Introduction
Myelodysplastic syndromes (MDS) are disorders
characterized by ineffective bone marrow hematopoiesis, clonal
proliferation of hematopoietic stem cells and increased apoptosis
(1), resulting in peripheral
cytopenias. The incidence of MDS increases with age and it is most
prevalent in male Caucasians. A recent study using the
SEER-Medicare database revealed that the incidence of MDS was as
high as 75 per 100,000 individuals aged ≥65 years; however, the
incidence was <5 per 100,000 individuals aged ≤50 years
(2). With the growth of the aging
population worldwide, the MDS burden of disease is expected to
escalate in the near future. To date, the most definitive risk
factors for MDS are age, ethnicity and smoking (3,4).
However, clear differences between ethnic and geographical
populations have yet to be defined. Hence, further investigation of
additional risk and protective factors is required to improve
prevention and early diagnosis of MDS.
Alcohol consumption is commonly practiced worldwide.
Alcohol is associated with certain diseases and is estimated to
account for 3.2% of all cancer cases and 5% of cancer-related
deaths worldwide (5–7). In epidemiological studies, including
cohort and case-control studies, the possible association between
alcohol intake and the risk of MDS has been investigated, but the
findings are inconclusive. Two case-control studies reported a
positive correlation between alcohol intake and MDS (8,9),
whereas 6 case-control studies reported a non-significant
association between the two (10–15).
By contrast, one prospective cohort study suggested that alcohol
consumption may decrease the risk of MDS (16). These conflicting reports are
reflective of the complex effects of alcohol consumption on the
human body, as well as the heterogeneity of the parameters used
among the different studies. We performed a meta-analysis of all
relevant published literature in order to better define the
association between alcohol consumption and MDS.
Materials and methods
Literature research
A systematic literature search was conducted by two
independent reviewers (Chao Hu and Mengxia Yu) using PubMed, the
Cochrane Library and Web of Science. The following search
specifications were used: alcohol, or ethanol, or drinking
behavior, or alcoholic beverages, or wine, or beer or liquor, or
spirit and myelodysplastic syndrome, or MDS, or myelodysplastic, or
myelodysplasia, or preleukemia. The titles and abstracts of the
relevant articles were selected and full-text articles were
retrieved. We also reviewed the reference lists from original
articles for additional studies not identified in the database
search.
Study selection
The studies included in this meta-analysis were
required to meet all the following criteria: i) cohort or
case-control studies; ii) the exposure of interest was alcohol
intake; iii) the outcome of interest was MDS; iv) risk and
corresponding 95% confidence intervals (CIs) were reported or could
be calculated from the data; and v) the identified studies were
written in the English language. If there were multiple
publications from the same study or overlapping study populations,
only the study with the largest number of cases was included in the
meta-analysis.
Data extraction
The following data were extracted from each study
and included in the final analysis: first author’s name, year of
publication, country of origin, gender, age, study design, source
of patients, number of cases/controls, risk factor assessment,
matching and adjusted covariates. We contacted the corresponding
authors of the primary studies for missing information when
necessary. As MDS is a rare disease, the relative risk in
prospective cohort studies is approximately equivalent to the odds
ratio (OR) (17). This allows data
from cohort and case-control studies to be combined and OR to be
used as a measure of outcome (18). Data extraction was performed
independently by two reviewers using a predefined data collection
form. To resolve any discrepancies, a third reviewer also extracted
the data and the results were attained by consensus. The quality of
each study was appraised independently by two reviewers who used
the nine-score Newcastle-Ottawa Scale (19).
Statistical analysis
A fixed-effects model with the method of
Mantel-Haenszel was used to calculate a pooled OR with 95% CI when
there was no heterogeneity (20).
Otherwise, the random-effects model with the method of DerSimonian
and Laird (21) was used for
pooling ORs. Heterogeneity was assessed by using the Q statistic
and the I2 score. P>0.05 for the Q-test was
considered as a lack of heterogeneity among the studies. While
publication bias was not expected, we assessed this possibility
using Begg’s funnel plots (rank correlation method, where an
asymmetrical plot indicated a possible publication bias) (22) and Egger’s bias test (linear
regression method, where P<0.05 suggested the presence of
statistically significant publication bias) (23). A sensitivity analysis was conducted
by sequential omission of studies under various contrasts to
reflect the effect of individual data on the pooled ORs and
evaluate the stability of the results. Stratified analyses were
performed by alcohol history, gender, ethnicity, study design,
sample source, disease subtype and quantity of alcohol per day. All
the statistical analyses were conducted with STATA 11.0 software
(StataCorp, College Station, TX, USA) using two-tailed P-values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search and study
characteristics
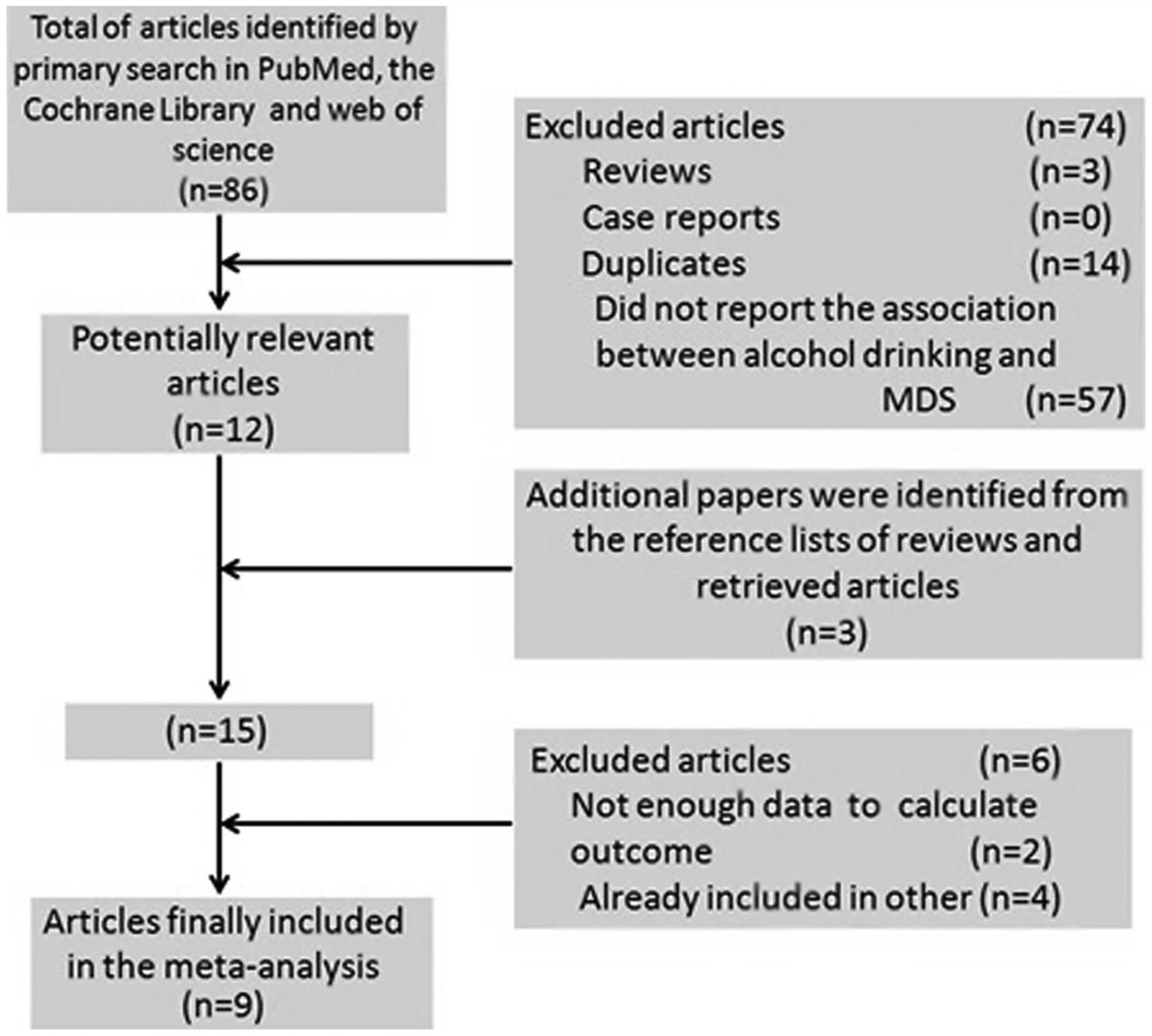
The detailed steps of our literature search are
illustrated in Fig. 1. A total of
9 studies were selected for the present meta-analysis, including 1
prospective cohort (16) and 8
case-control studies (8–15) published between 1991 and 2011.
These studies were conducted in the following regions: United
States (n=4), Europe (n=2) and Asia (n=3). A total of 1,448 MDS
patients were included in this meta-analysis. Information regarding
alcohol intake was collected by interviews, self-administered
questionnaires, or both. The study quality scores, evaluated by the
Newcastle-Ottawa Quality Assessment Scale, ranged between 6 and 8
(with a mean of 6.7). The general characteristics of the 9 studies
are summarized in Table I.
 | Table IMain characteristics of cohort and
case-control studies evaluating the association between alcohol
drinking and MDS. |
Table I
Main characteristics of cohort and
case-control studies evaluating the association between alcohol
drinking and MDS.
| Study (year) | Country | Gender | Age (years) | Study design | Source | No. of cases | No. of controls | Risk factor
assessment | Study quality | Matching and
adjustments | (Refs.) |
|---|
| Lv et al
(2011) | China | M/F | 20–88 | Case-control | HB | 403 | 806 | Face-to-face
interview | 6 | Age, gender, anti-TB
drugs, D860, TCM, alcohol intake, benzene, pesticides, gasoline,
glues, hair dye, education, new building | (10) |
| Ma et al
(2009) | United States | M/F | 50–78 | Cohort | PB | 193 | 471,799 | Mailed
questionnaire | 8 | Age, gender, race,
education, total energy intake | (16) |
| Pekmezovic et
al (2006) | Serbia
Montenegro | M/F | 18–85 | Case-control | HB | 80 | 160 | Interview | 6 | Age, gender | (8) |
| Strom et al
(2005) | United States | M/F | 24–89 | Case-control | HB | 352 | 443 | Mailed
questionnaire | 7 | Age, gender,
ethnicity, education, family history of hematopoietic cancer,
alcohol intake, fertilizer, herbicide, pesticide, benzene, solvent,
gasoline | (11) |
| Dalamaga et al
(2002) | Greece | M/F | 44–86 | Case-control | HB | 84 | 84 | Interview | 6 | Age, gender, marital
status, education, alcohol consumption, time since first diagnosis
of an autoimmune disorder | (12) |
| Nagata et al
(1999) | Japan | M/F | 20–74 | Case-control | PB | 111 | 815 | Telephone interview
and mailed questionnaire | 8 | Age, gender, living
area | (13) |
| Ido et al
(1996) | Japan | M/F | 20–75 | Case-control | HB | 116 | 116 | Interview | 6 | Age, gender,
hospital, hair dye use, occupational exposure to organic
solvents | (9) |
| Brown et al
(1992) | United States | M | ≤70 | Case-control | PB | 100 | 820 | Interview and
mailed questionnaire | 7 | Age, state,
tobacco | (14) |
| Crane and Keating
(1991) | United States | NR | ≥18 | Case-control | HB | 46 | 224 | Telephone interview
and mailed questionnaire | 6 | Age, gender,
alcohol intake, benzene, metal fume, dyes, glues, lacquers,
varnishes, radiation, pesticides, paints, spray paints | (15) |
Risk assessment
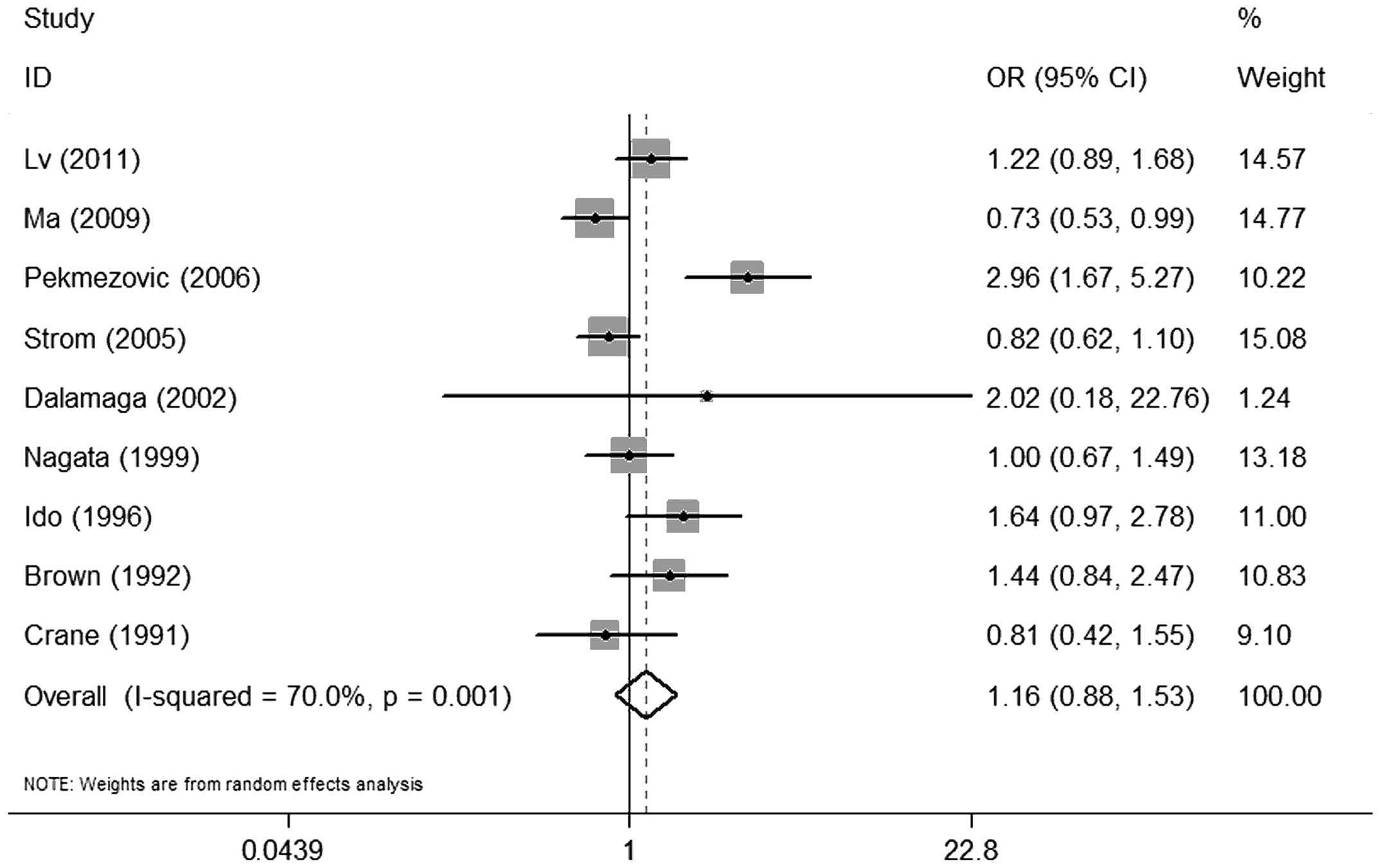
There was no significant association between alcohol
consumption and MDS when comparing individuals with any history of
alcohol consumption to non-drinkers (OR=1.16, 95% CI: 0.88–1.53)
(Fig. 2). A statistically
significant heterogeneity was found among the 9 studies (P=0.001)
and, thus, a random-effects analysis was performed
(I2=70.0%).
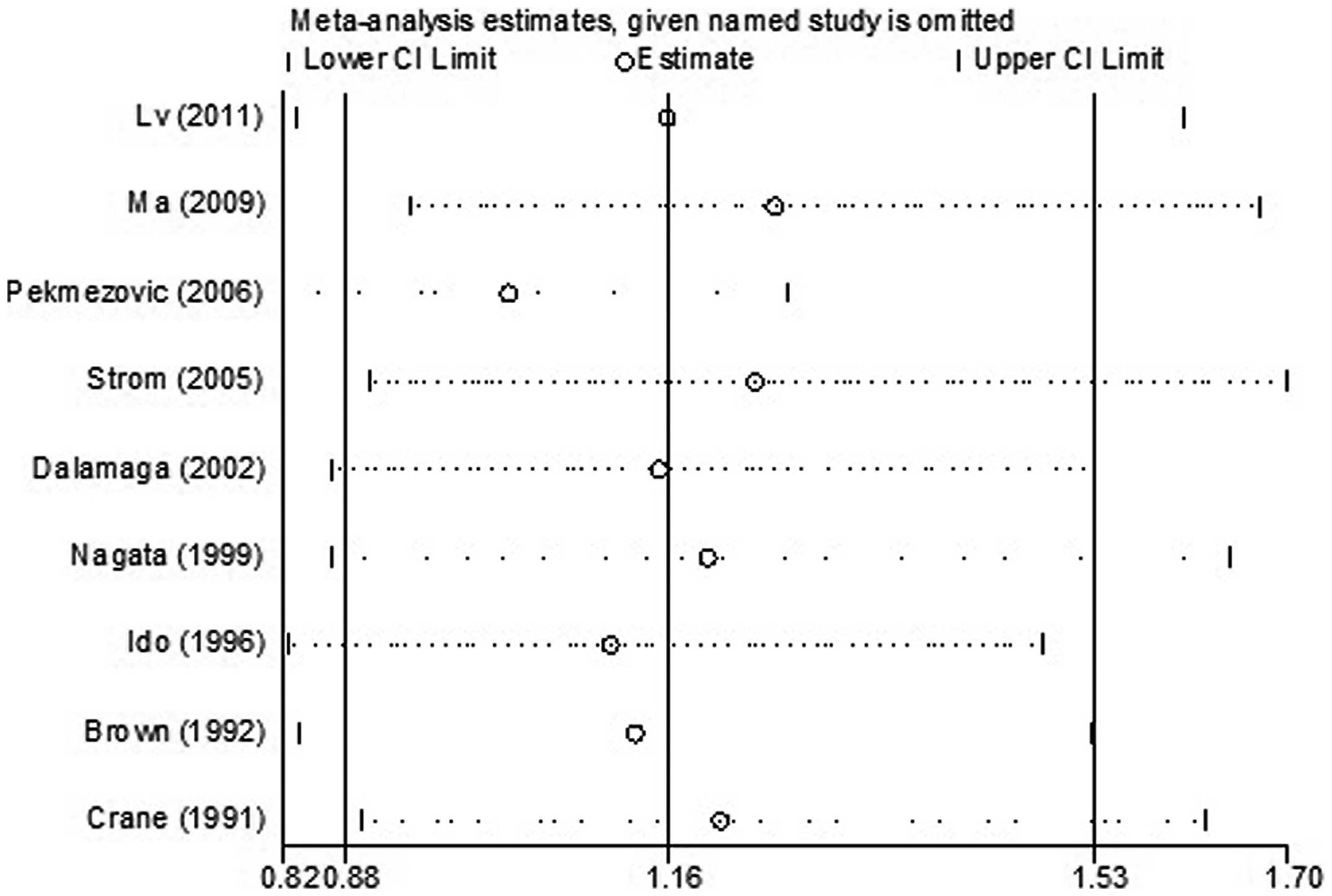
For the sensitivity analysis, the effect of any
single study on the overall estimate was assessed by repeating the
meta-analysis while omitting one study at a time. The results
indicated that no study considerably affected the summary of the
risk estimate. The 9 study-specific ORs ranged from 0.98 (95% CI:
0.85–1.14) to 1.25 (95% CI: 0.93–1.68) when the studies by
Pekmezovic et al (8) and by
Ma et al (16) were
omitted, respectively (Fig.
3).
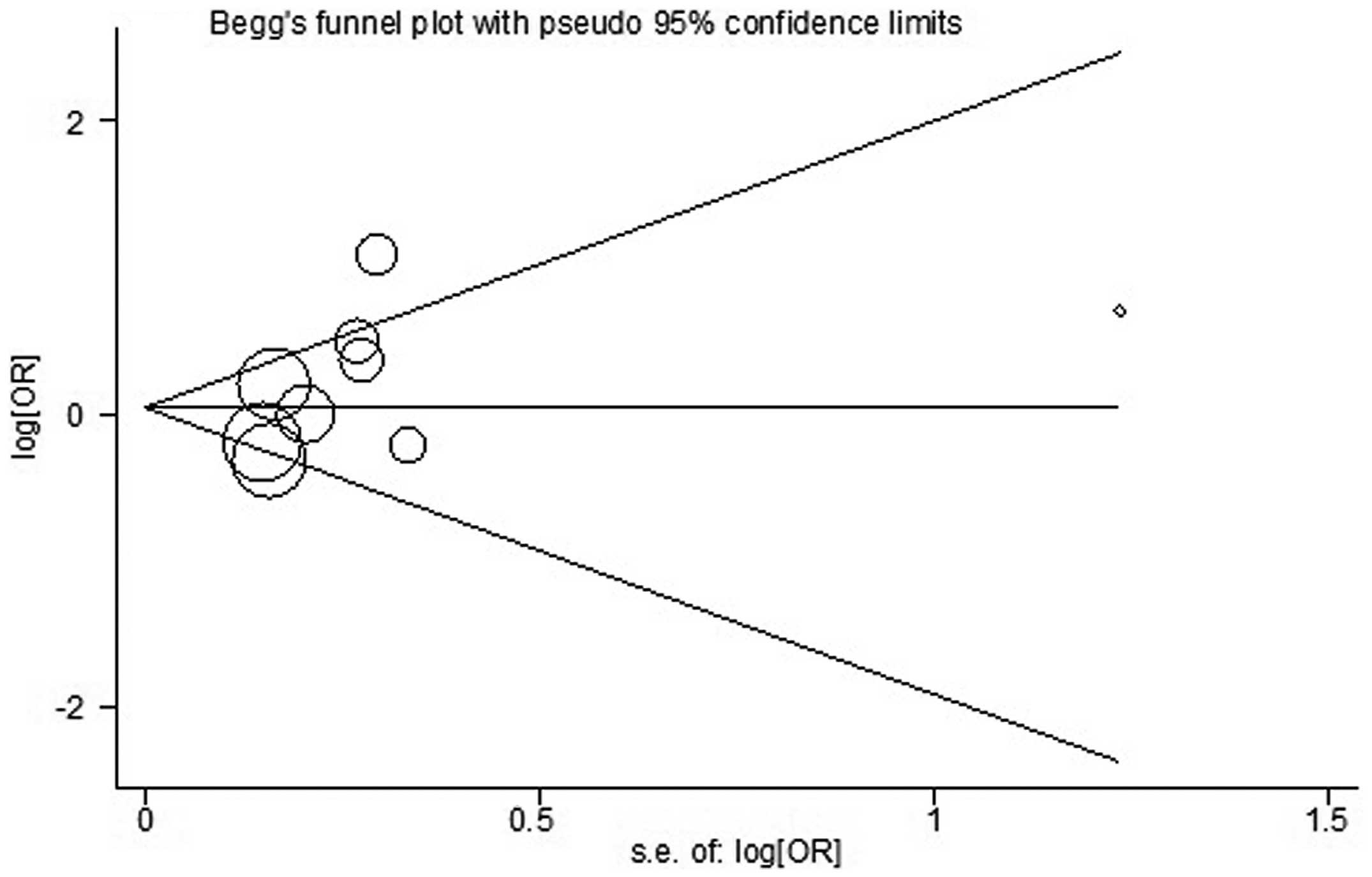
Begg’s and Egger’s tests were used to assess the
publication bias (Fig. 4). No
publication bias was identified by either test (P=0.251 and
P=0.170, respectively).
Subgroup analyses
Stratified group analyses were performed for the
following subgroups: drinking status (current or former drinkers),
gender, ethnicity (Asian or Caucasian), study design (case-control
or cohort), patient source (population- or hospital-based) and
disease subtypes [refractory anemia (RA)/RA with ringed
sideroblasts or RA with excess blasts (RAEB)/RAEB in
transformation]. The ORs are shown in Table II. There were no significant
differences between the subgroups. However, when we analyzed the
amount of alcohol ingested per day, we observed a statistically
significant hazardous effect on MDS (OR=1.55, 95% CI: 1.08–2.21)
with mild heterogeneity (I2=16.2%) when the consumed
amount of alcohol was ≥10 g/day. There was no publication bias in
the subgroup analyses with either the Begg’s or Egger’s test.
 | Table IIStratified pooled odds ratios of the
association between alcohol consumption and risk of MDS. |
Table II
Stratified pooled odds ratios of the
association between alcohol consumption and risk of MDS.
| Subgroup | No. of studies
(refs.) | Pooled OR (95%
CI) | Q-test for
heterogeneity P-value (I2 score, %) | Egger’s test
P-value | Begg’s test
P-value |
|---|
| Drinking
status |
| Current | 2 (10,13) | 1.05
(0.73–1.52) | 0.151 (51.4) | - | 1.000 |
| Former | 2 (10,13) | 1.21
(0.80–1.83) | 0.684 (0.0) | - | 1.000 |
| Gender |
| Male | 4 (9,11,13,14) | 1.07
(0.83–1.38) | 0.059 (56.0) | 0.347 | 0.806 |
| Female | 3 (9,11,13) | 0.78
(0.52–1.18) | 0.368 (0.0) | 0.612 | 1.000 |
| Ethnicity |
| Asian | 3 (9,10,13) | 1.31
(0.85–1.51) | 0.149 (43.7) | 0.700 | 1.000 |
| Caucasian | 6 (8,11,12,14–16) | 0.91
(0.65–1.28) | <0.001
(72.7) | 0.609 | 0.251 |
| Study design |
| Cohort | 1 (16) | 0.67
(0.51–0.84) | - | - | - |
| Case-control | 8 (8–16) | 1.16
(0.89–1.52) | 0.002 (61.1) | 0.290 | 0.088 |
| Source of
patients |
|
Population-based | 3 (13,14,16) | 0.93
(0.58–1.49) | 0.017 (75.5) | 0.247 | 0.296 |
|
Hospital-based | 6 (8–12,15) | 1.24
(0.78–1.96) | <0.001
(75.6) | 0.351 | 0.368 |
| MDS subtype |
| RA/RARS | 2 (9,11) | 0.74
(0.51–1.09) | 0.008 (79.4) | - | 1.000 |
| RAEB/RAEBt | 2 (10,11) | 1.59
(1.21–2.10) | 0.771 (0) | - | 1.000 |
| Quantity of
drinking (g/day) |
| 0 | 4 (9,10,13,14) | 1 | - | - | - |
| >0 and
<10 | 3 (9,10,14) | 1.09
(0.78–1.53) | 0.722 (0.0) | 0.176 | 1.000 |
| ≥10 | 4 (9,10,13,14) | 1.55
(1.08–2.21) | 0.311 (16.2) | 0.163 | 0.089 |
Discussion
MDS are myeloid neoplasms characterized by dysplasia
in one or more cell lines, with a significant impact on quality of
life and survival (24). The
pathophysiology of MDS has not been fully elucidated. Alcohol
consumption is a common practice worldwide and previous
epidemiological studies have suggested that daily alcohol intake
may be a risk factor for MDS; however, findings to date have been
inconclusive. We attempted to clarify this potential association
through a meta-analysis of 9 studies.
In this meta-analysis, there was a non-significant
16% risk of MDS with alcohol intake. Subgroup analyses also failed
to demonstrate any significant correlations, except with an alcohol
consumption of ≥10 g/day. Those individuals exhibited a 55%
increased risk of MDS.
The effect of alcohol and its role in MDS is likely
multifactorial. Our meta-analysis attests to the complicated nature
of the mechanisms by which alcohol consumption may be involved the
pathogenesis of MDS, which remain largely unknown at present.
Several potential mechanisms have been proposed. First, alcohol may
exert a direct toxic effect by causing bone marrow failure
(25). Second, alcohol or one of
its metabolites, such as methyl parathion, may induce chromosomal
aberrations in hematopoietic cells (26). Third, hematological and
immunological changes are known to be associated with alcohol.
Alcohol was found to impair both the innate and acquired immune
systems in heavy drinkers, thus increasing their susceptibility to
infection (27). However, alcohol
consumption may exert beneficial effects as well. In
light-to-moderate drinkers, a recent study reported that alcohol
may be beneficial for the immune system (27). At the molecular level, xanthohumol,
a major prenylated flavonoid present in hops and beer, attracted
considerable interest due to its latent cancer chemopreventive
effect (28). In addition,
resveratrol, an antioxidant found in the skin of grapes and
abundant in red wine, has been shown to negatively affect
initiation, promotion and progression of different types of cancer,
including leukemia, in in vitro and in vivo studies
(29,30). Since MDS shares several
characteristics with leukemia (31), resveratrol may also decrease the
risk of MDS. Indeed, one recent study reported that wine drinkers
had a 46% reduced risk of MDS (11). Hence, the effects of alcohol
consumption may be dependent on the dose and type of alcoholic
beverages. However, further studies are required to elucidate the
underlying mechanisms.
The major strength of our meta-analysis is the
number of large studies selected for review and analysis. This also
allowed us to have enough data to perform multiple subgroup
analyses. However, as with all meta-analyses of observational
studies, our study had several limitations. First, the majority of
the studies included in our meta-analysis were case-control
studies, which depended on retrospective data, thus introducing the
possibility of recall bias. Furthermore, combining data from
different study designs may also be a source of bias. Second, the
inclusion of articles only published in the English language may be
a source of publication bias, despite the fact that by Begg’s or
Egger’s tests, our results exhibited no evidence of publication
bias. Third, there were three major types of alcoholic beverages
(beer, wine, or spirits), which may exert different effects on MDS
patients. However, the majority of the studies included in our
analysis provided general data on alcohol drinking rather than
detailed information on the specific types of alcoholic beverages.
Fourth, our meta-analysis was likely affected by the inaccuracy of
self-reported alcohol consumption. Alcohol intake was recorded as
number of glasses of beverage per day or week. However, glass size
may vary considerably. Moreover, the accuracy of self-reported
alcohol use has been known to be highly variable as well and one
must consider telescopic or recall bias when evaluating this type
of data.
In summary, our meta-analysis suggests that alcohol
intake may increase the risk of developing MDS in a dose-dependent
manner and heavy alcohol consumption is associated with a higher
risk of MDS. Due to the limited number of studies, additional
well-designed cohort or intervention studies are required to
confirm these findings and help elucidate the pathophysiology of
MDS.
Acknowledgements
This study was supported by grants from the Zhejiang
Province Fund for Distinguished Young Scholars (no. LR12H08001),
the Foundation of Key Innovation Team of Zhejiang Province (no.
2011R50015), the National Public Health Grand Research Foundation
(no. 201202017), the Major Program of the Science Technology
Department of Zhejiang Province Fund (no. 2013c03043-2) and the
National Natural Science Foundation of China (nos. 30870914 and
81270582)
References
|
1
|
Raza A, Gezer S, Mundle S, et al:
Apoptosis in bone marrow biopsy samples involving stromal and
hematopoietic cells in 50 patients with myelodysplastic syndromes.
Blood. 86:268–276. 1995.PubMed/NCBI
|
|
2
|
Cogle CR, Craig BM, Rollison DE and List
AF: Incidence of the myelodysplastic syndromes using a novel
claims-based algorithm: high number of uncaptured cases by cancer
registries. Blood. 117:7121–7125. 2011. View Article : Google Scholar
|
|
3
|
Tong H, Hu C, Yin X, Yu M, Yang J and Jin
J: A meta-analysis of the relationship between cigarette smoking
and incidence of myelodysplastic syndromes. PLoS One. 8:e675372013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma X: Epidemiology of myelodysplastic
syndromes. Am J Med. 125(Suppl 7): S2–S5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boffetta P and Hashibe M: Alcohol and
cancer. Lancet Oncol. 7:149–156. 2006. View Article : Google Scholar
|
|
6
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M; Comparative Risk Assessment collaborating group
(Cancers). Causes of cancer in the world: comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Menezes RF, Bergmann A and Thuler LC:
Alcohol consumption and risk of cancer: a systematic literature
review. Asian Pac J Cancer Prev. 14:4965–4972. 2013.PubMed/NCBI
|
|
8
|
Pekmezovic T, Suvajdzic Vukovic N, Kisic
D, et al: A case-control study of myelodysplastic syndromes in
Belgrade (Serbia Montenegro). Ann Hematol. 85:514–519. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ido M, Nagata C, Kawakami N, et al: A
case-control study of myelodysplastic syndromes among Japanese men
and women. Leuk Res. 20:727–731. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv L, Lin G, Gao X, et al: Case-control
study of risk factors of myelodysplastic syndromes according to
World Health Organization classification in a Chinese population.
Am J Hematol. 86:163–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strom SS, Gu Y, Gruschkus SK, Pierce SA
and Estey EH: Risk factors of myelodysplastic syndromes: a
case-control study. Leukemia. 19:1912–1918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalamaga M, Petridou E, Cook FE and
Trichopoulos D: Risk factors for myelodysplastic syndromes: a
case-control study in Greece. Cancer Causes Control. 13:603–608.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagata C, Shimizu H, Hirashima K, et al:
Hair dye use and occupational exposure to organic solvents as risk
factors for myelodysplastic syndrome. Leuk Res. 23:57–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown LM, Gibson R, Burmeister LF, Schuman
LM, Everett GD and Blair A: Alcohol consumption and risk of
leukemia, non-Hodgkin’s lymphoma, and multiple myeloma. Leuk Res.
16:979–984. 1992.
|
|
15
|
Crane MM and Keating MJ: Exposure
histories in acute nonlymphocytic leukemia patients with a prior
preleukemic condition. Cancer. 67:2211–2214. 1991. View Article : Google Scholar
|
|
16
|
Ma X, Lim U, Park Y, et al: Obesity,
lifestyle factors, and risk of myelodysplastic syndromes in a large
US cohort. Am J Epidemiol. 169:1492–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J and Yu KF: What’s the relative
risk? A method of correcting the odds ratio in cohort studies of
common outcomes. JAMA. 280:1690–1691. 1998.
|
|
18
|
Hu ZH, Lin YW, Xu X, et al: No association
between tea consumption and risk of renal cell carcinoma: a
meta-analysis of epidemiological studies. Asian Pac J Cancer Prev.
14:1691–1695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wells GA, Shea B, O’Connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Accessed October, 19, 2009
|
|
20
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
21
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newman K, Maness-Harris L, El-Hemaidi I
and Akhtari M: Revisiting use of growth factors in myelodysplastic
syndromes. Asian Pac J Cancer Prev. 13:1081–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casagrande G and Michot F: Alcohol-induced
bone marrow damage: status before and after a 4-week period of
abstinence from alcohol with or without disulfiram. A randomized
bone marrow study in alcohol-dependent individuals. Blut.
59:231–236. 1989. View Article : Google Scholar
|
|
26
|
Sunil Kumar KB, Ankathil R and Devi KS:
Chromosomal aberrations induced by methyl parathion in human
peripheral lymphocytes of alcoholics and smokers. Hum Exp Toxicol.
12:285–288. 1993.PubMed/NCBI
|
|
27
|
Diaz LE, Montero A, Gonzalez-Gross M,
Vallejo AI, Romeo J and Marcos A: Influence of alcohol consumption
on immunological status: a review. Eur J Clin Nutr. 56(Suppl 3):
S50–S53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerhauser C: Beer constituents as
potential cancer chemopreventive agents. Eur J Cancer.
41:1941–1954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsan MF, White JE, Maheshwari JG and
Chikkappa G: Anti-leukemia effect of resveratrol. Leuk Lymphoma.
43:983–987. 2002.PubMed/NCBI
|
|
30
|
Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G
and Lee SJ: Resveratrol, an antioxidant present in red wine,
induces apoptosis in human promyelocytic leukemia (HL-60) cells.
Cancer Lett. 140:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Steensma DP: Are myelodysplastic syndromes
‘cancer’? Unexpected adverse consequences of linguistic ambiguity.
Leuk Res. 30:1227–1233. 2006.
|