Introduction
Lung cancer remains the most common cause of
cancer-related mortality worldwide. Non-small-cell lung carcinoma
(NSCLC) accounts for ~85% of lung cancers and the incidence of
adenocarcinomas has recently increased (1,2).
Lung adenocarcinoma has been found to harbor several kinds of
driver mutations and mutational analyses are required for the
development of novel targeted chemotherapies, particularly in
unresectable or recurrent lung adenocarcinoma. Epidermal growth
factor receptor (EGFR) and Kirsten rat sarcoma 2 viral
oncogene homolog (KRAS) are two proto-oncogenes that are
frequently mutated in primary lung adenocarcinoma and the
prognostic effect of their mutation status in advanced lung
adenocarcinoma has been widely investigated (3–5).
The post-recurrence survival of surgically resected
NSCLC patients was previously reported (6–10).
However, those studies investigated NSCLC patient cohorts, whereas
the number of available studies on adenocarcinoma patients is
limited (5,11–14).
Furthermore, there have been no studies on the effect of
EGFR and KRAS mutations on postrecurrence survival
following surgical resection, or the association of driver
mutations with relapse sites in patients with recurrent lung
adenocarcinoma.
The aim of this retrospective study was to
investigate the prognosis of lung adenocarcinoma patients following
postoperative recurrence, according to EGFR and KRAS
mutation status and the relapse site.
Patients and methods
Patient selection and follow-up
Between July, 2002 and December, 2011, a total of
297 consecutive patients underwent complete surgical resectioning
of primary pulmonary adenocarcinomas at the Department of Thoracic
and Visceral Organ Surgery, Gunma University Graduate School of
Medicine, Gunma, Japan. Two patients with resected stage IV disease
were also included: one with a single brain metastasis treated with
CyberKnife therapy and one with a single adrenal metastasis
resected simultaneously with the primary lesion. Following surgical
resection, a portion of each sample was immediately frozen and
stored at −80ºC until DNA extraction. All patients provided
Institutional Review Board-approved informed consent. Of the 297
patients, 58 (18.7%) developed recurrence and were retrospectively
reviewed in this study. Adjuvant chemotherapy was administered to
cases with pathological stages >IB, according to postoperative
performance status and age. Whenever possible, platinum-based
chemotherapy was administered to stage II or III cases.
The patients were followed up at 3-month intervals
for the first 2 years and at 6-month intervals thereafter, on an
outpatient basis. The follow-up evaluation included a physical
examination, chest radiography and blood analysis, including
analysis of pertinent tumor markers. Computed tomography (CT) scans
of the chest and abdomen or positron emission tomography and CT
(PET-CT) were performed annually. Whenever symptoms or signs of
recurrence were detected, further evaluations, including PET-CT,
brain magnetic resonance imaging and bone scintigraphy, were
performed. Recurrence was diagnosed on the basis of compatible
physical examination and diagnostic imaging findings and the
diagnosis was histologically confirmed when clinically feasible.
Local recurrence was defined as tumor reappearance at a local site,
including regional hilar and mediastinal lymph nodes, surgical
margins and ipsilateral hemithorax. Distant recurrence was defined
as tumor recurrence in the lung or outside the hemithorax.
DNA extraction and mutation analysis
All the surgical specimens were fixed with 10%
formalin and embedded in paraffin. Representative sections were
stained with hematoxylin and eosin and were reviewed by an
experienced pathologist. Genomic DNA was extracted from a 3–5 mm
cube of tumor tissue using a DNA Mini kit (Qiagen, Hilden, Germany)
and subsequently diluted to a concentration of 20 ng/μl.
KRAS and EGFR mutations in lung cancer tissue were
analyzed by peptide nucleic acid-enriched sequencing, as previously
described (15–17).
Statistical analysis
The patients were divided into three groups: those
with EGFR mutations (EGFR mutant), those with
KRAS mutations (KRAS mutant) and those negative for
those types of mutation (wild-type). The correlations between the
groups were evaluated using the Chi-square or Fisher’s exact tests,
as appropriate. The means were compared by one-way analysis of
variance. All pairs of groups were compared using the Bonferroni
test. Post-recurrence survival was defined as the time interval
between the date recurrence was confirmed and the date of death
from any cause or the last follow-up appointment. For univariate
analyses, postrecurrence survival rates were estimated by the
Kaplan-Meier method and differences in survival between the
subgroups were compared by the log-rank test. Multivariate analyses
were performed using the Cox proportional hazards model. Forward
and backward stepwise procedures were performed to determine the
combination of prognostic factors.
All reported P-values are two-sided and P<0.05
was considered to indicate a statistically significant difference.
The analyses were performed with SPSS 11.0 software (Dr. SPSS II
for Windows, standard version 11.0; SPSS, Inc., Chicago, IL,
USA).
Results
Patient characteristics and
post-recurrence therapy
The characteristics of the 297 patients according to
postoperative recurrence are listed in Table I. The proportion of patients with
pathologically advanced disease was significantly higher in the
recurrence (+) compared to that in the recurrence (−) group. There
were no significant differences in gender, smoking history, or
driver mutations. In the recurrence (+) group, EGFR
mutations were detected in 26 patients (44.8%). Of the EGFR
mutations detected, the L858R point mutation in exon 21 (observed
in 18 cases) was the most frequent, followed by a deletion in exon
19 (8 cases). No alterations were detected in exons 18 and 20.
KRAS alterations were detected in 11 patients (19.0%) and
all cases were a single amino acid substitution in codon 12.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Recurrence (+) | Recurrence (−) | |
|---|
|
|
| |
|---|
|
Characteristics | (n=58) | % | (n=239) | % | P-value |
|---|
| Age at surgery,
years median (range) | 69 (39–80) | - | 69 (36–87) | - | 0.222a |
| Gender |
| Male | 25 | 43.1 | 112 | 46.9 | 0.661b |
| Female | 33 | 56.9 | 127 | 53.1 | |
| Smoking
history |
| Never | 28 | 48.2 | 123 | 51.5 | 0.770b |
| Ever | 30 | 51.8 | 116 | 48.5 | |
| Pathological
stage |
| I | 21 | 36.2 | 200 | 83.7 | <0.001b |
| II | 9 | 15.5 | 19 | 8.0 | |
| III | 26 | 44.8 | 18 | 7.5 | |
| IV | 2 | 3.5 | 2 | 0.8 | |
| Driver
mutation |
| EGFR
mutant | 26 | 44.8 | 104 | 43.5 | 0.262b |
| Exon 21
L858R | 18 | 31.0 | 46 | 19.2 | |
| Exon 19
deletion | 8 | 13.8 | 57 | 23.9 | |
| Other | 0 | | 1 | 0.4 | |
| KRAS mutant
(codon 12) | 11 | 19.0 | 28 | 11.7 | - |
| Wild-type | 21 | 36.2 | 107 | 44.8 | - |
Table II shows the
characteristics of patients who developed recurrence. Recurrence
was initially detected in local sites in 17 (29.3%), in distant
sites in 25 (43.1%) and in both local and distant sites in 16
patients (27.6%). A total of 19 patients received EGFR-tyrosine
kinase inhibitor (TKI) treatment following recurrence: gefitinib,
15; erlotinib, 2; and both gefitinib and erlotinib, 2 patients.
Platinum-based chemotherapies, with cisplatin or carboplatin, were
administered to 21 patients and non-platinum-based chemotherapies,
such as pemetrexed, S-1, docetaxel, gemcitabine and tegafur-uracil
(UFT), were administered to 5 patients. Metastectomy was performed
in only 2 patients (3.4%).
 | Table IICharacteristics of patients with
recurrence. |
Table II
Characteristics of patients with
recurrence.
| Patients
(n=58) |
|---|
|
|
|---|
|
Characteristics | No. | % |
|---|
| Recurrence
site |
| Local | 17 | 29.3 |
| Distant | 25 | 43.1 |
| Local +
distant | 16 | 27.6 |
| Adjuvant
chemotherapy (+)/(−) | 23/35 | 39.7/60.3 |
|
Platinum-based | 5 | 8.6 |
| S-1 | 11 | 19.0 |
| UFT | 7 | 12.1 |
| Chemotherapy for
recurrence (+)/(−) | 37/21 | 63.8/36.2 |
|
Platinum-based | 21 | 36.2 |
|
Non-platinum-based | 5 | 8.6 |
| EGFR-TKI | 19 | 32.8 |
| Radiation therapy
(+)/(−) | 23/35 | 39.7/60.3 |
| Metastasectomy
(+)/(−) | 2/56 | 3.4/96.6 |
Correlation of EGFR and KRAS mutation
status with characteristics and recurrence sites
The patient characteristics according to mutation
status are presented in Table
III. Gender was the only variable exhibiting a significant
difference according to driver mutations, with female gender being
correlated with EGFR mutations. Other characteristics,
including smoking history, pathological stage, recurrence site and
number of recurrent lesions, were not statistically significant. Of
the 26 EGFR-mutant cases, 12 (46%) received EGFR-TKI
treatment.
 | Table IIIPatient characteristics according to
EGFR and KRAS mutation status. |
Table III
Patient characteristics according to
EGFR and KRAS mutation status.
|
Characteristics | EGFR mutant
(n=26) | KRAS mutant
(n=11) | Wild-type
(n=21) | P-value |
|---|
| Age at recurrence,
years median (range) | 67 (42–77) | 71 (57–82) | 71 (48–80) | 0.124a |
| Gender |
| Male | 6 | 6 | 13 | 0.020b |
| Female | 20 | 5 | 8 | |
| Smoking
history |
| Never | 16 | 3 | 8 | 0.101b |
| Ever | 10 | 8 | 13 | |
| Pathological
stage |
| I/II | 9/5 | 4/1 | 8/3 | 0.970b,c |
| III/IV | 11/1 | 6/0 | 9/1 | |
| Recurrence
site |
| Local | 4 | 5 | 8 | 0.155b |
| Distant | 15 | 2 | 8 | |
| Local +
distant | 7 | 4 | 5 | |
| Number of recurrent
lesions |
| One | 9 | 4 | 8 | 0.970b |
| Multiple | 17 | 7 | 13 | |
| Chemotherapy |
|
Platinum-based | 12 | 3 | 9 | - |
|
Non-platinum-based | 0 | 4 | 1 | |
| EGFR-TKI | 12 | 1 | 6 | |
| Radiation therapy
(+)/(−) | 9/17 | 5/6 | 9/12 | 0.771b |
| Recurrence-free
survival time, months median (range) | 16.4 (0–56.6) | 14.7
(1.0–54.7) | 10.1
(3.3–56.6) | 0.920a |
| 2-year
post-recurrence survival rate, % (median, months) | 81.0 (38.0) | 18.2 (3.3) | 46.5 (23.3) | <0.001d |
A comparison of initial sites of recurrence
according to driver mutation is presented in Table IV. The patients with
EGFR-mutated tumors exhibited significantly more bilateral
or contralateral lung recurrences compared to the other groups
(P=0.023). No differences were observed in first recurrence sites
other than the bilateral or contralateral lung.
 | Table IVComparison of recurrent organs
according to EGFR and KRAS mutation status. |
Table IV
Comparison of recurrent organs
according to EGFR and KRAS mutation status.
| Recurrence
site | EGFR mutant
(n=26) | KRAS mutant
(n=11) | Wild-type
(n=21) | P-valuea |
|---|
| Local | | | | |
| Regional lymph
nodes | | | | |
| + (n=23) | 9 | 4 | 10 | 0.643 |
| − (n=35) | 17 | 7 | 11 | |
| Ipsilateral
hemothorax (effusion or dissemination) | | | | |
| + (n=11) | 3 | 4 | 4 | 0.212 |
| − (n=47) | 23 | 7 | 17 | |
| Distant | | | | |
| Lung | | | | |
| + (n=23) | 14 | 3 | 6 | 0.137 |
| − (n=35) | 12 | 8 | 15 | |
| Ipsilateral | | | | |
| + (n=6) | 2 | 0 | 4 | - |
| − (n=52) | 24 | 11 | 17 | |
|
Bilateral/contralateral | | | | |
| + (n=17) | 12 | 3 | 2 | 0.023 |
| − (n=41) | 14 | 8 | 19 | |
| Brain | | | | |
| + (n=10) | 6 | 1 | 3 | 0.532 |
| − (n=48) | 20 | 10 | 18 | |
| Bone | | | | |
| + (n=12) | 5 | 4 | 3 | 0.332 |
| − (n=46) | 21 | 7 | 18 | |
| Liver | | | | |
| + (n=4) | 3 | 0 | 1 | - |
| − (n=54) | 23 | 11 | 20 | |
Correlation between EGFR and KRAS
mutation status and postrecurrence survival and prognostic factors
for postrecurrence survival
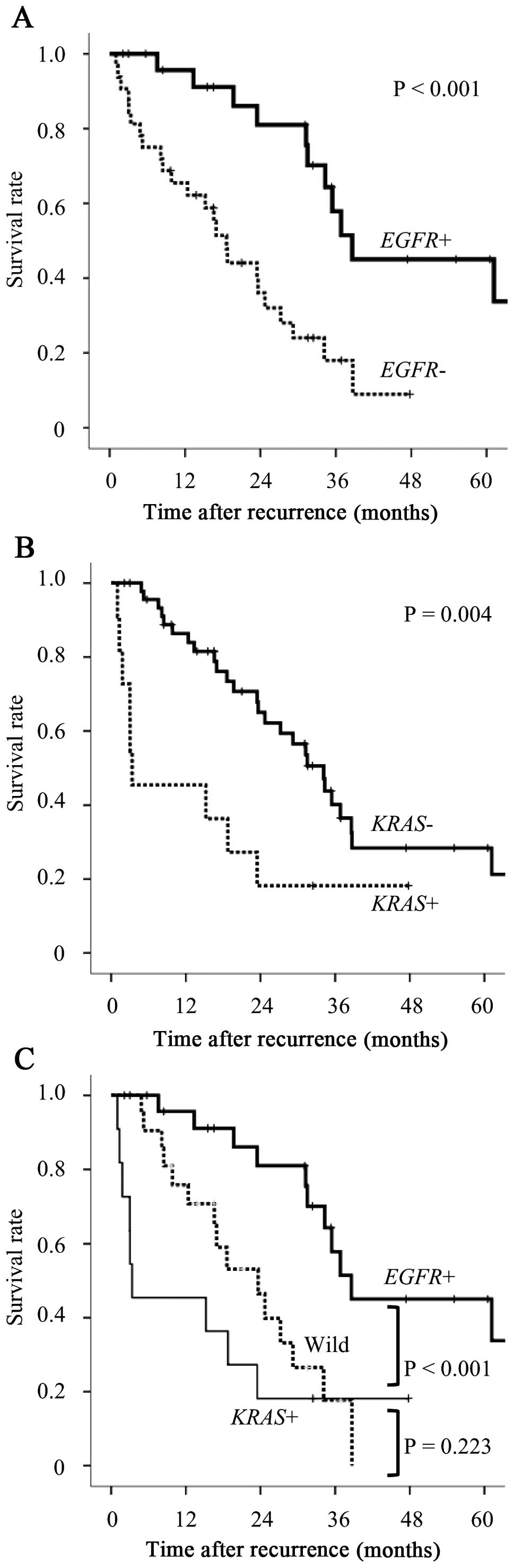
Postrecurrence survival curves according to driver
mutations were drawn by the Kaplan-Meier method (Fig. 1). Statistical significance was
assessed using the log-rank test. The survival of
EGFR-mutated (EGFR+) cases was significantly longer
compared to that of EGFR wild-type (EGFR−) cases
(P<0.001, Fig. 1A). By
contrast, KRAS-mutated (KRAS+) cases exhibited
significantly worse outcomes compared wiht those of KRAS
wild-type (KRAS−) cases (P=0.004, Fig. 1B). The patients with EGFR+
tumors exhibited significantly better outcomes compared to those
with KRAS+ tumors and those with wild-type tumors
(P<0.001, Fig. 1C). The
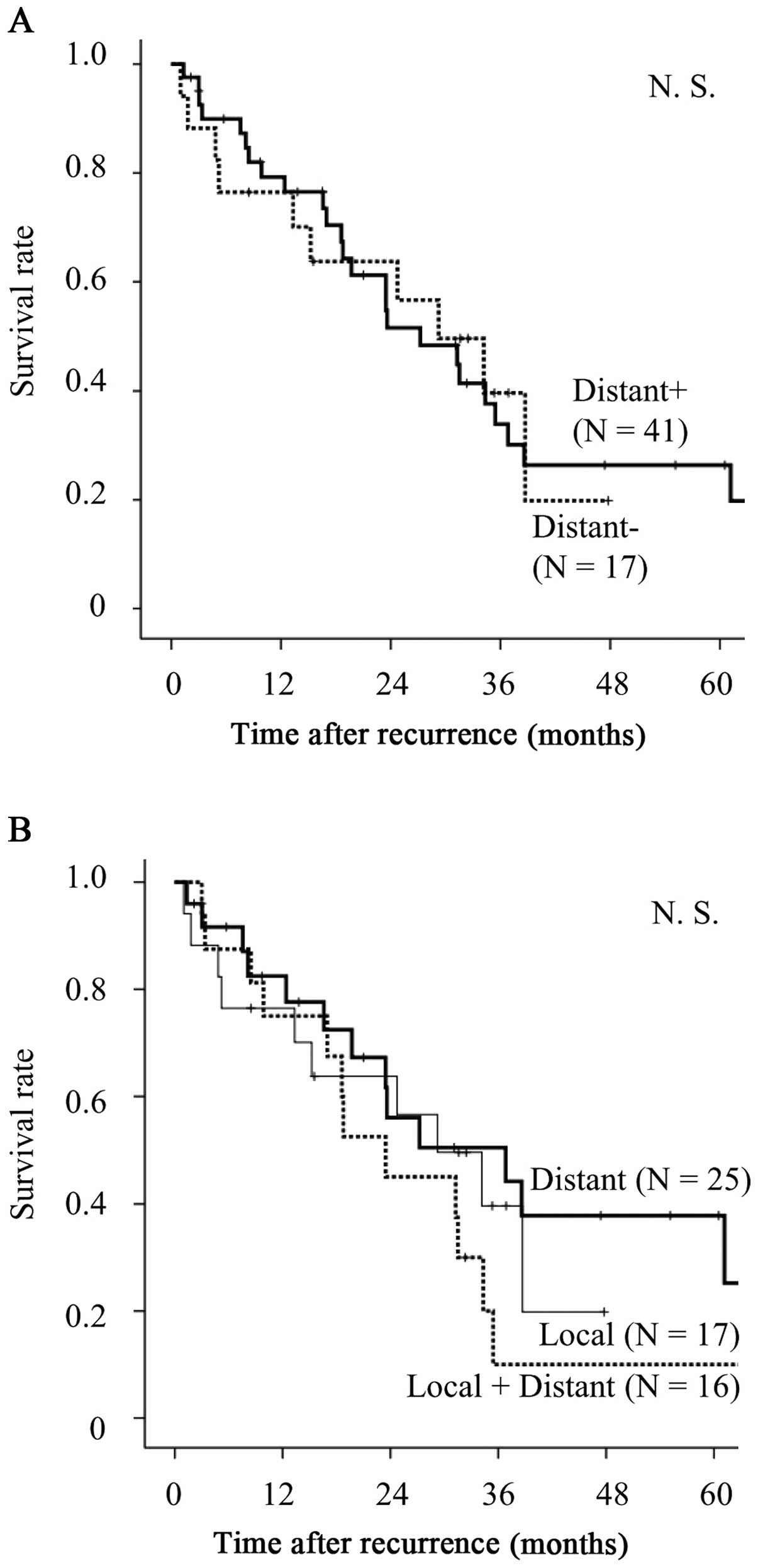
post-recurrence survival curves according to initial recurrence
sites are shown in Fig. 2A and B.
No significant differences were observed in the entire study
cohort, regardless of the presence or absence of distant organ
metastases.
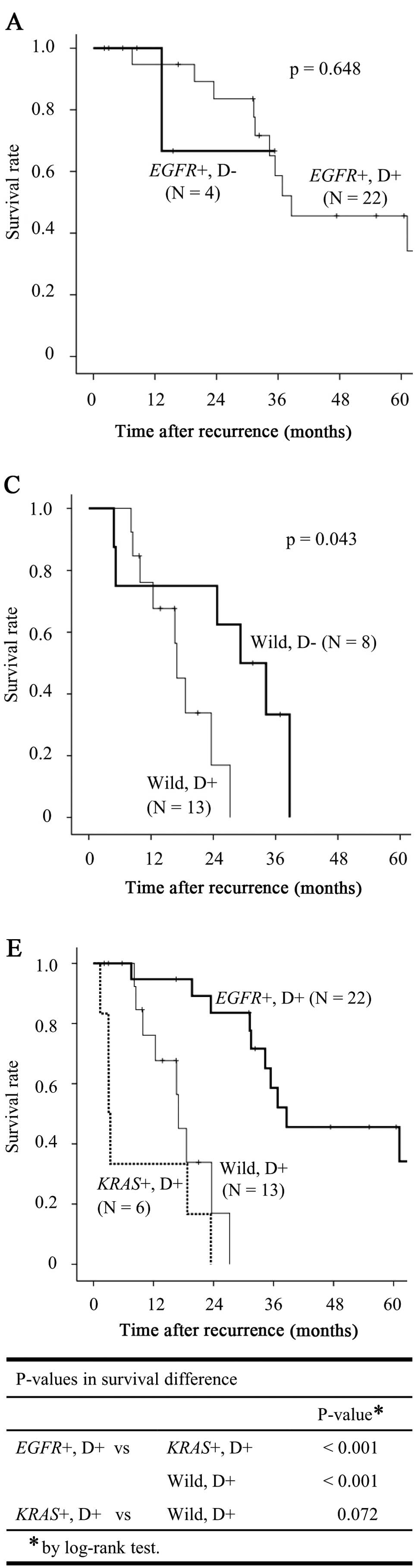
Survival according to driver mutation status and
distant sites of recurrence is shown in Fig. 3. Fig.
3A–C shows the survival for each driver mutation according to
the presence of distant site recurrence. In EGFR+ cases,
there were no survival differences between patients with (D+) and
without (D−) distant recurrence, although D− patients tended to
have an improved prognosis compared with that of D+ patients
(Fig. 3A). In KRAS+ cases,
although D− patients tended to have an improved prognosis compared
with that of D+ patients, there were no survival differences
between the two groups (Fig. 3B).
In wild-type cases, D− patients exhibited a significantly better
prognosis compared with that of D+ patients (Fig. 3C). Fig. 3D shows the overall survival curves
for all D− cases (n=17) according to driver mutation status. In the
D− cohort, the EGFR+ cases exhibited an improved prognosis
compared with the other groups, although the differences were not
statistically significant. Fig. 3E
shows the overall survival curves for all the D+ cases (n=41)
according to driver mutation status. In the D+ cohort, the
EGFR+ cases exhibited a significantly improved prognosis
compared with that of the KRAS+ and wild-type groups
(P<0.001 and <0.001, respectively). In the D− cohort,
survival did not differ significantly between the KRAS+ and
wild-type groups (Fig. 3D).
However, the KRAS+ cases tended to have a worse prognosis
compared with that of the wild-type group in the D+ cohort
(Fig. 3E).
The results of the univariate analyses of all
patients for postrecurrence survival are shown in Table V. There were significant survival
differences according to EGFR and KRAS mutations
(P<0.001 and =0.004, respectively). The multivariate analysis
revealed that multiple recurrences and the EGFR wild-type
status were statistically significant predictors of a worse
postrecurrence prognosis [multiple recurrences: hazard ratio
(HR)=2.80; 95% confidence interval (CI): 1.24–6.34; P=0.013;
EGFR wild-type: HR=3.69; 95% CI: 1.60–8.54; P=0.002]. The
KRAS mutation status also exhibited a tendency to affect
survival, albeit not statistically significantly (P=0.140).
 | Table VUnivariate and multivariate analyses
of prognostic factors for postrecurrence survival. |
Table V
Univariate and multivariate analyses
of prognostic factors for postrecurrence survival.
|
Characteristics | n=58 | 2-year OS rate
(%) | Univariate analysis
P-valuea | Multivariate
analysis |
|---|
|
|---|
| HR (95% CI) | P-valueb |
|---|
| Age at recurrence
(years) |
| <70 | 27 | 61.9 | 0.579 | - | - |
| ≥70 | 31 | 50.2 | | - | |
| Gender |
| Male | 25 | 41.7 | 0.137 | - | - |
| Female | 33 | 65.3 | | - | |
| Smoking
history |
| Never | 28 | 73.0 | 0.147 | - | - |
| Ever | 30 | 36.8 | | - | |
| Pathological
stage |
| I/II | 30 | 69.4 | 0.119 | - | - |
| III/IV | 28 | 43.4 | | - | |
| Recurrence
site |
| Local | 17 | 63.7 | 0.413 | - | - |
| Distant | 25 | 56.1 | | - | |
| Local +
distant | 16 | 45.0 | | - | |
| Recurrent
lesions |
| One | 21 | 75.4 | 0.082 | 1.00 | 0.013 |
| Multiple | 37 | 45.6 | | 2.80
(1.24–6.34) | |
| Adjuvant
chemotherapy |
| (+) | 23 | 54.9 | 0.675 | - | - |
| (−) | 35 | 55.5 | | - | |
| EGFR-TKI |
| (+) | 19 | 57.9 | 0.636 | - | - |
| (−) | 39 | 56.9 | | - | |
| Radiation
therapy |
| (+) | 23 | 45.4 | 0.518 | - | - |
| (−) | 35 | 61.8 | | - | |
| EGFR
mutation |
| (+) | 26 | 81.0 | < 0.001 | 1.00 | 0.002 |
| (−) | 32 | 36.0 | | 3.69
(1.60–8.54) | |
| KRAS
mutation |
| (+) | 11 | 18.2 | | 1.89
(0.81–4.42) | 0.140 |
| (−) | 47 | 65.0 | 0.004 | 1.00 | |
Characteristics of patients who survived
for >5 years
The characteristics of the patients who survived for
>5 years following recurrence are listed in Table VI. EGFR mutations were
detected in all primary tumors (exon 19 deletion, 4 patients; exon
21 L858R mutation, 2 patients). Relapse at distant sites was also
detected in all cases. Gefitinib was administered to 4 patients,
whereas the 2 remaining patients received radiotherapy alone for
localized distant metastases, without additional EGFR-TKI
administration.
 | Table VICharacteristics of patients who
survived for >5 years following recurrence. |
Table VI
Characteristics of patients who
survived for >5 years following recurrence.
| Case | Age, gender | Pathological
stage | Adjuvant
treatment | First recurrence
site and organ | EGFR
mutation | Treatment for
recurrence | Survival time
(years) | Outcome |
|---|
| 1 | 67, M | T2aN0M0 stage
IB | UFT | Distant: lung (Bi),
bone | Exon 21 L858R | RT, gefitinib,
CBDCA+PTX+Bev | 7.2 | Alive |
| 2 | 71, F | T2aN0M1b
(braina) stage IV | Gefitinib | Distant: bone
(braina) | Exon 19 del | Gefitinib
(RTa) | 7.2 | Alive |
| 3 | 76, F | T2aN0M0 stage
IB | None | Distant: brain | Exon 19 del | RT | 5.8 | Alive |
| 4 | 64, F | T2aN0M0 stage
IB | S-1 | Distant: lung
(Bi) | Exon 19 del | CDDP+GEM,
gefitinib, surgery | 5.6 | Alive |
| 5 | 57, M | T2aN2M0 stage
IIIA | None | Local + distant:
lung (Bi), brain, mediastinal LN | Exon 19 del | CDDP+DOC,
gefitinib, RT, surgery | 5.3 | Alive |
| 6 | 77, F | T1aN2M0 stage
IIIA | None | Distant: subclavian
LN | Exon 21 L858R | RT | 5.0 | Dead |
Discussion
In this study, we investigated the correlations
between EGFR and KRAS mutations, relapse site and
prognosis in lung adenocarcinoma patients with postoperative
recurrence.
Although several previous studies reported the
postrecurrence survival of NSCLC patients (7–10,18),
only a few reported survival and the effect of EGFR and
KRAS mutation status on postrecurrence survival following
surgical resection or investigated the association of driver
mutations with relapse site in lung adenocarcinoma patients with
recurrence. Johnson et al (19) reported that KRAS mutations
may be predictors of shorter survival and that EGFR
mutations were associated with longer overall survival in patients
with stage IV lung adenocarcinoma. In this study, we demonstrated
that the survival of patients with recurrent lung adenocarcinoma
was also associated with driver mutations, similar to advanced,
inoperable cases.
Although Endo et al (20) reported that distant or
extrathoracic recurrence was an unfavorable factor following
recurrence, other studies, including ours, demonstrated that it was
not significant (7–9). In this study, the EGFR+ cases
exhibited a significantly improved prognosis compared with that of
the KRAS+ and wild-type groups, particularly the D+
patients, whereas the D+ patients had a significantly higher
proportion of EGFR-mutant cases compared with the D− group
(54 vs. 24%). These findings may explain the lack of a significant
difference between the D+ and D− groups.
Despite the high frequency of distant organ
recurrence in the EGFR-mutant cases, the patients with
EGFR-mutated tumors exhibited significantly more favorable
outcomes compared to those with EGFR wild-type
adenocarcinomas. In EGFR-mutant cases, EGFR-TKIs would be
expected to be effective and long-term survival could be expected
with local treatment of cases of localized recurrence, such as
cases 3 and 6 in Table VI.
Our study demonstrated that bilateral/contralateral
lung recurrence was significantly more frequent among EGFR+
cases. In those cases, long-term survival may be achieved with
combination therapy, consisting of EGFR-TKI treatment, cytotoxic
chemotherapy and local treatment, for each lesion (21–24).
In general, long post-recurrence survival may be expected in
patients with slow-growing tumors or long recurrence-free survival;
however, no association between post-recurrence survival time and
recurrence-free survival time according to EGFR mutations
was observed in this study.
By contrast, KRAS mutations were found to be
predictors of worse prognosis following postoperative recurrence,
although the association was not significant. Notably, no patients
with KRAS-mutated tumors with distant recurrence survived
for >2 years after the recurrence, except 1 patient with pure
invasive mucinous adenocarcinoma. It was demonstrated that
KRAS-mutated adenocarcinomas may be divided into two groups
according to lepidic histological growth pattern, with those
patients without a lepidic component exhibiting a poor prognosis
(17). In this study, 6
adenocarcinoma patients with no lepidic component were included,
all of whom succumbed to the disease within 2 years of recurrence,
whereas patients with tumors with a lepidic component also
exhibited poor postrecurrence prognosis. The KRAS mutation
was previously reported to be a predictor of poor prognosis, with a
worse overall survival of KRAS-mutated patients (25,26).
The poor postrecurrence survival may explain the poor
prognosis.
The limitations of this study included the limited
patient sample. Notably, there was no significant difference in
survival with EGFR-TKI treatment in either the entire patient
cohort or the EGFR-mutant cases (P=0.21 and 0.35,
respectively, data not shown). This may be due to the small sample
size. Other driver mutations, such as ALK, BRAF and
HER2 mutations, were not analyzed in this study; however,
their involvement should be investigated in future studies.
Mutational analyses were not conducted for metastatic sites in this
study. Munfus-McCray et al (5) demonstrated acquisition of KRAS
mutations and loss of EGFR mutations at metastatic sites.
Therefore, driver mutations must also be confirmed in metastatic
lesions.
In conclusion, we demonstrated distinct survival
differences in recurrent pulmonary adenocarcinoma patients
according to the presence of driver mutations. Notably, the
patients with EGFR-mutated tumors may achieve long survival,
regardless of recurrence at distant sites. By contrast, patients
with KRAS-mutated adenocarcinoma exhibited poor outcomes
following postoperative recurrence. Therefore, it is considered
essential for the prediction of postrecurrence survival to consider
the driver mutation status, as well as the site of recurrence.
Acknowledgements
The authors would like to thank Mr. Yuki Kaneko and
Ms. Naoko Yoshizumi for their technical support.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Asamura H, Goya T, Koshiishi Y, et al;
Japanese Joint Committee of Lung Cancer Registry. A Japanese Lung
Cancer Registry study: prognosis of 13,010 resected lung cancers. J
Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paik PK, Johnson ML, D’Angelo SP, Sima CS,
Ang D, Dogan S, Miller VA, Ladanyi M, Kris MG and Riely GJ: Driver
mutations determine survival in smokers and never-smokers with
stage IIIB/IV lung adenocarcinomas. Cancer. 118:5840–5847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suda K, Tomizawa K and Mitsudomi T:
Biological and clinical significance of KRAS mutations in lung
cancer: an oncogenic driver that contrasts with EGFR mutation.
Cancer Metastasis Rev. 29:49–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munfus-McCray D, Harada S, Adams C, Askin
F, Clark D, Gabrielson E and Li QK: EGFR and KRAS mutations in
metastatic lung adenocarcinomas. Hum Pathol. 42:1447–1453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung JJ, Jeng WJ, Hsu WH, Wu KJ, Chou TY,
Hsieh CC, Huang MH, Liu JS and Wu YC: Prognostic factors of
postrecurrence survival in completely resected stage I non-small
cell lung cancer with distant metastasis. Thorax. 65:241–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa T, Okumura N, Ohata K, Igai H,
Matsuoka T and Kameyama K: Postrecurrence survival in patients with
stage I non-small cell lung cancer. Eur J Cardiothorac Surg.
34:499–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugimura H, Nichols FC, Yang P, Allen MS,
Cassivi SD, Deschamps C, Williams BA and Pairolero PC: Survival
after recurrent nonsmall-cell lung cancer after complete pulmonary
resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams BA, Sugimura H, Endo C, Nichols
FC, Cassivi SD, Allen MS, Pairolero PC, Deschamps C and Yang P:
Predicting postrecurrence survival among completely resected
nonsmall-cell lung cancer patients. Ann Thorac Surg. 81:1021–1027.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang
MH, Liu JS and Wu YC: Post-recurrence survival in completely
resected stage I non-small cell lung cancer with local recurrence.
Thorax. 64:192–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosaka T, Yatabe Y, Onozato R, Kuwano H
and Mitsudomi T: Prognostic implication of EGFR, KRAS, and TP53
gene mutations in a large cohort of Japanese patients with
surgically treated lung adenocarcinoma. J Thorac Oncol. 4:22–29.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katayama T, Matsuo K, Kosaka T, Sueda T,
Yatabe Y and Mitsudomi T: Effect of gefitinib on the survival of
patients with recurrence of lung adenocarcinoma after surgery: a
retrospective case-matching cohort study. Surg Oncol. 19:e144–e149.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu HP, Isaac Wu HD, Chang JW, Wu YC, Yang
HY, Chen YT, Hsieh WY, Chen YT, Chen YR and Huang SF: Prognostic
implications of epidermal growth factor receptor and KRAS gene
mutations and epidermal growth factor receptor gene copy numbers in
patients with surgically resectable non-small cell lung cancer in
Taiwan. J Thorac Oncol. 5:1175–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YT, Kim TY, Lee DS, Park SJ, Park JY,
Seo SJ, Choi HS, Kang HJ, Hahn S, Kang CH, et al: Molecular changes
of epidermal growth factor receptor (EGFR) and KRAS and their
impact on the clinical outcomes in surgically resected
adenocarcinoma of the lung. Lung Cancer. 59:111–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamae Y, Shimizu K, Mitani Y, et al:
Mutation detection of epidermal growth factor receptor and KRAS
genes using the smart amplification process version 2 from
formalin-fixed, paraffin-embedded lung cancer tissue. J Mol Diagn.
12:257–264. 2010. View Article : Google Scholar
|
|
16
|
Araki T, Shimizu K, Nakamura K, et al:
Usefulness of peptide nucleic acid (PNA)-clamp smart amplification
process version 2 (SmartAmp2) for clinical diagnosis of KRAS codon
12 mutations in lung adenocarcinoma: comparison of PNA-clamp
SmartAmp2 and PCR-related methods. J Mol Diagn. 12:118–124. 2010.
View Article : Google Scholar
|
|
17
|
Kakegawa S, Shimizu K, Sugano M, Miyamae
Y, Kaira K, Araki T, Nakano T, Kamiyoshihara M, Kawashima O and
Takeyoshi I: Clinicopathological features of lung adenocarcinoma
with KRAS mutations. Cancer. 117:4257–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung JJ, Jeng WJ, Hsu WH, Huang BS and Wu
YC: Time trends of overall survival and survival after recurrence
in completely resected stage I non-small cell lung cancer. J Thorac
Oncol. 7:397–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson ML, Sima CS, Chaft J, Paik PK, Pao
W, Kris MG, Ladanyi M and Riely GJ: Association of KRAS and EGFR
mutations with survival in patients with advanced lung
adenocarcinomas. Cancer. 119:356–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endo C, Sakurada A, Notsuda H, Noda M,
Hoshikawa Y, Okada Y and Kondo T: Results of long-term follow-up of
patients with completely resected non-small cell lung cancer. Ann
Thorac Surg. 93:1061–1068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hotta K, Kiura K, Toyooka S, Takigawa N,
Soh J, Fujiwara Y, Tabata M, Date H and Tanimoto M: Clinical
significance of epidermal growth factor receptor gene mutations on
treatment outcome after first-line cytotoxic chemotherapy in
Japanese patients with non-small cell lung cancer. J Thorac Oncol.
2:632–637. 2007. View Article : Google Scholar
|
|
22
|
Kalikaki A, Koutsopoulos A, Hatzidaki D,
Trypaki M, Kontopodis E, Stathopoulos E, Mavroudis D, Georgoulias V
and Voutsina A: Clinical outcome of patients with non-small cell
lung cancer receiving front-line chemotherapy according to EGFR and
K-RAS mutation status. Lung Cancer. 69:110–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takano T, Fukui T, Ohe Y, Tsuta K,
Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Furuta K
and Tamura T: EGFR mutations predict survival benefit from
gefitinib in patients with advanced lung adenocarcinoma: a
historical comparison of patients treated before and after
gefitinib approval in Japan. J Clin Oncol. 26:5589–5595. 2008.
View Article : Google Scholar
|
|
24
|
Toyooka S, Takano T, Kosaka T, et al:
Epidermal growth factor receptor mutation, but not sex and smoking,
is independently associated with favorable prognosis of
gefitinib-treated patients with lung adenocarcinoma. Cancer Sci.
99:303–308. 2008. View Article : Google Scholar
|
|
25
|
Sonobe M, Kobayashi M, Ishikawa M, et al:
Impact of KRAS and EGFR gene mutations on recurrence and survival
in patients with surgically resected lung adenocarcinomas. Ann Surg
Oncol. 19(Suppl 3): S347–S354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guan JL, Zhong WZ, An SJ, et al: KRAS
mutation in patients with lung cancer: a predictor for poor
prognosis but not for EGFR-TKIs or chemotherapy. Ann Surg Oncol.
20:1381–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|