there
is significant dispute over the treatment of primary intestinal
malignant melanoma, since this type of tumor is associated with a
very poor prognosis. There are usually no clinical manifestations
in the early stages of this tumor; therefore, diagnosis is often
delayed until the emergence of complications (1). This is the case report of a primary
small intestinal malignant melanoma, complicated by intestinal
obstruction, in a patient with a history of rectal cancer
resection. Furthermore, the relevant literature was reviewed in
order to improve our understanding of primary intestinal malignant
melanoma.
Case report
A 65-year-old man was admitted to the Emergency
Department of a local hospital, complaining of persistent abdominal
pain and obstipation. Abdominal computed tomography (CT) revealed
incomplete intestinal obstruction and the patient underwent
fasting, gastrointestinal decompression, parenteral nutrition and
inhibition of gastric acid secretion. However, there was no
significant improvement in the symptoms.
Since the administered treatment was ineffective,
the patient was admitted to our hospital. We found that the patient
had undergone Miles’ colorectal cancer resection 2 years earlier,
followed by adjuvant chemotherapy (FOLFOX4) and regular inspection.
However, there were no signs of recurrence and/or metastasis.
Significant findings were limited to the abdomen, which was mildly
tender to palpation in the periumbilical region. There was no
tenderness or rebound phenomenon, the bowel sounds were hyperactive
and there was no palpable mass. The rectal examination was normal.
The admission laboratory values were normal, except for the low
levels of albumin and potassium ion concentration. Following
admission, the re-examination of the abdominal plain film indicated
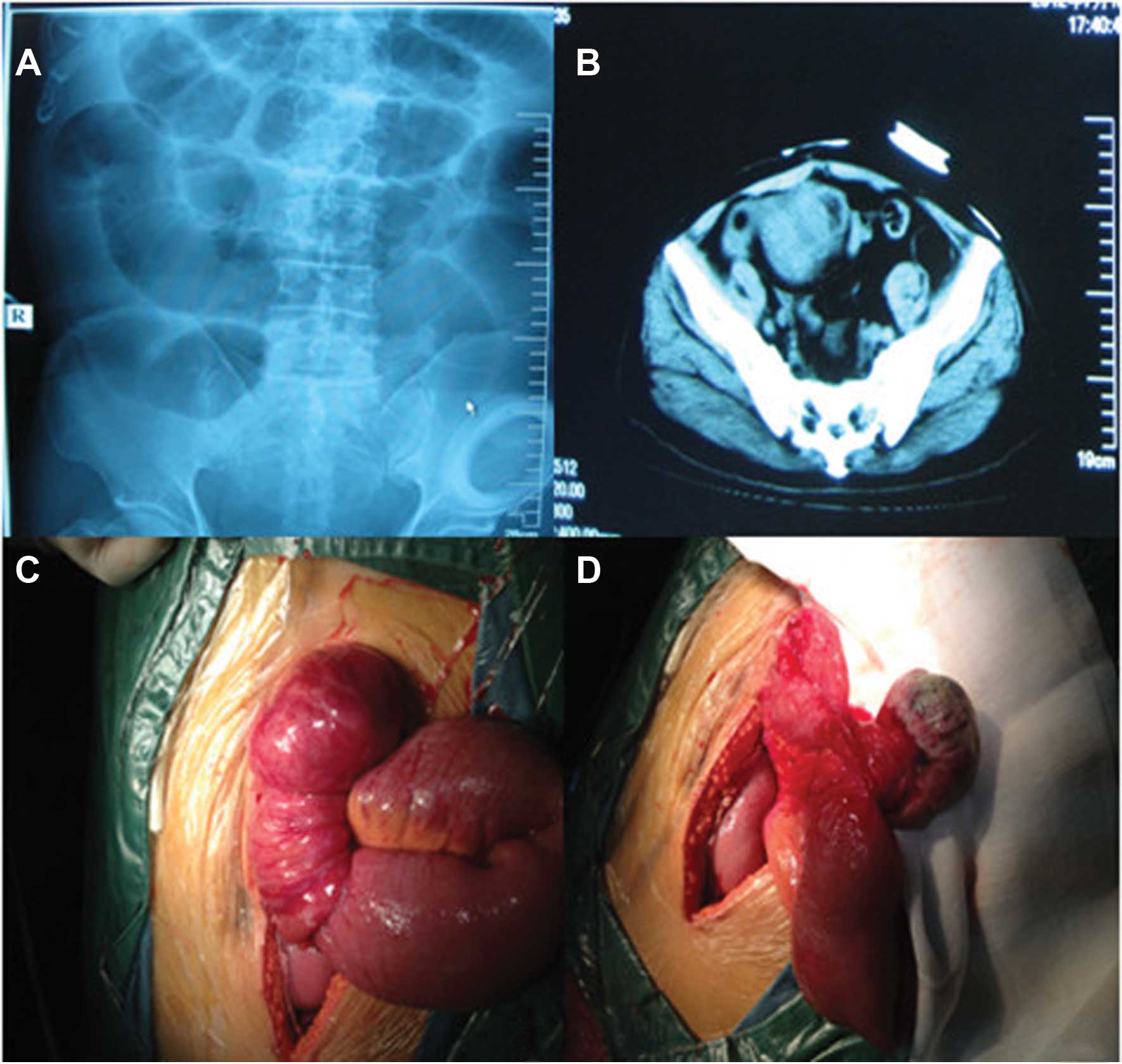
complete small intestinal obstruction (Fig. 1A) and re-examination of the
abdominal CT revealed small intestinal obstruction, expansion and
pneumatosis, absence of the rectum and presence of a pelvic mass
(Fig. 1B).
Abdominal ultrasonography revealed a distension of
the intestinal loops, without additional signs of parenchymatous
organ pathology. In order to exclude the recurrence of colon cancer
a colonoscopy was performed and the findings were normal. Head and
chest CT scan revealed no pathological changes. Therefore, an
urgent surgical exploration was performed.
During laparotomy, a 6-cm mass was identified 15 cm
from the distal end of the ileum. There was no other evidence of
abdominal metastases and the liver was normal to palpation. The
mass was pedunculated, leading to intestinal canal invagination and
intestinal obstruction. The intestinal obstruction was relieved by
surgical resection of the mass, including a segment of the ileum
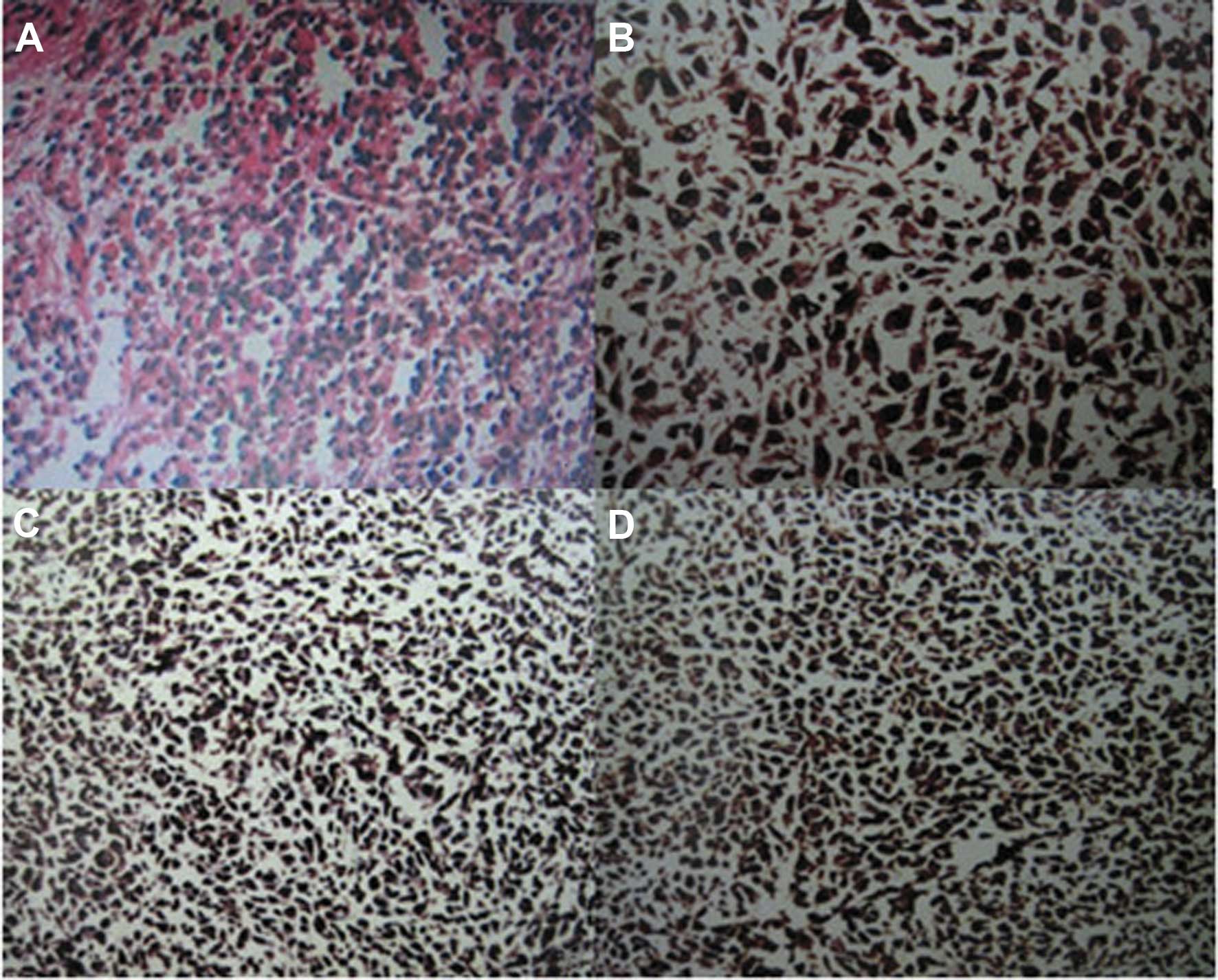
and the corresponding mesenterium (Fig. 1C and D). The pathological
examination of the resected specimen confirmed the diagnosis of a
small intestinal malignant melanoma, with invasion of the small
intestinal muscle layer and absence of metastasis to the mesenteric
lymph nodes (Fig. 2A). The
immunohistochemical invetigation confirmed that the tumor cells
were positive for S-100, anti-melanoma antibody (HMB-45) and
melanocyte/melanoma tumor antigen (Melan-A) (Fig. 2B–D). The postoperative course was
uneventful and the patient was discharged after 10 days. He was
administered adjuvant chemotherapy and high-dose interferon (IFN)
therapy. At the 3-month, 6-month and 1-year follow-up, the patient
remained alive, with no signs of skin melanoma and/or tumor
recurrence.
Discussion
Primary small intestinal melanoma is extremely rare.
In a large series investigating 84,836 cases of malignant melanoma,
only 1.3% originated from the gastrointestinal mucosa and the
relevant literature is sparse (2).
Malignant melanoma mainly occurs in the skin, eye, vulva,
occasionally in the rectum and anus, the genital and upper
gastrointestinal tracts, paranasal sinuses and the parotid
gland.
It has been demonstrated (3) that cutaneous malignant melanoma
originates from epidermal melanocytes or dermal nevus cells, both
of which are derived from the neural crest. Race, genetic factors,
trauma, irritation, viral infections, sun exposure and immune
disturbances may contribute to its progression. The origin of the
mucous membrane malignant melanoma has not been elucidated. It was
previously reported that the reason for the occurrence of malignant
melanoma in non-exposed areas may be the effect of the sunlight on
the skin, leading to the release of a substance into the
circulation, thus affecting the melanocytes of the non-exposed skin
areas or the mucous membranes (3).
It was also hypothesized that radiation and growth factors lead to
melanocyte hyperplasia and the subsequent development of malignant
melanoma (4). The etiology of
primary intestinal melanomas has not been determined. The proposed
primary sources are the melanoblasts of the neural crest or the
amine precursor uptake and decarboxylation (APUD) cells, which
migrate to the ileum through the omphalomesenteric canal and
undergo neoplastic transformation in non-cutaneous sites (5). However, this theory may explain
rectal melanoma (6). Malignant
melanoma usually metastasizes via hematogenous and lymphatic routes
to the liver, lung, bone and brain. Malignant melanomas of the
digestive system are usually metastatic lesions. In one case
series, melanoma commonly metastasized to the liver (68%), small
intestine (58%), colon (22%) and stomach (20%) (7). Small intestinal malignant melanoma is
usually asymptomatic. Tumor growth is often accompanied by
gastrointestinal tract hemorrhage, intussusception, obstruction,
abdominal pain, nausea, vomiting and weight loss. The time between
primary melanoma diagnosis and the development of digestive system
metastases varies between 2 and 180 months (8). In the case of small intestinal
malignant melanoma, surgical resection is the first-line treatment
option, due to acceptable mortality and morbidity rates and the
beneficial effect over the course of the disease (9–11).
In this case, the patient had a history of rectal
cancer resection, leading to the preoperative suspicion of rectal
cancer recurrence as the cause of intestinal obstruction. During
laparotomy, a small, pedunculated, highly mobile intestinal tumor
was identified. Intussusception was the result of the movement of
the tumor after reaching a certain size. The patient was eventually
diagnosed with primary small intestinal malignant melanoma. We
hypothesize that the immunosuppressive state associated with
colorectal cancer may have induced the development of the primary
small intestinal malignant melanoma postoperatively. However, there
is not enough evidence to support this hypothesis (12). To determine whether the small
intestinal malignant melanoma was a primary lesion, Sachs et
al (13) established three
diagnostic criteria: i) single lesion; ii) other organs free of
primary lesions and absence of enlargement of the draining lymph
nodes; and iii) survival time >1 year after diagnosis. In the
present case, the patient was eventually diagnosed with intestinal
obstruction caused by a tumor, according to the preoperative
systemic CT and abdominal plain film, with no detection of
metastatic lesions in other viscera. The postoperative pathological
examination confirmed the diagnosis of primary small intestinal
melanoma and there was no association between the colorectal cancer
history and intestinal obstruction. The pathological examination
also confirmed that there was no metastasis to the draining
regional lymph nodes. The patient’s skin, mucous membranes and eyes
were thoroughly examined postoperatively and no pathological
changes were identified. Furthermore, the survival period exceeded
1 year. Therefore, the patient was diagnosed with primary small
intestinal malignant melanoma.
Melanomas arising on mucosal surfaces appear to be
more aggressive and are associated with worse prognosis compared to
cutaneous melanomas. The poorer prognosis may be associated with
the delay in diagnosis, more aggressive behaviour, or earlier
dissemination due to the rich lymphatic and vascular supply of the
gastrointestinal mucosa (14). For
isolated intestinal malignant melanoma, surgery may prolong
survival (15). Previous studies
demonstrated that postoperative adjuvant chemotherapy and IFN
treatment may be beneficial for the patients, although their role
is limited (16–18). Therefore, early diagnosis and
surgical resection of the primary small intestinal malignant
melanoma is crucial in improving the prognosis.
In conclusion, the patient presented with intestinal
obstruction after undergoing resection of colorectal cancer and it
was first considered that the reason was tumor recurrence.
Therefore, a full preoperative evaluation was performed, which
enabled timely diagnosis and treatment. Surgical resection and
postoperative adjuvant therapy may significantly improve the
prognosis of patients with primary small intestinal malignant
melanoma. Therefore, a full preoperative assessment of cancer
patients is recommended, possibly including positron emission
tomography/CT, to ensure optimal surgical planning.
References
|
1
|
Jorge E, Harvey HA, Simmonds MA, Lipton A
and Jaehl RJ: Symptomatic malignant melanoma of the
gastrointestinal tract. Operative treatment and survival. Ann Surg.
199:328–331. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang AE, Karnell LH and Menck HR: The
National Cancer Data Base report on cutaneous and noncutaneous
melanoma: a summary of 84,836 cases from the past decade. The
American College of Surgeons Commission on Cancer and the American
Cancer Society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takubo K, Kanda Y, Ishii M, et al: Primary
malignant melanoma of the esophagus. Hum Pathol. 14:727–730. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capizzi PJ and Donohue JH: Metastatic
melanoma of the gastrointestinal tract: a review of the literature.
Compr Ther. 20:20–23. 1994.PubMed/NCBI
|
|
5
|
Amar A, Jougon J, Edouard A, Laban P,
Marry JP and Hillion G: Primary malignant melanoma of the small
intestine. Gastroenterol Clin Biol. 16:365–367. 1992.(In
French).
|
|
6
|
Clemmensen OJ and Fenger C: Melanocytes in
the anal canal epithelium. Histopathology. 18:237–241. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klausner JM, Skornick Y, Lelcuk S, Baratz
M and Merhav A: Acute complications of metastatic melanoma to the
gastrointestinal tract. Br J Surg. 69:195–196. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arneja JS and Gosain AK: Giant congenital
melanocytic nevi. Plast Reconstr Surg. 120:26e–40e. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atmatzidis KS, Pavlidis TE, Papaziogas BT
and Papaziogas TB: Primary malignant melanoma of the small
intestine: report of a case. Surg Today. 32:831–833. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Branum GD and Seigler HF: Role of surgical
intervention in the management of intestinal metastases from
malignant melanoma. Am J Surg. 162:428–431. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Butte JM, Meneses M, Waugh E, et al: Ileal
intussusception secondary to small bowel metastases from melanoma.
Am J Surg. 198:e1–2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oosterling SJ, van der Bij GJ, Mels AK,
Beelen RH, Meijer S, van Egmond M and van Leeuwen PA: Perioperative
IFN-alpha to avoid surgically induced immune suppression in
colorectal cancer patients. Histol Histopathol. 21:753–760.
2006.PubMed/NCBI
|
|
13
|
Sachs DL, Lowe L, Chang AE, et al: Do
primary small intestinal melanomas exist? Report of a case. J Am
Acad Dermatol. 41:1042–1044. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lagoudianakis EE, Genetzakis M, Tsekouras
DK, Papadima A, Kafiri G, Toutouzas K, Katergiannakis V and
Manouras A: Primary gastric melanoma: a case report. World J
Gastroenterol. 12:4425–4427. 2006.
|
|
15
|
Sanki A, Scolyer RA and Thompson JF:
Surgery for melanoma metastases of the gastrointestinal tract:
indications and results. Eur J Surg Oncol. 35:313–319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ollila DW, Essner R, Wanek LA and Morton
DL: Surgical resection for melanoma metastatic to the
gastrointestinal tract. Arch Surg. 131:975–980. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kadivar TF, Vanek VW and Krishnan EU:
Primary malignant melanoma of the small bowel: a case study. Am
Surg. 58:418–422. 1992.PubMed/NCBI
|
|
18
|
Letovanec I, Vionnet M and Bouzourene H:
Primary appendiceal melanoma: fiction or reality? Hum Pathol.
35:627–629. 2004. View Article : Google Scholar : PubMed/NCBI
|