Introduction
Gastric cancer (GC) is the third most common
malignancy and the second leading cause of cancer-related mortality
worldwide, particularly in East Asian countries. In China, the
majority of the GC patients are diagnosed at an advanced stage and,
despite the advances in chemotherapy and surgical techniques, the
5-year overall survival rate of patients with advanced-stage GC is
<20% (1–2). In GC cases, physicians are faced with
the following challenges: lack of markers for early diagnosis, weak
prognostic significance of histological indicators, limited
efficiency of current treatment for advanced GC and lack of
molecular markers for targeted therapy (3–5).
Therefore, it is of great clinical significance to achieve a better
understanding of gastric carcinogenesis and to identify novel
markers for the improvement of clinical management of patients with
GC.
Nucleophosmin (NPM), also referred to as protein
B23, numatrin and NO38, is a nucleolar phosphoprotein (6). As a multifunctional protein, NPM
participates in ribosome biosynthesis and centrosome duplication
and also acts as a molecular chaperone, promoting cell
proliferation and regulating apoptosis via the nuclear factor
(NF)-κB pathway (7–8). Positive expression of NPM has been
identified in several tumor types, including colorectal adenoma
(9), hepatocellular carcinoma
(10), prostate cancer (11), bladder cancer (12), ovarian cancer (13) and bronchial tumors (14). As regards GC, Tanaka et al
(15) reported that advanced GC
appears to exhibit higher NPM mRNA levels. However, the protein
expression and prognostic value of NPM in GC has not been
extensively investigated.
Trefoil factor 3 (TFF3), also referred to as
intestinal trefoil factor, is a member of the trefoil peptide
family. TFF3 is mainly secreted by goblet cells in the small and
large intestine. There is almost no TFF3 expression in normal
gastric mucosa. However, TFF3 expression may be detected in cases
with intestinalization or GC (16–17).
It was previously demonstrated that TFF3 may inhibit cell adhesion
and promote cell invasion by downregulating E-adhesin expression
(18), as well as blocking
apoptosis through the NF-κB signaling pathway (19). Previous studies also reported that
the positive expression of TFF3 was correlated with a poor
prognosis (20–21).
Based on the abovementioned knowledge, we
hypothesized that NPM and TFF3 played a key role in carcinogenesis.
It was recently demonstrated that there is a close association of
human epidermal growth factor receptor 2 with NPM and TFF3
expression and that their co-expression may play a pivotal role in
GC (22–23). Furthermore, there is evidence
demonstrating that NPM and TFF3 are involved in the regulation of
apoptosis via the NF-κB signaling pathway, prompting us to
determine the significance of their co-expression in GC. Therefore,
in the present study, we investigated the expression of NPM and
TFF3 in GC tissues and analyzed their correlation with
clinicopathological variables and prognosis.
Patients and methods
Patients and samples
GC and adjacent tissues were obtained from patients
who underwent radical gastrectomy at the Affiliated Hospital of
Qingdao University Medical College between November, 2007 and
March, 2009. All the patients met the following criteria: i) All
the tumors had been histologically confirmed as adenocarcinomas;
ii) no patients had received preoperative chemotherapy or
radiotherapy; and iii) all the patients were available for
follow-up. Finally, 108 patients were enrolled in this study.
Clinicopathological information, including gender, age (<60 vs.
≥60 years), tumor size (≤4 vs. >4 cm), tumor differentiation
(high, moderate or poor), lymph node metastasis, tumor stage and
primary vs. recurrent tumor, were obtained from the patients’
medical records and are summarized in Table I. The tumor-node-metastasis (TNM)
stage was assessed according to the 7th edition of the TNM
Classification of Malignant Tumors (24). Stages I and II were classified as
early, whereas stages III and IV were classified as advanced. The
median duration of follow-up was 31 months (range, 3–53 months).
For patients who remained alive until the cut-off date of
follow-up, survival duration was recorded as 53+
months.
 | Table INPM or TFF3 staining and
clinicopathological characteristics. |
Table I
NPM or TFF3 staining and
clinicopathological characteristics.
| | NPM staining | | TFF3 staining | | Co-expression of NPM
and TFF3 | |
|---|
| |
| |
| |
| |
|---|
| Characteristics | Cases (n=108) | Positive (n=57) | Negative (n=51) | P-value | Positive (n=54) | Negative (n=54) | P-value | Positive (n=25) | Negative (n=83) | P-value |
|---|
| Gender | | | | 0.1891 | | | 0.3993 | | | 0.4262 |
| Male | 76 | 37 | 39 | | 36 | 40 | | 16 | 60 | |
| Female | 32 | 20 | 12 | | 18 | 14 | | 9 | 23 | |
| Age (years) | | | | 0.5020 | | | 0.0195 | | | 0.7649 |
| <60 | 46 | 26 | 20 | | 17 | 29 | | 10 | 36 | |
| ≥60 | 62 | 31 | 31 | | 37 | 25 | | 15 | 47 | |
| Tumor size (cm) | | | | 0.4343 | | | 0.0005 | | | 0.0002 |
| ≤4 | 53 | 30 | 23 | | 17 | 36 | | 4 | 49 | |
| >4 | 55 | 27 | 28 | | 32 | 22 | | 21 | 34 | |
|
Differentiation | | | | 0.5652 | | | 0.0435 | | | 0.1529 |
| High | 5 | 2 | 3 | | 1 | 4 | | 0 | 5 | |
| Moderate | 10 | 4 | 6 | | 2 | 8 | | 1 | 9 | |
| Poor | 93 | 51 | 42 | | 51 | 42 | | 25 | 58 | |
| Lymph node
metastasis | | | | 0.2754 | | | 0.0116 | | | 0.0156 |
| Present | 61 | 35 | 26 | | 37 | 24 | | 23 | 38 | |
| Absent | 47 | 22 | 25 | | 17 | 30 | | 2 | 45 | |
| Tumor stage | | | | 0.0333 | | | 0.0244 | | | 0.0320 |
| Early | 26 | 9 | 17 | | 8 | 18 | | 2 | 24 | |
| Advanced | 82 | 48 | 34 | | 46 | 36 | | 23 | 59 | |
|
Primary/recurrence | | | | <0.0001 | | | 0.0116 | | | <0.0001 |
| Primary tumor | 80 | 33 | 47 | | 33 | 47 | | 10 | 70 | |
| Recurrence
tumor | 28 | 24 | 4 | | 21 | 7 | | 15 | 13 | |
The study protocol was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University Medical
College and informed consent was obtained from all the patients
prior to enrolment.
Immunohistochemistry
Paraffin-embedded tissue blocks were cut in 4-μm
sections. Following dewaxing and rehydration, the slides were
incubated in peroxidase-blocking solution to block endogenous
peroxidase activity. For antigen retrieval, all the sections were
incubated in citrate buffer solution (pH 6.0) in a microwave oven
for 20 min. Subsequently, the specimens were incubated at 37°C for
90 min in anti-NPM mouse monoclonal antibody (dilution, 1:100;
Abcam, Cambridge, MA, USA) or in TFF3 monoclonal mouse antibody
(dilution, 1:100; Abcam). The sections were then incubated with
reagent 1 and 2 of the PV9005 mouse hypersensitivity two-step
immunohistochemical kit (Beijing fir Jinqiao, Beijing, China) for a
total duration of 60 min at 37°C in a humid chamber. Finally, the
staining was visualized with diaminobenzidine. The slides were
counterstained with hematoxylin, washed in tap water for 10 min and
then mounted. Between each pair of steps, the sections were rinsed
with phosphate-buffered saline (PBS). A negative control reaction
was set with PBS replacing the anti-NPM or anti-TFF antibody, while
known positive-stained sections were used as positive control.
Scoring of immunostaining
Each slide was scored by two experienced
pathologists who were blinded to the clinical outcome.
Immunohistochemical staining was assessed semiquantitatively by
measuring the extent of staining (0, 0%; 1, 0–10%; 2, 10–50%; and
3, 50–100%) as well as the intensity of staining (0, no staining;
1, yellow; 2, brown-to-yellow; and 3, brown staining). The weighted
score for each case according to the intensity and extent of
staining were multiplied [0, negative (−); 1–4, weakly positive
(+); 5–8, moderately positive (++); and 9–12, strongly positive
(+++)]. The weighted scores of 0–4 were considered as negative and
5–12 as positive.
Statistical analysis
The Chi-square test was used to analyze the
association between the expression of NPM and TFF3 and
clinicopathological characteristics. Survival curves were plotted
with the Kaplan-Meier method. The significance of the difference
between groups was assessed with the log-rank test. Multivariate
analyses (Cox proportional hazard regression models) were performed
to assess the prognostic value of NPM and TFF3. The clinical
variables included gender, age, tumor size, differentiation, lymph
node metastasis and stage. SAS 9.2 software (SAS Institute, Inc.,
Cary, NC, USA) was used for statistical analysis. P≤0.05 was
considered to indicate a statistically significant difference.
Results
NPM and TFF3 immunohistochemical
staining
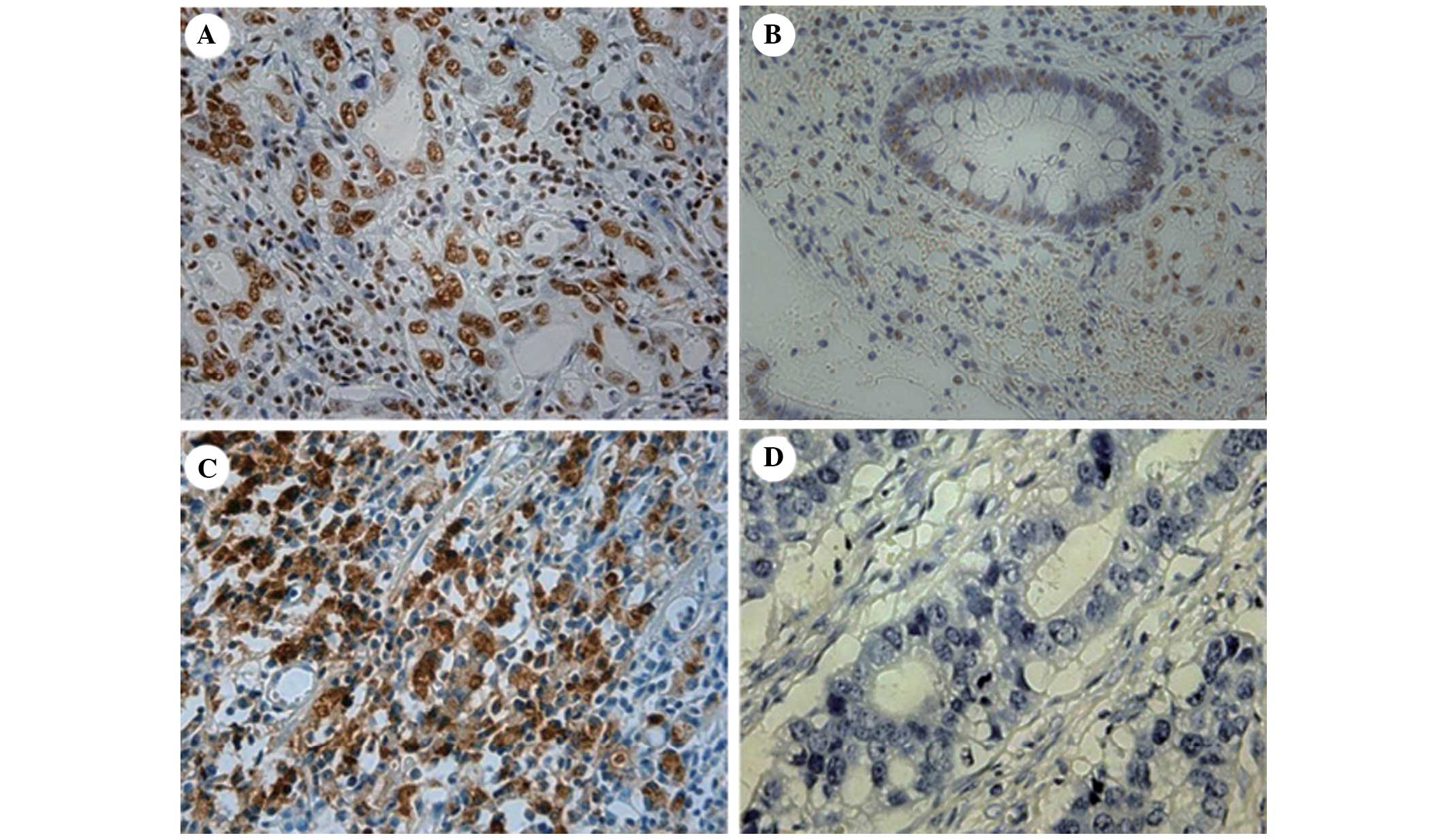
NPM was mainly expressed in the nucleoli, nuclei and
cytoplasm of tumor epithelial cells (Fig. 1A and B) and TFF3 was mainly
expressed in the cytoplasm (Fig. 1C
and D). Of the 108 specimens, the positive expression rates of
NPM in neoplastic tissue and adjacent gastric mucosa were 53 and
37% (P<0.05), respectively. Of the 57 NPM-positive neoplastic
tissue specimens, 25 were TFF3-positive and 32 TFF3-negative. Of
the 54 TFF3-positive neoplastic tissue specimens (50%), 25 were
NPM-positive and 29 NPM-negative (Table II). No moderate or strong positive
expression was identified in the adjacent gastric mucosa. By
correlation analysis, no significant association was found between
the expression of NPM and TFF3 (r=0.11119, P=0.2520).
 | Table IIExpression of NPM and TFF3. |
Table II
Expression of NPM and TFF3.
| TFF3
expression | NPM expression | Total |
|---|
|
|---|
| Positive | Negative |
|---|
| Positive | 25 | 29 | 54 |
| Negative | 32 | 22 | 54 |
| Total | 57 | 51 | 108 |
Correlation between NPM, TFF3 and
clinicopathological characteristics
We analyzed the associations between NPM and TFF3
expression and clinical characteristics (Table I)and demonstrated that NPM-positive
expression was correlated with advanced tumor stage (P=0.0333). Of
the 80 cases with primary tumor, 33 exhibited positive staining for
NPM, whereas of the 28 cases with recurrence, 24 exhibited positive
staining for NPM, suggesting that NPM was associated with
recurrence (P<0.0001). There was no statistically significant
association between NPM expression and age (P=0.5020), gender
(P=0.1891), tumor size (P=0.4343) or differentiation
(P=0.5652).
As regards TFF3, there were significant correlations
between TFF3-positive expression and advanced age (P=0.0195),
larger tumor size (P=0.0005) and poor differentiation (P=0.0435),
whereas, by further pairwise comparison, a significant difference
was found between moderate and poor differentiation (P=0.0312, data
not shown), lymph node metastasis (P=0.0116), advanced tumor stage
(P=0.0244) and recurrence (P=0.0116). There was no significant
association between TTF3 and gender (P=0.3993).
The co-expression of NPM and TFF3 was associated
with large tumor size (P=0.0002), lymph node metastasis (P=0.0156),
advanced tumor stage (P=0.0320) and recurrence (P<0.0001). There
was no significant association between the co-expression of these
two factors and other clinicopathological parameters.
Prognostic significance of NPM and
TFF3
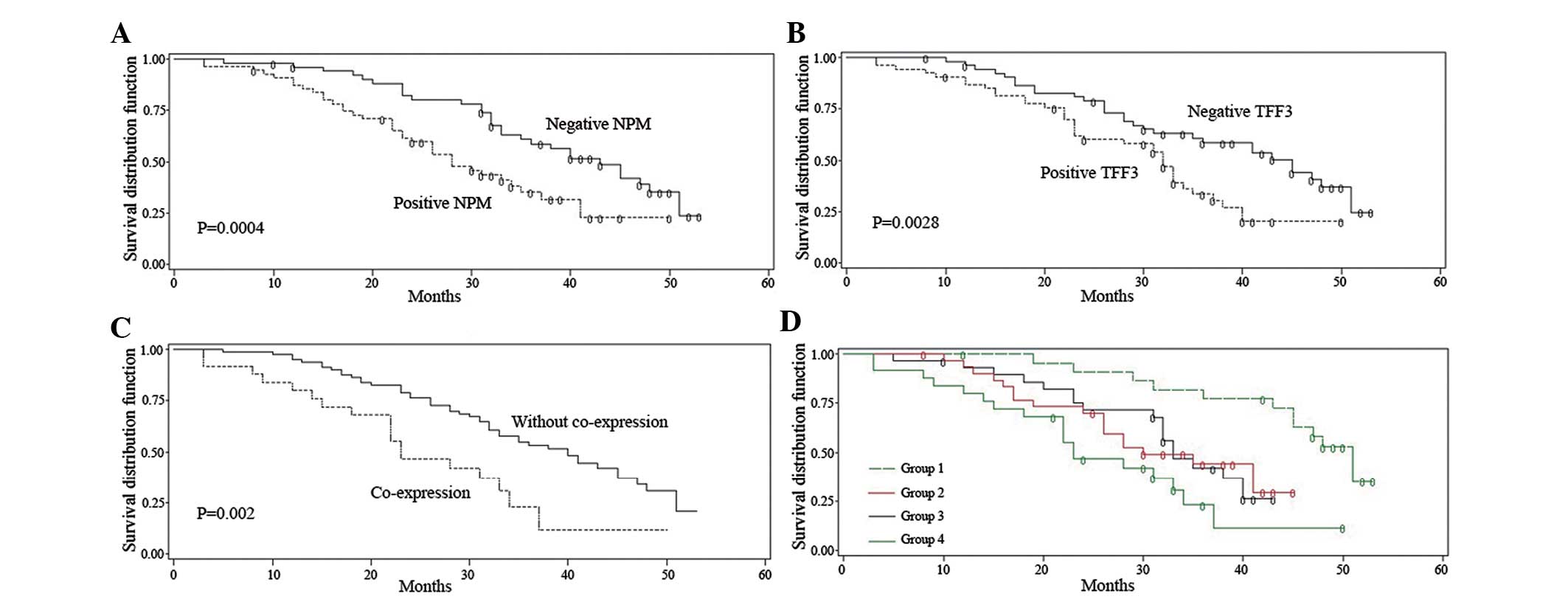
To assess the prognostic significance of NPM and
TFF3, Kaplan-Meier survival curves were constructed. A
statistically significant correlation was observed between
NPM-positive expression and poor survival (P=0.0004, log-rank test,
Fig. 2A). TFF3-positive expression
was associated with a poor survival rate compared to TFF3-negative
expression (P=0.0028, log-rank test, Fig. 2B). Patients with co-expression of
NPM and TFF3 exhibited lower survival rates compared to patients
without co-expression of the two factors (P=0.0020, log-rank test,
Fig. 2C).
We also divided the samples into 4 subgroups based
on the different expressions of NPM and TFF3. NPM-negative and
TFF3-negative was designated as group 1; NPM-negative and
TFF3-positive was designated as group 2; NPM-positive and
TFF3-negative was designated as group 3; and NPM-positive and
TFF3-positive was designated as group 4. The survival curves
indicated that patients with co-expression of NPM and TFF3
exhibited the lowest survival rate when compared to the other 3
groups (Fig. 2D). Correspondingly,
patients with negative expression for both markers exhibited the
highest survival rate, while patients expressing either NPM or TFF3
exhibited intermediate survival rates (Fig. 2D). The detailed survival data of
the 4 subgroups are described in Table III.
 | Table IIISurvival data of 4 subgroups. |
Table III
Survival data of 4 subgroups.
| Subgroups | Median survival
time (months) | Survival time
interval (months) | Survival rate |
|---|
|
|---|
| 12 months | 24 months | 36 months | 48 months |
|---|
| NMP−, TFF3− | 39 |
12–53+ | 1.0000 | 0.8696 | 0.6857 | 0.3532 |
| NPM−, TFF3+ | 32 |
5–43+ | 0.9298 | 0.7152 | 0.4197 | 0.3257 |
| NPM+, TFF3− | 28 |
8–45+ | 0.9333 | 0.7000 | 0.4410 | N/A |
| NPM+, TFF3+ | 23 |
3–50+ | 0.8000 | 0.5100 | 0.2119 | 0.2119 |
The univariate analysis revealed that NPM-positive
expression, TFF3-positive expression, co-expression of NPM and
TFF3, age, tumor stage and lymph node metastasis were significantly
associated with poor prognosis. By Cox regression survival
analysis, NPM-positive expression, TFF3-positive expression,
co-expression of NPM and TFF3, age and tumor stage were identified
as independent prognostic factors in postoperative GC patients
(Table IV).
 | Table IVUnivariate and multivariate survival
analysis. |
Table IV
Univariate and multivariate survival
analysis.
| Parameters | Univariate analysis
P-value (Log-rank test) | Cox regression
survival analysis |
|---|
|
|---|
| Chi-square | P-value | Hazard ratio | 95% CI |
|---|
| NPM-positive
expression | 0.0004 | 57.849 | 0.0162 | 1.970 | 1.134–3.422 |
| TFF3-positive
expression | 0.0028 | 70.962 | 0.0077 | 2.021 | 1.204–3.391 |
| Co-expression of
NPM and TFF3 | 0.0020 | 88.712 | 0.0029 | 2.339 | 1.337–4.092 |
| Age | 0.0261 | 44.381 | 0.0351 | 1.765 | 1.040–2.994 |
| Tumor stage | 0.0004 | 174.061 | <.0001 | 5.539 | 2.478–12.379 |
| Tumor size | 0.2028 | 13.231 | 0.2500 | 1.351 | 0.809–2.257 |
|
Differentiation | 0.7221 | 0.5422 | 0.8775 | 1.056 | 0.530–2.103 |
| Lymph node
metastasis | 0.0356 | 94.943 | 0.0021 | 2.604 | 1.417–4.787 |
Discussion
Focusing on different molecular markers may help us
understand the histopathological characteristics of GC by detecting
the expression of different and specific genes in GC tissues. In
our study, we demonstrated that NPM overexpression was an
independent prognostic factor in postoperative GC patients. In
particular, we demonstrated that the co-expression of NPM and TFF3
was associated with a more aggressive biological behavior and poor
prognosis in GC patients.
NPM, the focus of several recent cancer studies,
plays a critical role in the development and progression of
malignant tumors. Although evidence demonstrates that NPM
expression is correlated with unfavorable clinical characteristics
and poor prognosis in several types of solid tumors, the precise
role of NPM in GC has not been clearly determined. In the present
study, we observed that the positive expression of NPM was mainly
localized to the nucleus and cytoplasm in GC cells, consistent with
a previous study reporting that NPM shuttled between the nucleus
and cytoplasm in other tumor types (6). It was previously demonstrated that a
higher NPM mRNA expression was detected in gastric tumors (12). In this study, we detected NPM
expression at the protein level and found that a positive
expression of NPM was inversely correlated with prognosis in
patients who underwent radical gastric resection. Moreover, in the
present study, we observed that NPM was associated with lymph node
metastasis and tumor recurrence, which was consistent with previous
findings that reported NPM overexpression in 73% of GC patients
with disease recurrence (25).
Therefore, downregulating the level of NPM expression in GC may
decrease the recurrence rate. Interestingly, we did not observe any
significant differences in NPM expression between
well-differentiated and poorly differentiated tumors, while Tsui
et al (26) reported that
NPM was also associated with differentiation in bladder tumors.
This discordance may due to the different histological type (the GC
type was adenocarcinoma, whereas the bladder carcinoma was of the
transitional cell type). Based on this difference, it is reasonable
to hypothesize that NPM may have different functions in different
histological tumor types. However, as the number of studies
focusing on NPM in gastric tumors is limited, the mechanism of NPM
in gastric tumorigenesis has not been clearly determined and
further studies are required to elucidate it.
As regards TFF3, we observed that the positive
expression of TTF3 was associated with tumor size, differentiation,
lymph node metastasis and tumor stage (Table I), suggesting that TFF3 may play an
important role in the development, progression and dissemination of
GC. Recently, Meng et al (27) demonstrated that the positive
expression of TFF3 was significantly associated with a lower
survival rate in GC compared to negative expression; however, in
that study, a multivariate analysis was not performed. In the
present study, by multivariate analysis, we confirmed that the
positive expression of TFF3 was an independent prognostic
indicator, consistent with the findings of previous studies
(20,21). However, Dhar et al (28) reported that TFF3 expression was
prognostically significant only in female patients, but not in the
overall patient population. In our study, we conducted an
additional survival analysis separately for female, male and
overall patients and demonstrated that TFF3 was of prognostic
significance in all three groups (data not shown), although a
larger sample size is required for validation.
We also demonstrated that the co-expression of NPM
and TFF3 predicted the poorest prognosis (Fig. 2D). Recent studies also demonstrated
that both NPM and TFF3 are involved in the regulation of apoptosis
via the NF-κB pathway (7,8,19).
Tobita et al (29) reported
that E2F1, which is implicated in hepatocarcinogenesis, may
increase the expression of TFF3 by upregulating DNA-binding protein
A, which is associated with advanced stages of human hepatocellular
carcinoma. In addition, the activation of E2F1 is regulated by
NPM/B23 via modulating the promoter binding of NF-κB (8). These findings, taken together with
ours, suggest that NPM and TFF3 may be involved in the occurrence
or development of GC, possibly through interacting with each other
in the NF-κB pathway. Therefore, the co-detection of NPM and TFF3
may serve as a more predictive index for GC patients. However,
whether NPM and TFF3 act independently or cooperatively to increase
the malignant potential of GC requires further investigation. In
the present study, the correlation analysis identified no
significant correlation between the protein level of NPM and TFF3
(r=0.11119, P=0.2520, Table II).
In the future, further studies on NPM and TFF3, possibly at the
nucleic acid level, should be conducted to elucidate whether there
is an interaction, as well as the type of that interaction between
these two factors in GC.
In conclusion, the present study provided direct
evidence that NPM is an independent prognostic factor in
postoperative GC patients. We also confirmed that TFF3 expression
is associated with poor prognosis. Of note, the co-expression of
NPM and TFF3 proved to be an independent prognostic factor in
postoperative GC patients. The combined detection of the
co-expression of the two factors may serve as a more predictive
index of GC patient prognosis. However, the molecular mechanism
underlying the association of NPM and TFF3 requires elucidation by
further studies.
Acknowledgements
This study was supported by the Shandong Natural
Science Foundation (grant no. 2009HW024), the Shandong Excellent
Young Scientist Research Award Fund Project (grant nos.
2006BSB14114 and BS2010YY013) and the Shandong Tackle Key Problems
in Science and Technology (grant no. 2010GSF10245).
References
|
1
|
He W, Tang B, Yang D, et al:
Double-positive expression of high-mobility group box 1 and
vascular endothelial growth factor C indicates a poorer prognosis
in gastric cancer patients. World J Surg Oncol. 11:1612013.
View Article : Google Scholar
|
|
2
|
Zhang YZ, Zhang LH, Gao Y, et al:
Discovery and validation of prognostic markers in gastric cancer by
genome-wide expression profiling. World J Gastroenterol.
17:1710–1717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia YF, Xiao DJ, Ma XL, et al:
Differentiated embryonic chondrocyte-expressed gene 1 is associated
with hypoxia-inducible factor 1alpha and Ki67 in human gastric
cancer. Diagn Pathol. 8:372013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin J, Jin T, Quan M, Piao Y and Lin Z:
Ezrin overexpression predicts the poor prognosis of gastric
adenocarcinoma. Diagn Pathol. 7:1352012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sotoudeh K, Hashemi F, Madjd Z,
Sadeghipour A, Molanaei S and Kalantary E: The clinicopathologic
association of c-MET overexpression in Iranian gastric carcinomas;
an immunohistochemical study of tissue microarrays. Diagn Pathol.
7:572012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grisendi S, Mecucci C, Falini B and
Pandolfi PP: Nucleophosmin and cancer. Nat Rev Cancer. 6:493–505.
2006. View
Article : Google Scholar
|
|
7
|
Khandelwal N, Simpson J, Taylor G, Rafique
S, Whitehouse A, Hiscox J and Stark LA: Nucleolar NF-kappaB/RelA
mediates apoptosis by causing cytoplasmic relocalization of
nucleophosmin. Cell Death Differ. 18:1889–1903. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CY, Liang YC and Yung BY:
Nucleophosmin/B23 regulates transcriptional activation of E2F1 via
modulating the promoter binding of NF-kappaB, E2F1 and pRB. Cell
Signal. 18:2041–2048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nozawa Y, Van Belzen N, Van der Made AC,
Dinjens WN and Bosman FT: Expression of nucleophosmin/B23 in normal
and neoplastic colorectal mucosa. J Pathol. 178:48–52. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yun JP, Miao J, Chen GG, et al: Increased
expression of nucleophosmin/B23 in hepatocellular carcinoma and
correlation with clinicopathological parameters. Br J Cancer.
96:477–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subong EN, Shue MJ, Epstein JI, Briggman
JV, Chan PK and Partin AW: Monoclonal antibody to prostate cancer
nuclear matrix protein (PRO:4–216) recognizes nucleophosmin/B23.
Prostate. 39:298–304. 1999.
|
|
12
|
Tsui KH, Cheng AJ, Chang Pe, Pan TL and
Yung BY: Association of nucleophosmin/B23 mRNA expression with
clinical outcome in patients with bladder carcinoma. Urology.
64:839–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y: The ARF-B23 connection:
implications for growth control and cancer treatment. Cell Cycle.
3:259–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mascaux C, Bex F, Martin B, et al: The
role of NPM, p14arf and MDM2 in precursors of bronchial squamous
cell carcinoma. Eur Respir J. 32:678–686. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka M, Sasaki H, Kino I, Sugimura T and
Terada M: Genes preferentially expressed in embryo stomach are
predominantly expressed in gastric cancer. Cancer Res.
52:3372–3377. 1992.PubMed/NCBI
|
|
16
|
Muskett FW, May FE, Westley BR and Feeney
J: Solution structure of the disulfide-linked dimer of human
intestinal trefoil factor (TFF3): the intermolecular orientation
and interactions are markedly different from those of other dimeric
trefoil proteins. Biochemistry. 42:15139–15147. 2003. View Article : Google Scholar
|
|
17
|
Katoh M: Trefoil factors and human gastric
cancer (Review). Int J Mol Med. 12:3–9. 2003.
|
|
18
|
Meyer zum Buschenfelde D, Hoschutzky H,
Tauber R and Huber O: Molecular mechanisms involved in TFF3
peptide-mediated modulation of the E-cadherin/catenin cell adhesion
complex. Peptides. 25:873–883. 2004.PubMed/NCBI
|
|
19
|
Chen YH, Lu Y, De Plaen IG, Wang LY and
Tan XD: Transcription factor NF-kappaB signals antianoikic function
of trefoil factor 3 on intestinal epithelial cells. Biochem Biophys
Res Commun. 274:576–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung WK, Yu J, Chan FK, et al: Expression
of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas,
intestinal metaplasia, and non-neoplastic gastric tissues. J
Pathol. 197:582–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamachika T, Werther JL, Bodian C, et al:
Intestinal trefoil factor: a marker of poor prognosis in gastric
carcinoma. Clin Cancer Res. 8:1092–1099. 2002.PubMed/NCBI
|
|
22
|
Zhou F, Qiu W, Sun L, et al: Clinical
significance of nucleophosmin/B23 and human epidermal growth factor
receptor 2/neu expressions in gastric cancers. APMIS. 121:582–591.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu CC, Yue L, Wei HJ, et al: Significance
of TFF3 protein and Her-2/neu status in patients with gastric
adenocarcinoma. Pathol Res Pract. 209:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu D, Huang Y, Geng Q, Guan Y, Li Y, et
al: Effect of lymph node number on survival of patients with lymph
node-negative gastric cancer according to the 7th edition UICC TNM
system. PLoS One. 7:e386812012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding A, Zhao W, Shi X, et al: Impact of
NPM, TFF3 and TACC1 on the prognosis of patients with primary
gastric cancer. PLoS One. 8:e821362013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsui KH, Juang HH, Lee TH, Chang PL, Chen
CL and Yung BY: Association of nucleophosmin/B23 with bladder
cancer recurrence based on immunohistochemical assessment in
clinical samples. Acta Pharmacol Sin. 29:364–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng JR, Tang HZ, Zhou KZ, Shen WH and Guo
HY: TFF3 and survivin expressions associate with a lower survival
rate in gastric cancer. Clin Exp Med. 13:297–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dhar DK, Wang TC, Tabara H, et al:
Expression of trefoil factor family members correlates with patient
prognosis and neoangiogenesis. Clin Cancer Res. 11:6472–6478. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tobita H, Kajino K, Inami K, et al: Gene
expression profile of DNA binding protein A transgenic mice. Int J
Oncol. 29:673–679. 2006.PubMed/NCBI
|