Introduction
Cancer is a major public health concern worldwide
and has been ranked first in the disease spectrum. A total of
1,638,910 new cancer cases and 577,190 cancer deaths were projected
to occur in the United States in 2012 (1). Despite significant advances in cancer
treatment strategies, surgical resection, chemotherapy and
radiotherapy remain the primary methods for cancer treatment.
However, the conventional first- or second-line chemotherapy
treatments, although they may be efficient, they are often
associated with toxicity, occasionally so severe that requires
treatment discontinuation. Furthermore, following a long period of
repeated treatment, tumor resistance may also develop. Therefore,
novel effective agents, with an acceptable toxicity profile, are
urgently required.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL), also referred to as Apo2L, was first discovered in
1995. It was observed that full-length TRAIL is expressed on the
cell surface and picomolar concentrations of soluble TRAIL rapidly
induce apoptosis in a wide variety of transformed cell lines of
diverse origin, without affecting normal cells (2,3).
TRAIL is a type II membrane protein consisting of 281 amino acids.
TRAIL may bind to the death receptor (DR)4 or DR5, leading to the
transduction of an apoptotic signal, which triggers transformed
cell death (4–8). However, normal cells survive due to
the function of the decay receptors, (DcR)1 and DcR2, which are
able to bind TRAIL without apoptotic signal transduction, due to
the absence of a death domain (5,7).
This suggests that TRAIL may be a promising strategy for cancer
treatment and may result in the development of cancer therapies
that target this apoptotic pathway. The TRAIL apoptotic pathway has
been targeted by at least two approaches: the recombinant human
TRAIL (rhTRAIL) ligand and its agonistic antibodies against DR4 and
DR5 (9). In this study, we applied
the term ‘TRAIL-related agents’ to refer to this type of drug.
Several randomized controlled trials (RCTs) were
conducted over the last few years and the majority of the results
did not support the addition of TRAIL-related agents to cancer
treatment regimens. However, to the best of our knowledge, no
systematic review or meta-analysis of the currently available
evidence has been conducted thus far. This meta-analysis aimed to
investigate the safety and efficacy of TRAIL-related agents.
Materials and methods
Study design and search strategy
All the published RCTs comparing TRAIL-related
agents with other therapies for cancer treatment were independently
searched for by two authors (Sun S and Sun L). Other therapies
included drugs that had been applied as first- or second-line
cancer treatment. Medline, the Cochrane Library and EMBASE
databases were searched. We also performed a manual search of the
reference abstracts in the Journal of Clinical Oncology for the
American Society of Clinical Oncology, in order to obtain the
latest data. RCTs were included if they were conducted between 1995
and May 31, 2013. The publication language was limited to English.
The following key words were used to search PubMed: ‘TNF-related
apoptosis-inducing ligand’, ‘tumor necrosis factor-related
apoptosis-inducing ligand’, ‘TRAIL’, ‘Apo2L’, ‘rhTRAIL’, ‘rhApo2L’,
‘HGS-ETR1’, ‘HGS-ETR2’, ‘Apomab’, ‘TRA-8’, ‘CS-1008’, ‘AMG 655’,
‘LBY135’, ‘PRO95780’, ‘drozitumab’, ‘HGS-TR2J’, ‘KMTR-2’,
‘lexatumumab’, ‘conatumumab’, ‘tigatuzumab’, ‘dulanermin’,
‘mapatumumab’, ‘death receptor 4’, ‘DR4’, ‘death receptor 5’,
‘DR5’, ‘neoplasms’, ‘neoplasm’, ‘tumor’, ‘tumour’, ‘cancer’,
‘clinical trial’ and ‘random controlled trial’.
Inclusion and exclusion criteria
Only the RCTs comparing TRAIL-related agents with
traditional therapies for cancer treatment were included in this
study, regardless of whether they were blinded.
Inclusion criteria of the original articles:
i) cancer patients with measurable or evaluable disease that had
been histologically or cytologically confirmed; ii) patients aged
≥18 years, without gender or race restrictions; iii) Eastern
Cooperative Oncology Group performance status 0–2; iv) no
contraindication of chemotherapy with regards to hepatic, renal and
hematopoietic function; v) life expectancy of ≥3 months; vi)
efficacy assessed by the Response Evaluation Criteria in Solid
Tumors; vii) no untreated or unstable central nervous system
metastases; viii) no prior chemotherapy or radiotherapy (except
adjuvant chemotherapy within 1 year of enrollment); and ix)
patients provided written informed consent.
Interventions and comparisons: i) the
articles investigated cancer treatment and included a complete
protocol; ii) TRAIL-related agents were used as an intervention,
without restriction of dose and usage methods; and iii) other
chemotherapies, including targeted therapy, were used as
control.
Outcome measurements: i) complete tumor
response and survival data; and ii) complete adverse event (AE)
data.
Exclusion criteria: i) retrospective, cohort,
clinically controlled or any type of study other than RCT; ii)
study not published in English; iii) study published without
effective reporting of the primary results or adequate data for a
meta-analysis and the missing data were not available after
contacting the authors; iv) only the abstract was available (no
full text); and v) the efficacy data were not accurate and
clear.
Study identification
Two reviewers (Sun S and Sun L) independently
screened the titles of all the retrieved articles. We first
reviewed the abstract of the articles that were relevant to the
topic. The full text was then obtained through different approaches
to retrieve more information on the selected clinical trials. A
third reviewer (Xie C) was consulted for the final decision in case
of any disagreement on eligibility between the first two
reviewers.
Data extraction
The extracted data consisted of two parts: i)
general information (study type, time, location, number of cases in
each group and gender ratio); and ii) data for the meta-analysis
(tumor response, survival and AEs). Two reviewers (Sun S and Li Z)
participated in the extraction of data from all the eligible RCTs.
The third reviewer (Xie C) was consulted if necessary.
Study assessment
The studies were independently assessed by two
reviewers (Sun S and Li Z). A third reviewer (Xie C) was consulted
in case of any disagreement. The study assessment included: i)
Assessing the risk of bias. The Cochrane Collaboration’s tool for
assessing the risk of bias was used (10), which consists of 6 items. Each
question was answered with yes (low risk of bias), no (high risk of
bias) or unclear (unclear risk of bias). ii) Evidence quality and
recommended grade. Based on the outcomes of the systematic review,
Grading of Recommendations Assessment Development and Evaluation
(GRADE) was used to assess the quality of the evidence. The quality
of the evidence for each outcome measurement falls into one of four
categories: high, moderate, low and very low (11). RCTs have high-quality evidence,
with 5 factors that may result in a lower rating of the quality of
evidence and 3 factors that may cause an increase in the rating.
The grading recommendation was described as strong (i.e., the
authors were confident that the intervention with TRAIL-related
agents has more advantages or disadvantages) or weak (i.e., the
authors were not certain whether the intervention has more
advantages or disadvantages or, regardless of the high or low
quality of the evidence, they all indicated that the advantages and
disadvantages were equal).
Outcomes for the meta-analysis
The outcomes considered in this meta-analysis
included the objective response rate (ORR), the clinical benefit
rate (CBR)/disease control rate (DCR) and AEs. ORR included
complete response (CR) and partial response (PR), whereas CBR/DCR
included CR, PR and stable disease (SD).
Statistical analysis
The meta-analysis was conducted with the Cochrane
Collaboration Review Manager 5.2 software (http://tech.cochrane.org/revman). P≤0.05 was
considered to indicate a statistically significant difference. For
continuous data, a weighted mean difference or standard mean
difference were used in this study, both with 95% confidence
intervals (CIs). For dichotomous outcomes, an odds ratio (OR) or
relative risk (RR) were calculated as the summary statistics, both
with 95% CIs. When the related data were calculated, two decimals
were kept. The statistical heterogeneity was tested with the
χ2 and I2 tests. P≥0.05 and I2≤50%
were considered to indicate low statistical heterogeneity and,
therefore, the fixed effects model was used. P<0.05 and
I2>50% were considered to indicate high heterogeneity
and the random effects model was adopted. The source of high
heterogeneity was investigated by subgroup analysis based on the
methodological quality after clinical heterogeneity was excluded.
GRADE Pro 3.6 software was used for the GRADE assessment.
Observed-expected (O-E) and its variance of progression-free
survival (PFS) and overall survival (OS) were calculated with a
method introduced by Tierney et al (12), assisted by the Engauge Digitizer
4.1 software (http://digitizer.sourceforge.net). A sensitivity
analysis was performed by excluding studies one by one. Data input
was executed by one reviewer under the supervision of a second
reviewer.
Results
Search for relevant articles and general
characteristics of the included RCTs
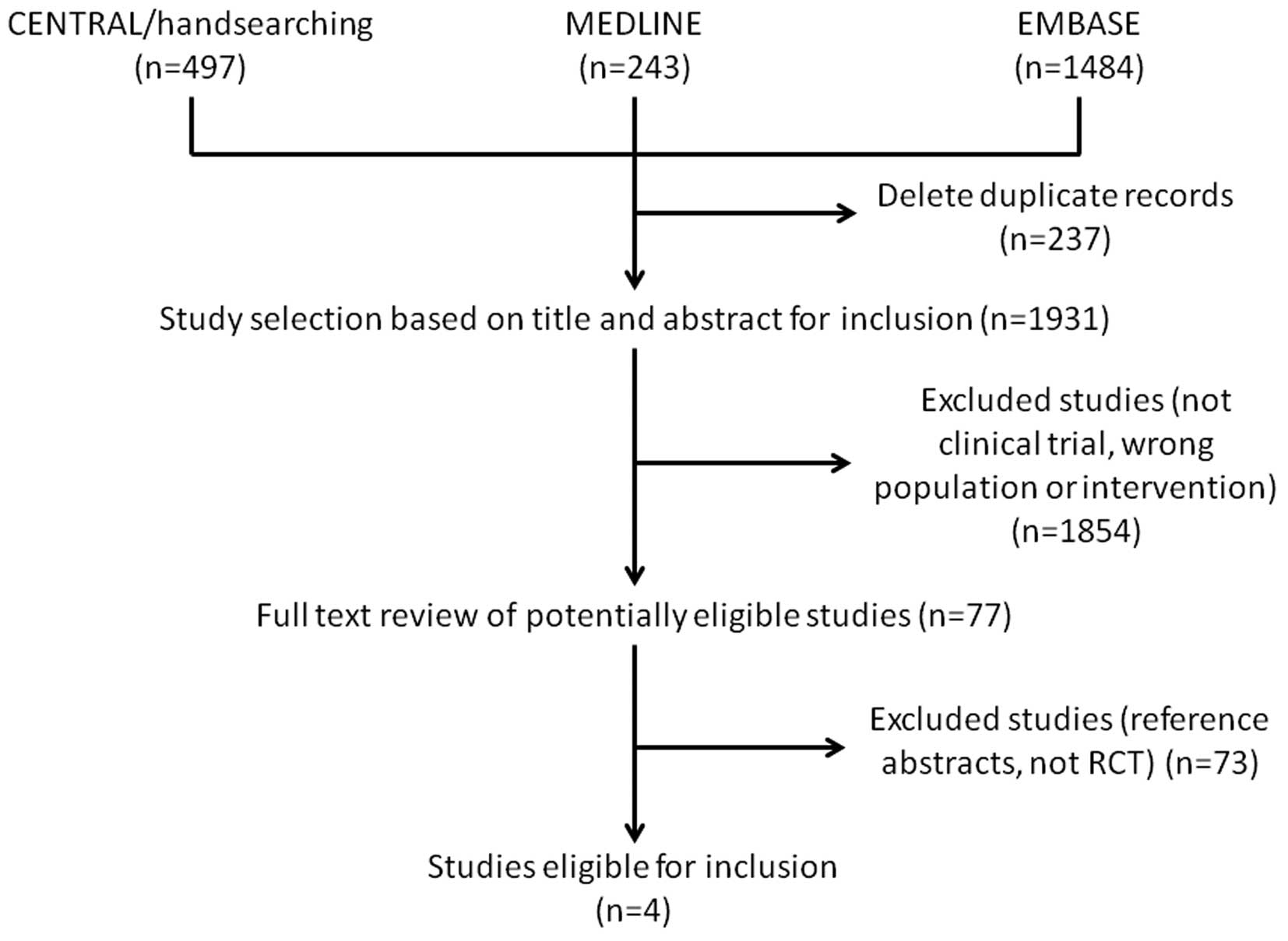
A total of 2,224 relevant articles were retrieved.
The study selection process is summarized in Fig. 1. We selected 77 clinical trials
that investigated TRAIL-related agents for cancer treatment, of
which 73 articles were excluded as they were reference abstracts
without a detailed data report or were studies other than RCTs. Two
reviewers confirmed 4 RCTs (13–16)
as being suitable for inclusion in this meta-analysis. The general
characteristics of the eligible RCTs are summarized in Table I.
 | Table I.General characteristics of the
included randomized controlled trials. |
Table I.
General characteristics of the
included randomized controlled trials.
| Studies (refs.) | Sample size
(T/C) | Type of tumor | Intervention and
comparison | Outcomes |
|---|
| Soria et al
(13) | 128/85 | Advanced NSCLC | Patients with
squamous NSCLC and/or CNS metastases received PC every 3 weeks
alone (arm 1; n=41) or with dulanermin 8 mg/kg for 5 days (arm 2;
n=39). Patients with non-squamous NSCLC received PCB alone (arm 3;
n=42) or with dulanermin 8 mg/kg for 5 days ‘(arm 4; n=40) or 20
mg/kg for 2 days (arm 5; n=41) | ORR, PFS, OS, safety,
pharmacodynamics biomarker analysis, GaINT 14 expression biomarker
analysis |
| Demetri et al
(14) | 86/42 | Metastatic or locally
advanced unresectable soft tissue sarcomas | This clinical trial
consists of three parts: phase I, phase II and rollover. In phase
II, patients were randomized (2:1) to receive doxorubicin with
either double-blind conatumumab 15 mg/kg (conatumumab-doxorubicin;
n=86) or placebo (placebo-doxorubicin; n=42) | PFS as primary
efficacy variable; OS, subgroup analysis of PFS according to FCGR3A
genotype, patient-reported outcomes, time to response and duration
of response as secondary efficacy variables; safety, biomarker
analysis, pharmacokinetics |
| Kindler et al
(15) | 41/42 | Metastatic pancreatic
cancer | Patients with
previously untreated metastatic pancreatic adenocarcinoma were
randomized 1:1:1 to i.v. gemcitabine 1,000 mg/m2 (days
1, 8 and 15 of each 28-day cycle) combined with open-label
ganitumab (12 mg/kg Q2W, n=42), double-blind conatumumab (10 mg/kg
Q2W, n=41), or double-blind placebo Q2W (n=42) | Survival and
response, safety |
| Paz-Ares et al
(16) | 113/59 | Advanced or recurrent
NSCLC | Patients (aged >18
years) with previously untreated advanced or recurrent NSCLC were
randomized 1:1:1 (stratified by ECOG performance status and disease
stage) to receive up to six 3-week cycles of PC combined with
conatumumab (arm 1, 3 mg/kg; arm 2, 15 mg/kg) or placebo (arm 3)
every 3 weeks Arm 1, n=57; arm 2, n=56; arm 3, n=59 | PFS as primary
end-point, ORR, toxicity, pharmacokinetics, subgroup analysis of OS
according to FCGR3A 158 polymorphisms |
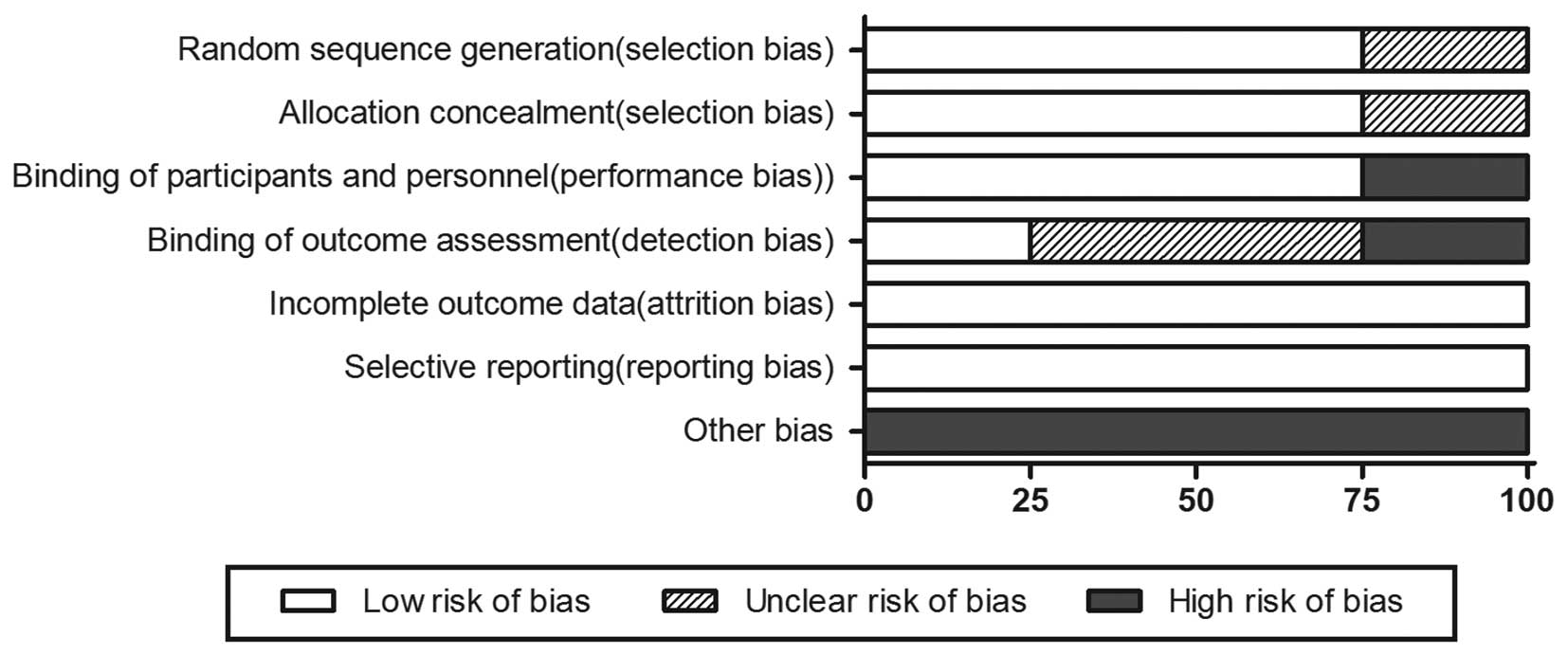
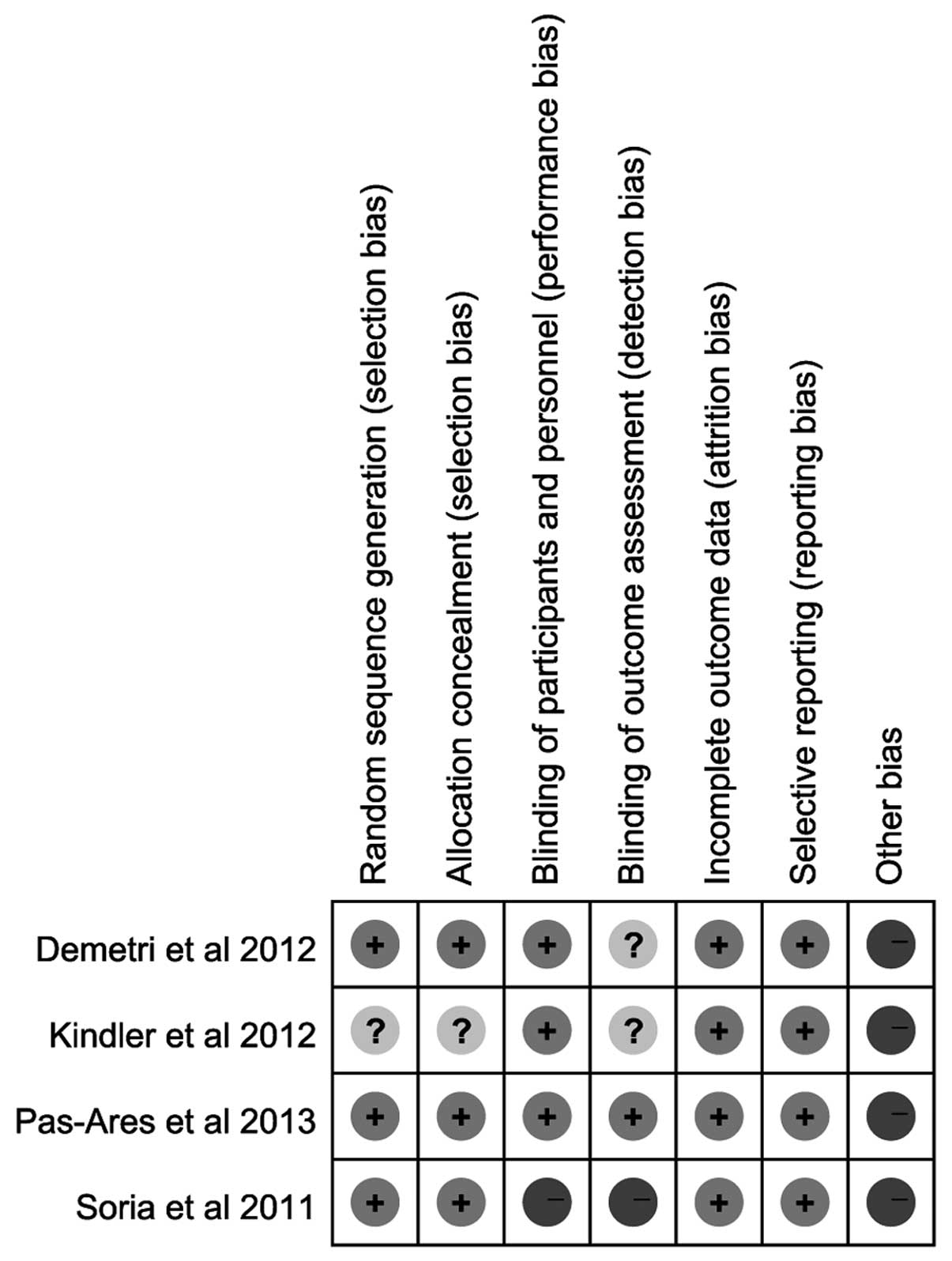
Risk of bias of the included RCTs
As shown in Figs. 2
and 3, according to the Cochrane
Collaboration’s tool for assessing the risk of bias, random
sequence generation and allocation concealment of 3 RCTs were
considered as low-risk for the proper application of the
stratification strategy and randomization methods (centralized
randomization). One RCT was considered to have an unclear risk of
bias. One RCT was described as an open-label trial, whereas the
remaining were described as double-blind trials. All the RCTs
provided complete outcome measurements that were assessed in this
meta-analysis. Other bias were considered as high risk, as there
were clear statements of company funding.
Meta-analysis
In this meta-analysis, data from 4 RCTs were
included. As the subgroup of the 4 RCTs was not consistent, we
considered the subgroups as independent clinical trials and
analyzed them separately. The data from Soria et al
(13) were divided into three
parts: i) paclitaxel plus carboplatin (PC) vs. PC + dulanermin; ii)
PC and bevacizumab (PCB) vs. PCB + dulanermin (8 mg/kg); and iii)
PCB vs. PCB + dulanermin (20 mg/kg). The data from Paz-Ares et
al (16) were divided into two
parts: i) PC + placebo vs. PC + conatumumab (3 mg/kg); and ii) PC +
placebo vs. PC + conatumumab (20 mg/kg).
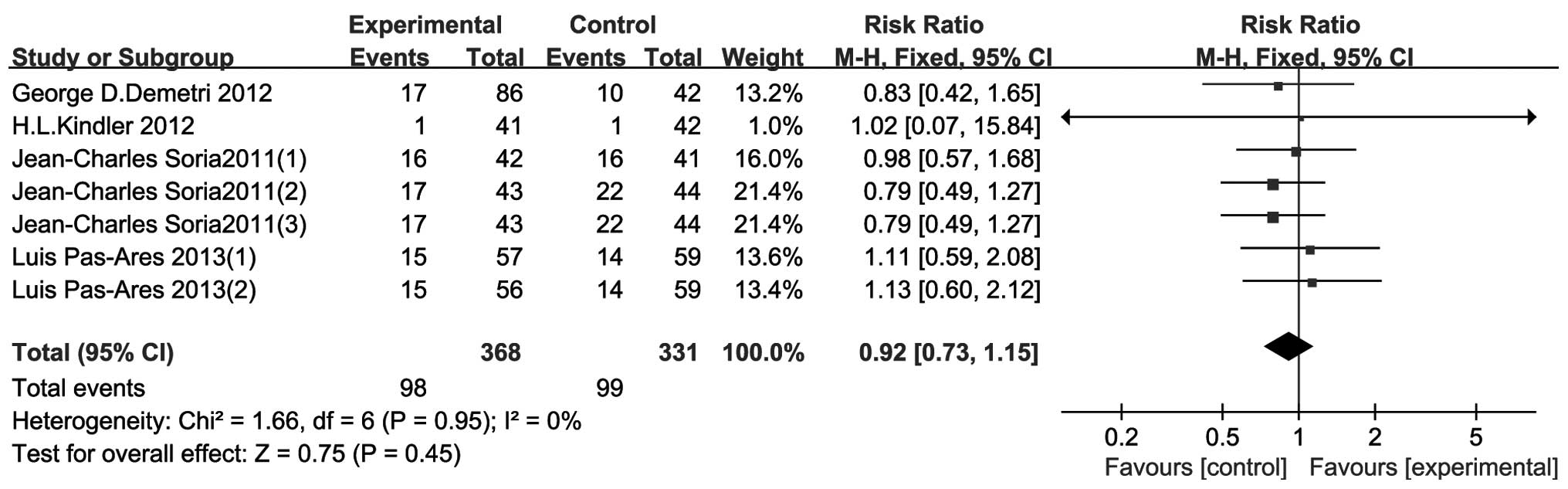
ORR
The results of the 4 included RCTs are shown in
Fig. 4. There was no heterogeneity
between studies (P=0.95, I2=0%). The results indicated
that treatment with TRAIL-related agents conferred no statistically
significant differences in the ORR compared to that of the control
group (RR=0.92, 95% CI: 0.73–1.15, P=0.45).
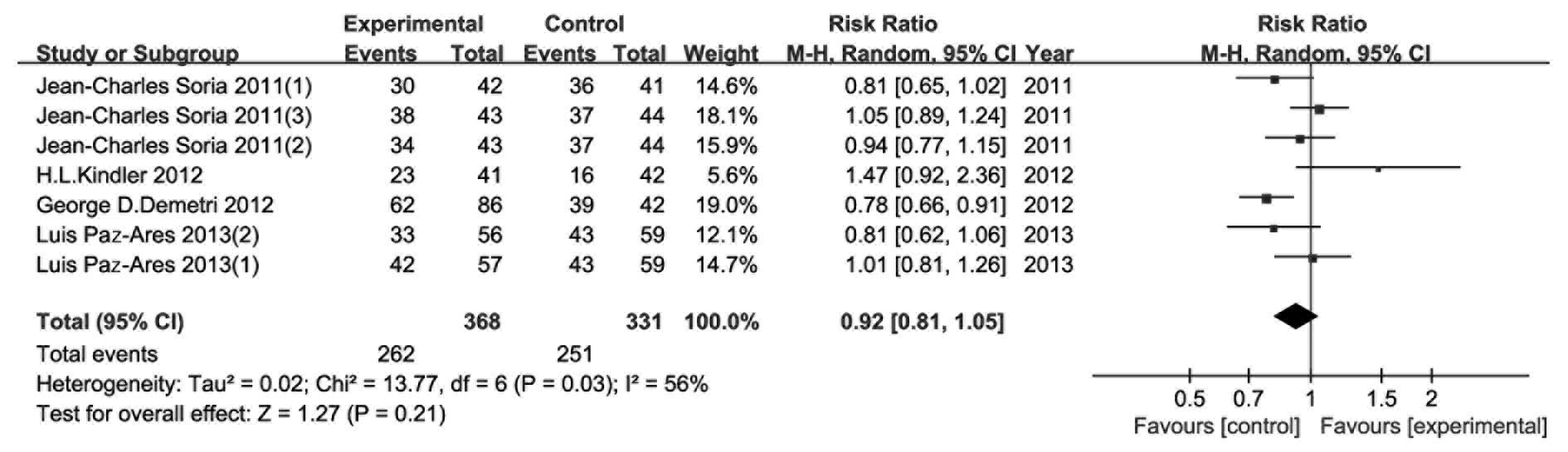
CBR/DCR
The 4 included RCTs provided CBR/DCR data and the
analysis of the outcome is shown in Fig. 5. The random effects model was
applied due to the median heterogeneity among the included studies
(P=0.03, I2=56%). The results indicated that the
addition of TRAIL-related agents conferred no significant benefits
to CBR/DCR (RR=0.92, 95% CI: 0.81–1.05, P=0.21).
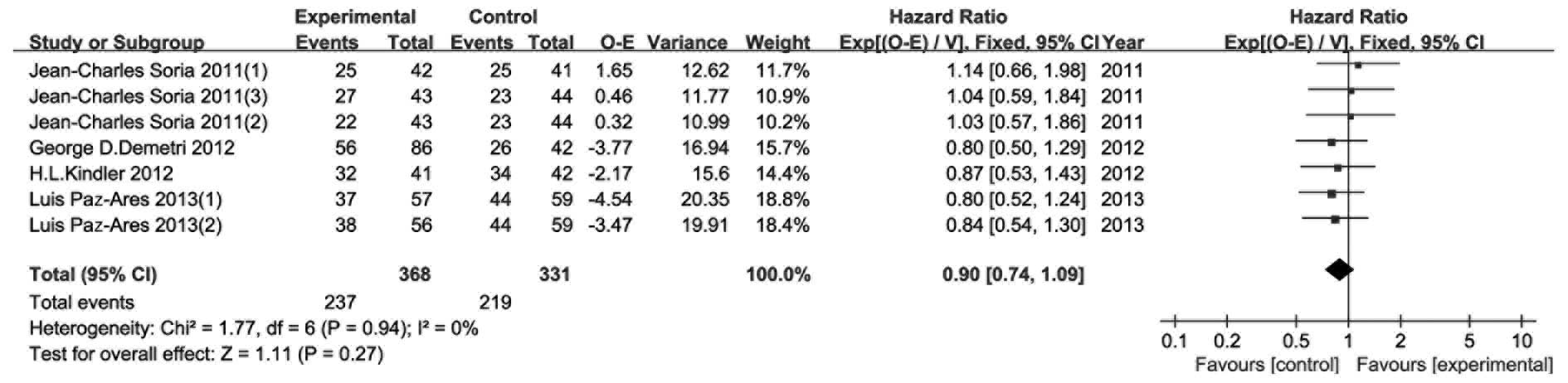
PFS
As the main endpoint of all 4 RCTs, PFS was reported
with PFS events, hazard ratio (HR) and 95% CI. According to the
method introduced by Tierney et al (12), we calculated two types of data (O-E
and its variance) in this analysis. As shown in Fig. 6, no heterogeneity was observed
(P=0.73, I2=0%) and a fixed effects model was used.
There were no statistically significant differences in PFS between
the experimental and control groups (HR=0.89, 95% CI: 0.75–1.05,
P=0.16).
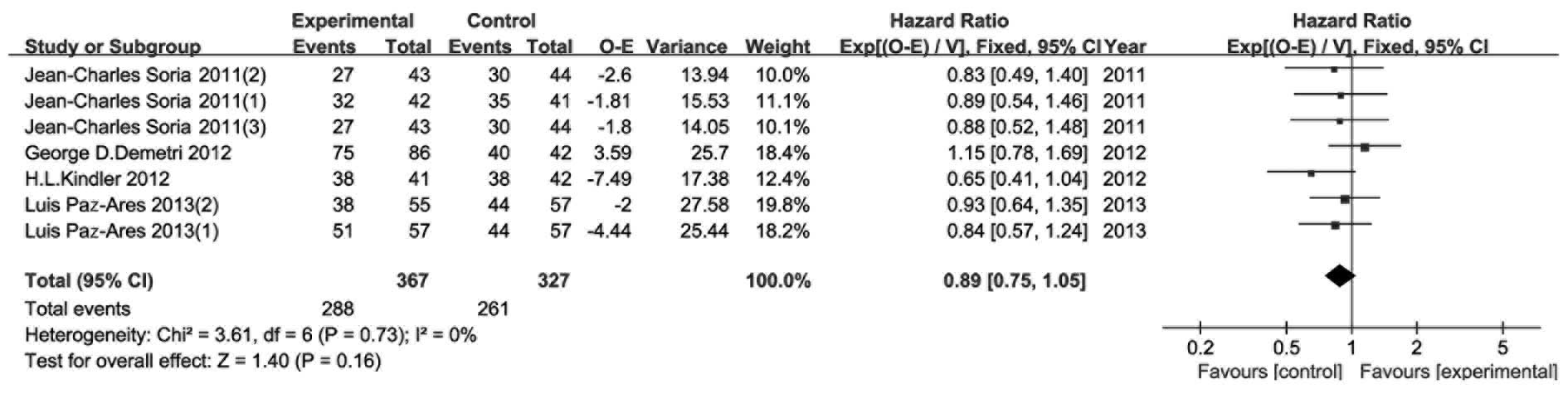
OS
The results of the OS meta-analysis are shown in
Fig. 7. OS was considered as a
secondary endpoint. The included RCTs reported OS events and HR
with 95% CI, except for one. We then calculated the required data
using the abovementioned methods. The fixed effects model was used,
as there was no observed heterogeneity (P=0.94, I2=0%).
There were no notable difference between the experimental and
control groups in terms of OS (HR=0.90, 95% CI: 0.74–1.09,
P=0.27).
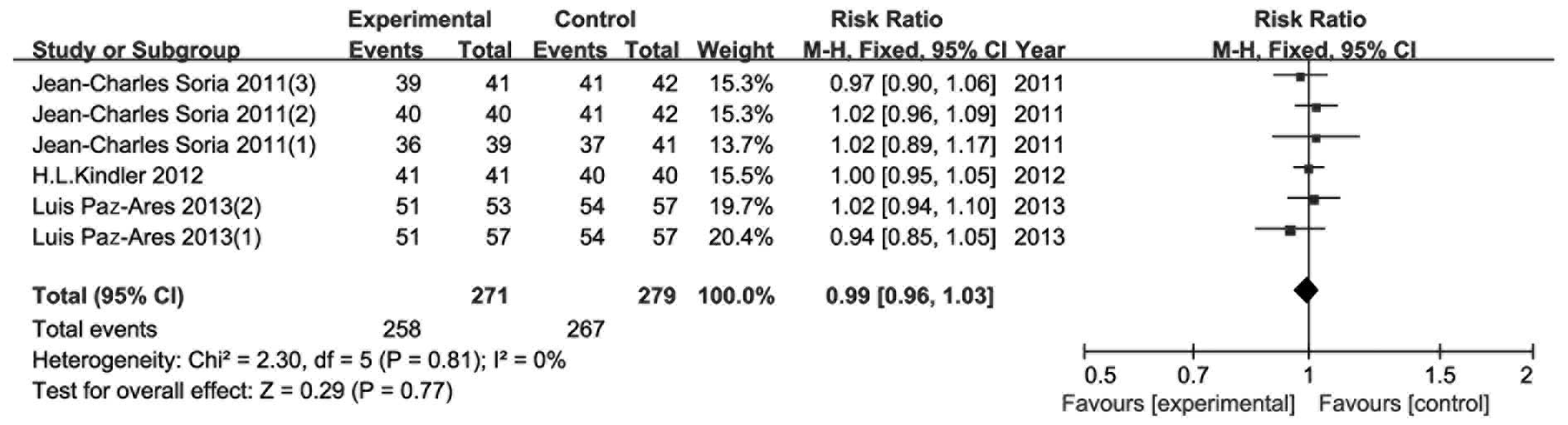
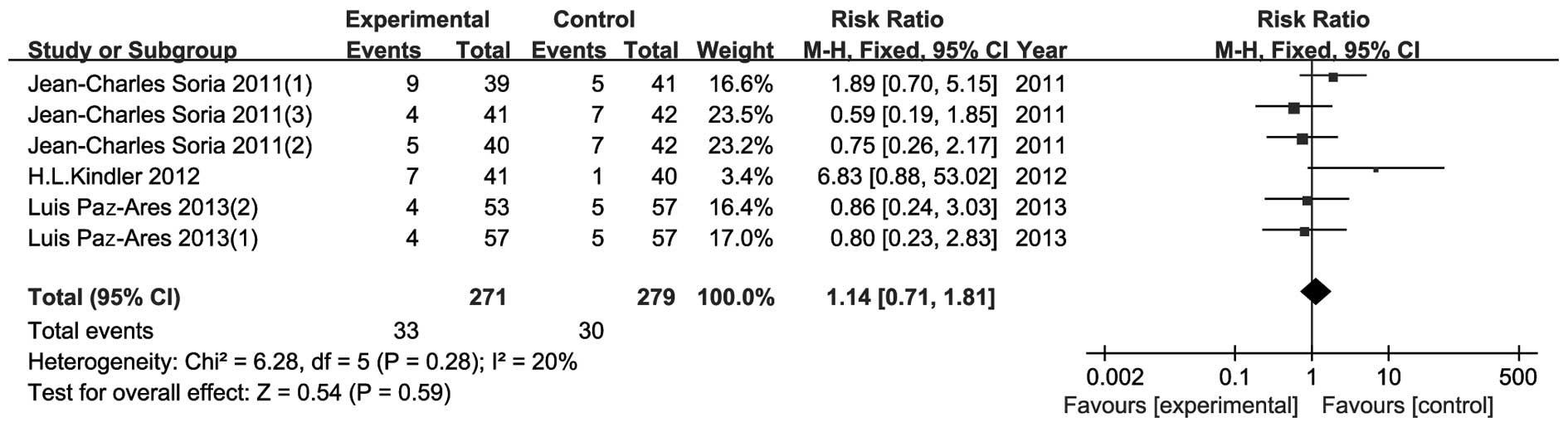
AEs
As shown in Figs.
8–11, AE data were presented
in four parts: i) number of patients with any AEs; ii) number of
patients with any severe AEs; iii) number of patients with ≥grade 3
AEs; and iv) fatal AEs. However, Demetri et al (14) presented their results differently
and therefore data from that study could not be included in this
meta-analysis. There were no significant differences in all four
parts between the experimental and the control groups. The risk
ratio (95% CI) was 0.99 (0.96–1.03, P=0.77), 1.13 (0.93–1.38,
P=0.22), 0.95 (0.78–1.15, P=0.58) and 1.14 (0.71–1.81, P=0.59) for
AEs, severe AEs, ≥grade 3 AEs and fatal AEs, respectively.
Sensitivity analysis
A sensitivity analysis was performed by excluding
studies one by one. The RRs, HRs, 95% CIs and P-values for ORR,
CBR/DCR, PFS, OS and AEs were similar to the results of the all-set
analysis. This indicated that no single study had bias on the
results of our meta-analysis.
GRADE assessment
The results of evidence quality and grade of
recommendation of the included RCTs are summarized in Table II. There were 8 outcome measures in
this analysis: i) ORR; ii) CBR/DCR; iii) PFS; iv) OS; v) number of
patients with any AEs; vi) number of patients with any severe AEs;
vii) number of patients with ≥grade 3 AEs; and viii) fatal AEs.
 | Table II.Evidence quality and recommendation
grade. |
Table II.
Evidence quality and recommendation
grade.
| Evidence quality
assessment | Evidence quality | Importance | Recommendation
grade |
|---|
|
|---|
| Outcome | No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias |
|---|
| ORR | 4 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| CBR | 4 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| PFS | 4 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| OS | 4 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| Patients with any
AEs | 3 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| Patients with any
severe AEs | 3 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| Patients with ≥
grade 3 AEs | 3 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
| Fatal AEs | 3 | RCT | Higha | No | No | No | Undetected | +++, moderate | Critical | Weak |
Discussion
Several phase I or II clinical trials assessing the
tolerability, pharmacokinetics (13–18),
safety and efficacy of TRAIL-related agents for the treatment of
cancer patients were conducted from 2004 onwards, reporting that
medicines based on TRAIL were tolerable and human anti-TRAIL
antibody was rarely detected (13–16).
However, the efficacy of TRAIL-related agents was not found to be
satisfactory. The TRAIL-based agents that were previously used in
clinical trials are summarized in Table III.
 | Table III.TRAIL-related agents in cancer
therapeutics. |
Table III.
TRAIL-related agents in cancer
therapeutics.
| Reagents | Target | Description | Company |
|---|
| rhTRAIL/Apo2L
(dulanermin) | DR4/DR5 | rhTRAIL | Amgena/Genentechb |
| HGS-ETR1
(mapatumumab) | DR4 | Fully human
MAb | Human Genome
Sciencesc |
| HGS-ETR2
(lexatumumab) | DR5 | Fully human
MAb | Human Genome
Sciencesc |
| HGS-TR2J
(KMTR-2) | DR5 | Fully human
MAb | Human Genome
Sciencesc |
| CS-1008 (TRA-8,
tigatuzumab) | DR5 | Humanized mouse
MAb | Daiichi
Sankyod |
| AMG 655
(conatumumab) | DR5 | Fully human
MAb | Amgena |
| PRO95780
(apomab) | DR5 | Fully human IgG1
MAb | Genetechb |
| LBY135 | DR5 | Humanized MAb | Novartise |
ORR was the main outcome in the majority of stage
II/III clinical trials. The number of patients with CR and PR
reflected the efficacy of chemotherapy. In our analysis, we failed
to demonstrate substantial evidence supporting the addition of
TRAIL-related agents to first-line treatments for cancer (RR=0.92,
95% CI: 0.73–1.15, P=0.45). However, our study had certain
limitations, which are discussed below. CBR was also used as an
index of efficacy. The number of patients with SD did not affect
our results. The median heterogeneity may be a result of the
different types of cancer and treatment. Although the included RCTs
had recruited patients with advanced-stage cancer, different types
of cancer exhibit differences in chemo-sensitivity.
Survival data were analyzed using the methods
described by Tierney et al (12). The results of PFS and OS indicated
that the addition of TRAIL-related agents conferred no significant
benefits to the patients. Furthermore, there was a tendency in
favour of the control groups, suggesting that the addition of
TRAIL-related agents should be avoided. The patients who were lost
during follow-up were ≤10% of the total patients and the results of
the survival analysis were considered to be reliable. However, the
method applied for this data type was not accurate, particularly
the O-E and variance of OS from the Kindler et al study
(15), which were estimated from
the survival curve using Engauge Digitizer 4.1 software. This lack
of accuracy may result in bias.
We also assessed AEs as an endpoint. The common AEs
included nausea, alopecia, fatigue, dyspnea, anemia and
neutropenia. The majority of the AEs were not associated with the
administration of TRAIL-related agents. Although we did not detect
a significant difference in this analysis, we consider the data to
be reliable. Furthermore, patients with advanced cancer tolerated
TRAIL-related agents well, which is consistent with the results of
the majority of the phase I clinical trials (13–18).
There were several limitations to this study. First,
the meta-analysis was limited to articles published in English,
leading to a selection bias in language. Second, the control groups
from 2 studies were used twice, which may enhance the effect of the
control group. Third, the total number of patients was limited and
all patients were diagnosed with advanced-stage cancer; therefore,
the prognosis was worse compared to that of patients with
early-stage cancer, which may have masked the effects of
TRAIL-related agents. Moreover, the 4 included RCTs experimented on
different types of cancer and treatment strategies and we had to
overlook the differences among them in order to gain preliminary
results on this topic. Finally, the treatment cost was not taken
into consideration; this, however, is a factor that may affect the
patients’ perspective regarding this type of treatment.
In conclusion, this meta-analysis compared the
outcome of patients with advanced cancer who were treated with or
without TRAIL-related agents. There were no significant differences
in all 8 outcome measures, including ORR, CBR/DCR, PFS, OS, number
of patients with any AEs, number of patients with any severe AEs,
number of patients with ≥grade 3 AEs and fatal AEs. Therefore, the
benefits of the addition of TRAIL-related agents to standard
chemotherapy regimens for the treatment of cancer patients remain
uncertain.
Acknowledgements
This study was supported by grants
from the National Nature Science Foundation of China (grant no.
81071908).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pitti RM, Marsters SA, Ruppert S, et al:
Induction of apoptosis by Apo-2 ligand, a new member of the tumor
necrosis factor cytokine family. J Biol Chem. 271:12687–12690.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pan G, O’Rourke K, Chinnaiyan AM, et al:
The receptor for the cytotoxic ligand TRAIL. Science. 276:111–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Pan G, Ni J, Wei YF, et al: An antagonist
decoy receptor and a death domain-containing receptor for TRAIL.
Science. 277:815–818. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rowinsky EK: Targeted induction of
apoptosis in cancer management: the emerging role of tumor necrosis
factor-related apoptosis-inducing ligand receptor activating
agents. J Clin Oncol. 23:9394–9407. 2005. View Article : Google Scholar
|
|
7.
|
Marsters SA, Sheridan JP, Pitti RM, et al:
A novel receptor for Apo2L/TRAIL contains a truncated death domain.
Curr Biol. 7:1003–1006. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: the potential of
recombinant human apoptosis ligand 2/tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar
|
|
9.
|
Bellail AC, Qi L, Mulligan P, et al: TRAIL
agonists on clinical trials for cancer therapy: the promises and
the challenges. Rev Recent Clin Trials. 4:34–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0. The Cochrane
Collaboration. 2011, https://www.cochrane-handbook.org
Accessed, September 20, 2013.
|
|
11.
|
Schunemann H, Brozek J and Oxman A: GRADE
handbook for grading the quality of evidence and the strength of
recommendations Version 3.2 (updated March 2009). GRADE Working
Group. 2009, Available from http:/www.cc-ims.net/gradepro.
Accessed September 20, 2013.
|
|
12.
|
Tierney JF, Stewart LA, Ghersi D, et al:
Practical methods for incorporating summary time-to-event data into
meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Soria JC, Mark Z, Zatloukal P, et al:
Randomized phase II study of dulanermin in combination with
paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell
lung cancer. J Clin Oncol. 29:4442–4451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Demetri GD, Le Cesne A, Chawla SP, et al:
First-line treatment of metastatic or locally advanced unresectable
soft tissue sarcomas with conatumumab in combination with
doxorubicin or doxorubicin alone: a phase I/II open-label and
double-blind study. Eur J Cancer. 48:547–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kindler HL, Richards DA, Garbo LE, et al:
A randomized, placebo-controlled phase 2 study of ganitumab (AMG
479) or conatumumab (AMG 655) in combination with gemcitabine in
patients with metastatic pancreatic cancer. Ann Oncol.
23:2834–2842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Paz-Ares L, Balint B, de Boer RH, et al: A
randomized phase 2 study of paclitaxel and carboplatin with or
without conatumumab for first-line treatment of advanced
non-small-cell lung cancer. J Thorac Oncol. 8:329–337.
2013.PubMed/NCBI
|
|
17.
|
Plummer R, Attard G, Pacey S, et al: Phase
1 and pharmaco-kinetic study of lexatumumab in patients with
advanced cancers. Clin Cancer Res. 13:6187–6194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tolcher AW, Mita M, Meropol NJ, et al:
Phase I pharmaco-kinetic and biologic correlative study of
mapatumumab, a fully human monoclonal antibody with agonist
activity to tumor necrosis factor-related apoptosis-inducing ligand
receptor-1. J Clin Oncol. 25:1390–1396. 2007. View Article : Google Scholar
|