Introduction
Nasopharyngeal carcinoma (NPC) is the most commonly
diagnosed head and neck malignancy in Southeast Asia and 70% of
patients present with locally advanced-stage cancer at the time of
diagnosis (1–3). Moreover, NPC demonstrates the highest
incidence rates of distant metastasis among all head and neck
cancers. Due to anatomical restrictions, the standard treatment for
NPC is definitive radiotherapy. The benefits of chemotherapy
administered concurrently with radiation are supported by the
present data, demonstrating significant increases in
progression-free survival (PFS), as well as overall survival (OS)
(4,5).
Although considered essential for patients with
locally advanced NPC, the therapeutic value of chemotherapy
administered concurrently with intensity-modulated radiation
therapy (IMRT) and the optimal strategy of combining the two, has
not yet been sufficiently addressed. The rationale of concurrent
chemotherapy and IMRT in the treatment of NPC is mostly derived
from experience with conventional radiotherapy. Concurrent
chemoradiotherapy has shown significant benefits following
treatment; however, whether it offers equal therapeutic benefits to
all the patient subgroups with locally advanced NPC remains to be
determined. The objective of this study was to assess treatment
outcomes and elucidate the efficacy of concurrent
chemoradiotherapy, by analyzing results obtained from a relatively
large group of patients with locally advanced NPC, uniformly
treated with IMRT and concurrent chemotherapy.
Materials and methods
Patients and pretreatment evaluation
A total of 226 patients with histologically
diagnosed non-metastatic NPC were treated with concurrent
chemoradiotherapy at the First Affiliated Hospital of Soochow
University (Suzhou, China), between March, 2005 and March, 2007.
The pretreatment evaluation consisted of a complete history and
physical examination, flexible fiberoptic endoscopic examination,
blood chemistry tests, urinalysis, chest X-ray, electrocardiogram,
computed tomography (CT) scans of the nasopharynx and neck,
magnetic resonance imaging (MRI) scans of the head and neck, bone
emission computed tomography scans, liver and abdominal lymph node
ultrasounds and dental evaluation. Positron emission tomography
scans and CT scans of the chest and abdomen were optional and
performed when clinically indicated. Tumors were staged according
to the Guangzhou staging system (2008). Patients who presented with
evidence of distant metastasis were not eligible for this treatment
protocol. The characteristics of the 226 patients are listed in
Table I.
 | Table IPatient characteristics in the study
population of 226 nasopharyngeal carcinoma patients, according to
the Guangzhou staging system (2008). |
Table I
Patient characteristics in the study
population of 226 nasopharyngeal carcinoma patients, according to
the Guangzhou staging system (2008).
| Characteristics | No. | Percentage (%) |
|---|
| Patients | 226 | 100 |
| Age (years) | | |
| >60 | 71 | 31.4 |
| ≤60 | 155 | 68.6 |
| Gender | | |
| Male | 154 | 68.1 |
| Female | 72 | 31.9 |
| T-classification | | |
| T3 | 78 | 34.5 |
| T4 | 148 | 65.5 |
| N-classification | | |
| N0 | 36 | 15.9 |
| N1 | 72 | 31.9 |
| N2 | 112 | 49.6 |
| N3 | 6 | 2.7 |
| Clinical
classification | | |
| III | 78 | 34.5 |
| IV | 148 | 65.5 |
IMRT techniques
IMRT was performed using a commercial stereotactic
radiotherapy system (Varian Eclipse; Varian Medical Systems, Inc.,
Palo Alto, CA, USA), in order to deliver revolving conformal
radiation based on multileaf collimator to the target, using a 6-MV
linear accelerator (Varian 23EX; Varian Medical Systems, Inc.). The
patients were immobilized in the supine position with thermoplastic
masks. CT planning scans with intravenous contrast material were
performed, using 3-mm slices from the head to 2 cm below the level
of the sternoclavicular joints. The primary and nodal gross tumor
volumes (GTV-nx) and the cervical lymph node gross tumor volumes
(GTV-nd) included the gross diseases visualized on CT and MRI. The
high-risk clinical tumor volume (CTV-1) included GTV and a 5- to
10-mm margin, encompassing the entire nasopharyngeal mucosa, as
well as a 5-mm submucosal volume. CTV-2 was designed for
potentially involved regions, including the nasopharyngeal cavity,
maxillary sinus, pterygopalatine fossa, posterior ethmoid sinus,
parapharyngeal space, skull base, anterior third of the clivus and
cervical vertebrae, inferior sphenoid sinus and cavernous sinus, as
well as the retropharyngeal lymph nodal regions, from the base of
skull to the cranial edge of the second cervical vertebra. The
planning target volume was created based on each volume, with an
additional 2- to 3-mm margin, which allowed for setup variability.
Critical normal structures, including brainstem, spinal cord,
parotid glands, optic nerves, optic chiasm, lens, eyeballs,
temporal lobes, temporomandibular joints, mandible and hypophysis,
were contoured and set as organs at risk (OAR) during optimization.
The radiation doses prescribed in the protocol were as follows: a
total dose of 65–70 Gy in 32 fractions to the GTV-nx and GTV-nd,
56–60 Gy in 32 fractions to the CTV-1 and 50–52 Gy in 28 fractions
to the CTV-2. The patients were treated with one fraction daily for
five days per week. The dose received by each OAR should be less
than its tolerance limit, according to the radiation therapy
oncology group (RTOG) 0225 protocol.
Chemotherapy
Concurrent chemotherapy was administered to all
patients, in order to prevent disease progression during IMRT
treatment planning. Concurrent chemotherapy consisted of 6 cycles
of TPF regimen (cisplatin 80–100 mg/m2 i.v. on Day 1,
5-fluorouracil 500-600 mg/m2 i.v. on Days 1–5 and
docetaxel 135–175 mg/m2 i.v. on Day 1). The patients
received 2 courses of chemotherapy every 28 days during
radiotherapy, followed by an additional 2–4 cycles of chemotherapy
every 21 days after radiotherapy.
Follow-up
The patients were evaluated weekly during radiation
therapy and were required to be followed-up by their attending
radiation oncologist following the completion of their treatment,
every 3 months for the first 2 years, every 6 months from the
second through the fifth year and annually thereafter. Each
follow-up included a complete examination, basic serum chemistry
tests, chest X-ray and ultrasound of the liver and abdomen.
Flexible fiberoptic endoscopy was performed at every visit after
the treatment. MRI of the head and neck areas was performed every 6
months. Treatment-induced toxicities were assessed and scored
according to the RTOG radiation morbidity scoring criteria at each
follow-up.
Statistical analysis
The PFS and OS rates were calculated using the
Kaplan-Meier method. The duration of time to local failure and
distant metastasis was measured from the date of completion of the
radiation therapy (including boost irradiation) until documented
treatment failure. The OS duration was calculated from diagnosis
until death or until the date of the last follow-up visit for the
surviving patients. The statistical tests were performed using SPSS
version 17.0. P≤0.05 among the groups was considered to indicate a
statistically significant difference.
Results
Patients and treatment evaluation
A total of 226 patients diagnosed with locally
advanced NPC were included in this study. The patients received
concurrent chemoradiotherapy (IMRT and TPF). The mean age of the
patients was 43 years (range, 17–72). The majority of the patients
were male (68.1%) and Guangzhou stage III (34.5%) and IV (65.5%).
The patients received 4–6 planned cycles of chemotherapy.
Both the primary site and neck nodes achieved an
objective response rate of 98% for the response evaluation. The
primary site showed an 87% complete response and the partial
response was 11%.
PFS and OS
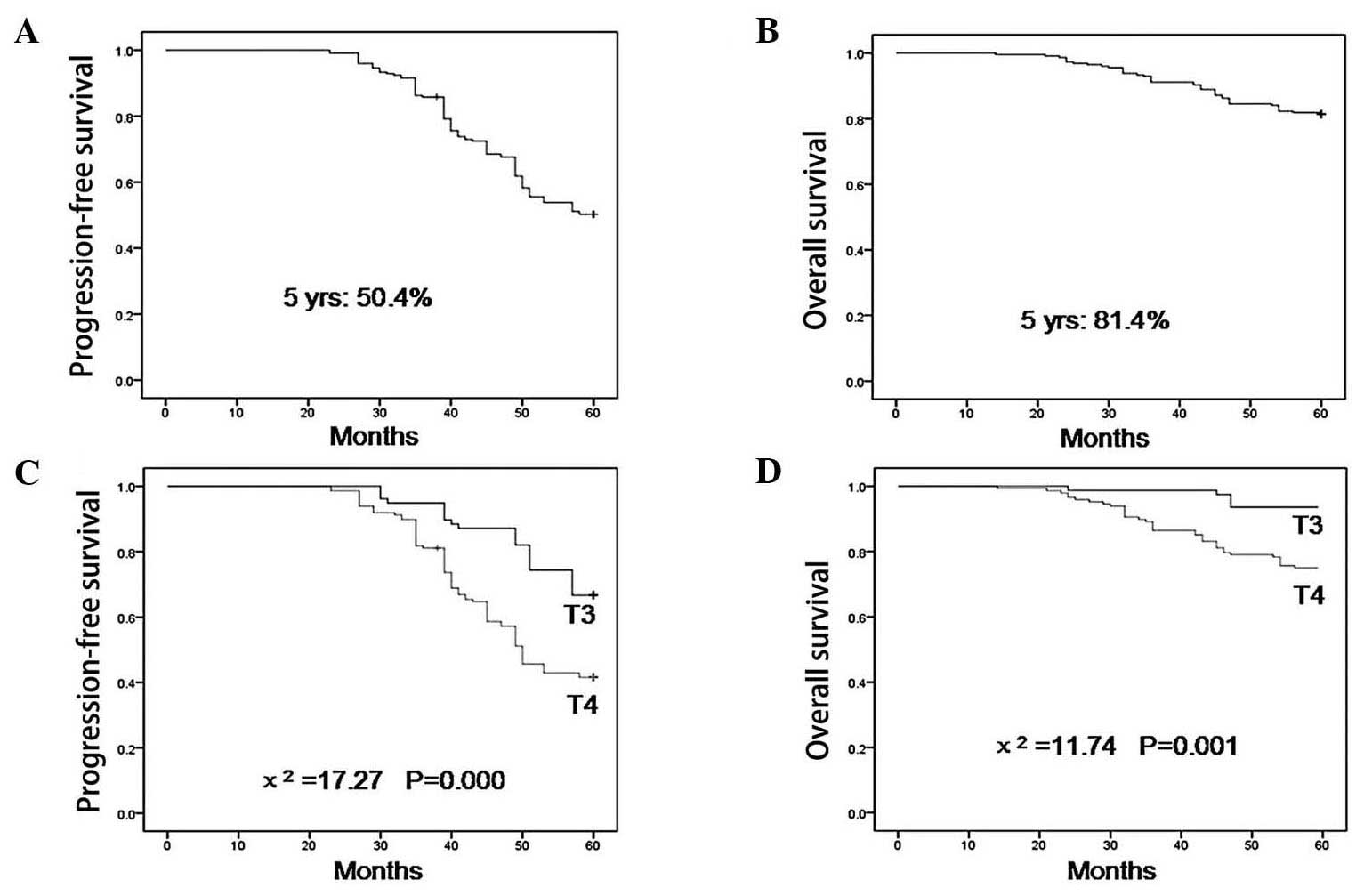
With a median follow-up time of 35 months (range,
7–60), the 5-year OS rate was 81.4%, with 93.6 and 75.0% for T3 and
T4 lesions, respectively (P=0.001, Fig. 1). The 5-year PFS was 50.4%, with
66.7 and 46.9% for T3 and T4 lesions, respectively (P<0.001,
Fig. 1). T-classification was a
significant prognostic factor for PFS and OS. The subgroup analysis
revealed that pterygopalatine fossa invasion was associated with a
significantly lower 5-year PFS (P=0.001, Table II) and OS (P=0.002, Table II), foramen rotundum invasion was
associated with a significantly lower 5-year PFS (P<0.001) and
OS (P=0.004), foramen ovale invasion was associated with a
significantly lower 5-year PFS (P=0.013, Table II) and OS (P=0.024, Table II) and foramen lacerum and
cavernous sinus invasion were associated with a significantly lower
5-year PFS (P<0.001 and P<0.001, respectively; Table II). By contrast, foramen lacerum
and cavernous sinus invasion had no impact on the 5-year OS
(P=0.148 and P=0.166, respectively; Table II). None of the patients was lost
during the follow-up period.
 | Table IIKaplan-Meier estimate of
progression-free survival (PFS) and overall survival (OS),
according to pterygopalatine fossa, foramen rotundum, foramen
ovale, foramen lacerum and cavernous sinus invasion, or lack
thereof. |
Table II
Kaplan-Meier estimate of
progression-free survival (PFS) and overall survival (OS),
according to pterygopalatine fossa, foramen rotundum, foramen
ovale, foramen lacerum and cavernous sinus invasion, or lack
thereof.
| Characteristic | No. | 5-year PFS (%) | χ2 | P-value | No. | 5-year OS (%) | χ2 | P-value |
|---|
| Pterygopalatine
fossa | | | | | | | | |
| + | 120 | 43.3 | 11.74 | | 71 | 74.2 | 9.68 | |
| − | 106 | 58.5 | | 0.001 | 155 | 89.6 | | 0.002 |
| Foramen rotundum | | | | | | | | |
| + | 38 | 7.9 | 47.73 | | 154 | 65.8 | 8.08 | |
| − | 188 | 59.9 | | 0.000 | 72 | 84.6 | | 0.004 |
| Foramen ovale | | | | | | | | |
| + | 102 | 43.1 | 6.12 | | 78 | 74.5 | 5.11 | |
| − | 124 | 53.5 | | 0.013 | 148 | 85.1 | | 0.024 |
| Foramen lacerum | | | | | | | | |
| + | 111 | 39.6 | 23.27 | | 36 | 77.5 | 2.10 | |
| − | 115 | 60.9 | | 0.000 | 72 | 85.2 | | 0.148 |
| Cavernous sinus | | | | | | | | |
| + | 112 | 38.4 | 18.91 | | 78 | 77.7 | 1.92 | |
| − | 114 | 62.3 | | 0.000 | 148 | 85.1 | | 0.166 |
Discussion
NPC is considered unresectable due to its anatomical
location and thus far radiation has been the conventional treatment
approach (6,7). Changing failure pattern has been
noted in several publications and distant metastasis rates may be
as high as 30%, even with the integration of aggressive concurrent
chemoradiotherapy schedules (8,9).
Concurrent chemoradiation is an attractive approach
to overcome the problem of distant metastases. However, it requires
further investigation, since available post-experience data are
sparse (10–13). China has reported the largest
series of concurrent chemotherapy and IMRT data, with 323
locoregionally advanced NPC patients. The overall 3-year OS rate
was 87.2%. In the present study, the estimated 5-year PFS and OS
rates were 50.4 and 80.4%, respectively (14). Concurrent chemotherapy for
locoregionally advanced NPC has been shown to be feasible and
effective for local control, with high compliance. Although all our
patients had locally advanced disease (stages III and IV), they
exhibited excellent local control rates following concurrent
chemotherapy or even salvage therapy.
The histopathological type and involvement of lymph
nodes in the lower neck have been well-established as prognostic
factors of NPC (15). In addition
to these factors, tumor invasive range has been recognized as an
important prognostic factor in the treatment of malignancy.
Recently, tumor invasive range has been actively studied in head
and neck malignancies. NPC is a tumor with a highly infiltrative
growth pattern and a propensity to spread along the parapharyngeal
space, as well as to the skull base and foramina (16). The invaded area may affect
prognosis and may vary from extensive invasion, involving multiple
sites, to only a small localized area, which, in some patients, may
be the only site of extranasopharyngeal spread. The skull base
foramina represent an unimpeded channel for tumor spread, although
direct invasion of the bones bordering these foramina often occurs
as well. The foramen ovale and foramen lacerum are the two most
commonly involved foramina, which provide a route for tumor spread
into the cranium. The inferior spread of the tumor, involving the
hypoglossal nerve canal and jugular foramen, is less common.
Orbital invasion usually occurs by direct tumor spread from the
pterygopalatine fossa; the tumor may also spread directly to the
cavernous sinus, leading to multiple cranial nerve palsies. This
phenomenon suggests that NPC has an infiltrative growth pattern,
often with a highly irregular tumor contour.
In this study, our results demonstrated that
T-classification was a significant prognostic factor for PFS and
OS. The subgroup analysis revealed that pterygopalatine fossa,
foramen rotundum, foramen ovale, foramen lacerum and cavernous
sinus invasion were all associated with the long-term results of
concurrent chemotherapy for patients with NPC.
Other factors that contribute to the apparent tumor
radioresistance must be considered. Although this study
demonstrated an inverse correlation between tumor control and
disease volume, several failures in small tumors and cures in cases
with massive disease have been observed. These observations suggest
that other factors, aside from those observed in this study,
contribute to radiation response. Moreover, cellular factors, such
as repopulation, intrinsic radioresistance, reoxygenation and
redistribution, have been suggested as important variables for
tumor control (17).
In conclusion, volumetric analysis of the primary
tumor and lymph nodes, anatomical sites involved and intracranial
extension should be included in the current TNM staging system for
NPC, in order to optimize the staging system.
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
IMRT
|
intensity-modulated radiation
therapy
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
OAR
|
organs at risk
|
|
CT
|
computed tomography
|
|
GTV
|
gross tumor volume
|
|
CTV
|
clinical tumor volume
|
|
RTOG
|
radiation therapy oncology group
|
References
|
1.
|
Perri F, Bosso D, Buonerba C, et al:
Locally advanced nasopharyngeal carcinoma: current and emerging
treatment strategies. World J Clin Oncol. 2:377–383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Al-Sarraf M and Reddy MS: Nasopharyngeal
carcinoma. Curr Treat Options Oncol. 3:21–32. 2002. View Article : Google Scholar
|
|
3.
|
Yi JL, Gao L, Xu GZ, et al: Treatment
results of intensity-modulated radiotherapy for nasophyngeal
carcinoma: an analysis of 147 patients. Chin J Radiat Oncol.
17:329–334. 2008.
|
|
4.
|
Al-Sarraf M, LeBlanc M, Giri PG, et al:
Chemoradiotherapy versus radiotherapy in patients with advanced
nasopharyngeal cancer: phase III randomized Intergroup study 0099.
J Clin Oncol. 16:1310–1317. 1998.PubMed/NCBI
|
|
5.
|
Baujat B, Audry H, Bourhis J, et al:
Chemotherapy in locally advanced nasopharyngeal carcinoma: an
individual patient data meta-analysis of eight randomized trials
and 1753 patients. Int J Radiat Oncol Biol Phys. 1:47–56. 2006.
View Article : Google Scholar
|
|
6.
|
Ishiki H and Tahara M: Induction
chemotherapy followed by chemoradiotherapy for the patients with
far-advanced nasopharyngeal carcinoma - our treatment strategy. Gan
To Kagaku Ryoho. 39:698–701. 2012.(In Japanese).
|
|
7.
|
Caponigro F, Longo F, Ionna F, et al:
Treatment approaches to nasopharyngeal carcinoma: a review.
Anticancer Drugs. 21:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Heng DMK, Wee J, Fong KW, et al:
Prognostic factors in 677 patients in Singapore with
nondisseminated nasopharyngeal carcinoma. Cancer. 86:1912–1920.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ma J, Mai HQ, Hong MH, et al: Is the 1997
AJCC staging system for nasopharyngeal carcinoma prognostically
useful for Chinese patient population? Int J Radiat Oncol Biol
Phys. 50:1181–1189. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhang L, Zhao C, Peng PJ, et al: Phase III
study comparing standard radiotherapy with or without weekly
oxaliplatin in treatment of locoregionally advanced nasopharyngeal
carcinoma: preliminary results. J Clin Oncol. 23:8461–8468. 2005.
View Article : Google Scholar
|
|
11.
|
Lee AW, Lau WH, Tung SY, et al:
Preliminary results of a randomized study on therapeutic gain by
concurrent chemotherapy for regionally-advanced nasopharyngeal
carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer
Study Group. J Clin Oncol. 23:6966–6975. 2005. View Article : Google Scholar
|
|
12.
|
Wee J, Tan EH, Tai BC, et al: Randomized
trial of radiotherapy versus concurrent chemoradiotherapy followed
by adjuvant chemotherapy in patients with American Joint Committee
on Cancer/International Union against cancer stage III and IV
nasopharyngeal cancer of the endemic variety. J Clin Oncol.
23:6730–6738. 2005. View Article : Google Scholar
|
|
13.
|
Lu H, Peng L, Yuan X, et al: Concurrent
chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a
treatment paradigm also applicable to patients in Southeast Asia.
Cancer Treat Rev. 35:345–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lin S, Pan J, Han L, et al: Nasopharyngeal
carcinoma treated with reduced-volume intensity-modulated radiation
therapy: report on the 3-year outcome of a prospective series. Int
J Radiat Oncol Biol Phys. 75:1071–1078. 2009.PubMed/NCBI
|
|
15.
|
Han L, Lin SJ, Pan JJ, et al: Prognostic
factors of 305 nasopharyngeal carcinoma patients treated with
intensity-modulated radiotherapy. Chin J Cancer. 29:145–150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lu CH, Yang CY, Wang CP, et al: Imaging of
nasopharyngeal inflammatory pseudotumours: differential from
nasopharyngeal carcinoma. Br J Radiol. 83:8–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Oehler C, Dickinson DJ, Broggini-Tenzer A,
et al: Current concepts for the combined treatment modality of
ionizing radiation with anticancer agents. Curr Pharm Des.
13:519–535. 2007. View Article : Google Scholar : PubMed/NCBI
|