Introduction
Extrahepatic cholangiocarcinoma (ECC) is a
relatively rare malignant tumor of the bile duct epithelium, with a
significantly increasing incidence among women (1). ECC is classified into two types
according to anatomical location, namely perihilar and distal
types, but does not include the papilla of Vater. Currently,
complete surgical resection is the only curative option for ECC
patients. However, the resectability rate of ECC cases has been
low, as the majority of the patients have advanced-stage disease at
diagnosis (2). Even following
complete resection, the majority of the patients develop local
recurrence or distant metastasis (3). The low overall survival (OS) rate of
ECC (4,5) is considered an oncologic
challenge.
The number of studies on survival outcomes and
prognostic factors of ECC patients following resection is limited
and the results vary among different countries (5-year survival
rate range, 16–54%), with a median survival time range of 13–47.2
months (6–9). A variety of factors have been used to
predict prognosis following surgical resection for ECC, but no
consensus has been reached. Although postoperative adjuvant therapy
(AT), including chemotherapy, radiotherapy, concurrent
chemoradiotherapy and sequential chemotherapy and radiotherapy,
have improved the disease-free survival and OS of patients in
various other malignancies, the effects of AT on the survival in
ECC patients have not yet been determined (10). The reasons may be as follows: Owing
to the rarity of ECC, it usually takes several decades to collect
the available data in most studies (11). Furthermore, it is generally
considered that traditional cytotoxic chemotherapeutic drugs and
radiotherapy are ineffective in ECC patients (4,12,13).
In addition, previous studies evaluated cholangiocarcinoma together
with cancer of the gallbladder and the ampulla of Vater, due to the
low incidence of ECC (14).
Therefore, the role of AT in patients who underwent radical
resection of ECC has not been clearly determined.
Therefore, this study aimed to investigate survival
outcomes and prognostic factors of ECC patients following surgical
treatment and determine the role of postoperative AT through a
comparison of survival outcomes between ECC patients with and those
without postoperative AT.
Materials and methods
Eligibility criteria
The eligibility criteria for the present study were
as follows: patients with histologically proven ECC, without
distant metastasis at diagnosis and without a history of malignancy
other than skin cancer. Patients who had received preoperative
chemotherapy and those with intrahepatic cholangiocarcinoma (ICC)
and/or ampullary carcinoma were excluded from the study. Concurrent
clinical and pathological data were retrospectively collected from
105 patients who underwent surgical resection of pathologically
confirmed ECC between March 3, 2008 and December 20, 2013 at the
First Affiliated Hospital of Xi’an Jiaotong University and the
Tangdu Hospital. Demographic data were collected for each patient,
including age, gender, imaging findings, laboratory test results
and pathological results. The study was approved by the Ethics
Committees of the two participating hospitals in March, 2008 and
all the patients signed an informed consent.
Pathological evaluation
All the resected specimens were examined
pathologically for tumor size, histological differentiation and the
presence of positive lymph nodes. The surgical margins were
examined for the presence of residual tumor, which was described by
the residual tumor (R) classification as follows: R0, no residual
tumor and resection margin >0 mm; R1, microscopic residual tumor
or nil resection margin; and R2, macroscopic residual tumor
(15). Each patient was staged
according to the 7th edition of the American Joint Committee on
Cancer (AJCC) staging system for ECC (16).
Preoperative cholangitis
Cholangitis was defined according to the
international consensus-revised Tokyo Guidelines (17) and it was diagnosed when one of the
three following conditions was present: i) purulent bile; ii)
clinical remission following bile duct drainage; or iii) remission
achieved by antibacterial therapy alone in patients in whom the
only site of infection was the biliary tree.
AT
AT was administered in 32 ECC patients undergoing
surgical resection. A total of 18 patients received systematic
intravenous chemotherapy and each patient completed at least 2
cycles of chemotherapy. All the regimens of intravenous
chemotherapy in our study were combinations of chemotherapeutic
agents (n=18), including gemcitabine/cisplatin (n=8),
gemcitabine/oxaliplatin (n=6) and gemcitabine/capecitabine (n=4).
As regards postoperative adjuvant radiotherapy, 11 patients
received three-dimensional conformal radiotherapy after surgical
resection, with a total dose of 45–50 Gy, in 5 fractions per week,
with 1.8 Gy per fraction, including the primary tumor bed as well
as the regional lymph nodes. In addition, 2 patients were
administered postoperative radiotherapy with a total dose of 45 Gy,
followed by single-agent capecitabine orally, 650 mg/m2
on days 1–14 q3w ×4 cycles and 1 patient received concurrent
chemoradiotherapy, with a total dose of 45 Gy and 5-fluorouracil
(5-FU) plus leucovorin as radiosensitizers.
Follow-up
After surgery, all the patients were regularly
followed up by ultrasound scan, liver function tests and
measurement of carbohydrate antigen 19-9 (CA19-9) at 1- to 3-month
intervals. Survival time was calculated from the date of surgery.
The patients were followed up until death or until the study
deadline date, which was December 10, 2013. By the end of the
study, 75 patients (71.4%) had succumbed to the disease.
Statistical analysis
OS rates were calculated with the Kaplan-Meier
method. The possible prognostic factors were analyzed by univariate
analysis and evaluated using the Kaplan-Meier method; differences
in survival curves were compared with the log-rank test. The
baseline characteristics were compared between patients who
received AT and those who did not using the Chi-square test. The
multivariate analysis was performed using the Cox proportional
hazards model to identify the independent prognostic factors for
survival. Statistical analysis was performed using the SPSS 18.0
software for windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate statistically significant differences.
Results
Demographics and clinicopathological
characteristics of ECC patients
A total of 105 postoperative ECC patients were
included in this study. The patients had a mean age of 62 years and
included 50 men and 55 women. A common underlying liver disease in
these patients was cholangitis (22/105, 20.95%). Elevated CA19-9
levels were detected in 65.71% (69/105) and lymph node metastasis
in 38.1% (40/105) of the patients. According to the 7th edition of
the AJCC staging system, 68.57% (72/105) of the patients had stage
1/2 and 31.43% (33/105) had stage 3/4 disease. The R0 resectability
rate was 59.05% (62/105). AT was administered to 30.5% (32/105) of
the patients after surgery in this series.
Univariate analysis of survival rates,
survival time and prognostic factors
At a median follow-up of 15.4 months, the median OS
time was 17.6 months, with 1- and 3-year survival rates of 67.9 and
19.5%, respectively, for the entire cohort, with corresponding
rates of 52.3 and 7.3% for the patients with lymphatic metastasis;
76.5 and 26.1% for the patients without lymphatic metastasis
(P=0.003); 44.8 and 13.6% for the surgical margin-positive
patients; and 83.2 and 22.4% for surgical margin-negative patients
(P=0.003), respectively (Table
I).
 | Table IUnivariate analysis of overall
survival following resection for ECC (n=105). |
Table I
Univariate analysis of overall
survival following resection for ECC (n=105).
| Clinical factors | No. | Survival rate
(%) | Median survival
(months) | P-value
(log-rank) | HR | 95% CI for
Exp(B) |
|---|
|
|---|
| 1-year | 3-year |
|---|
| Gender |
| Male | 50 | 60.4 | 16.6 | 17.2 | 0.79 | 0.94 | 0.60–1.48 |
| Female | 55 | 74.1 | 22.4 | 18.8 | | | |
| Age at surgery
(years) |
| ≤70 | 80 | 64.3 | 17.7 | 17.2 | 0.13 | 0.65 | 0.37–1.15 |
| >70 | 25 | 79.3 | 17.8 | 24.6 | | | |
| Preoperative
cholangitis |
| Yes | 22 | 59.9 | 8.2 | 12.3 | 0.047a | 1.70 | 1.00–2.90 |
| No | 83 | 70.0 | 23.5 | 20.2 | | | |
| CA19-9 (U/ml) |
| ≤39 | 36 | 76.9 | 25.2 | 23.0 | 0.23 | 1.35 | 0.82–2.21 |
| >39 | 69 | 61.6 | 16.5 | 15.9 | | | |
| Lymphatic
metastasis |
| Yes | 40 | 52.3 | 7.3 | 13.8 | 0.003a | 1.98 | 1.24–3.17 |
| No | 65 | 76.5 | 26.1 | 20.2 | | | |
| Surgical
margins |
| R0b | 62 | 83.2 | 22.4 | 23.8 | 0.003a | 1.97 | 1.25–3.12 |
| Non-R0 | 43 | 44.8 | 13.6 | 11.6 | | | |
| Child-Pugh
class |
| A | 54 | 65.3 | 15.8 | 17.6 | 0.39 | 0.82 | 0.52–1.29 |
| B | 51 | 70.6 | 23.2 | 18.1 | | | |
| Adjuvant
therapy |
| Yes | 32 | 78.6. | 19.3 | 21.6 | 0.57 | 0.87 | 0.52–1.44 |
| No | 73 | 62.0 | 18.3 | 15.9 | | | |
| Histological
grade |
| 1/2 | 68 | 74.8 | 22.7 | 21.6 | 0.02a | 1.70 | 1.07–2.72 |
| 3 | 37 | 55.4 | 14.6 | 12.3 | | | |
| AJCC stage |
| 1/2 | 72 | 80.0 | 28.4 | 24.0 | <0.01a | 3.47 | 2.11–5.71 |
| 3/4 | 33 | 39.6 | 0 | 11.5 | | | |
| All patients | 105 | 67.9 | 19.5 | 17.6 | | | |
The univariate analysis (Table I) identified the following adverse
prognostic factors for OS: preoperative cholangitis [hazard ratio
(HR)=1.70, P=0.047]; non-R0 surgical margins (HR=1.97, P=0.003),
poor differentiation grade (HR=1.70, P=0.02), stage 3/4 (HR=3.47,
P<0.01) and lymphatic metastasis (HR=1.98, P=0.003). The
univariate analysis demonstrated that AT was not significantly
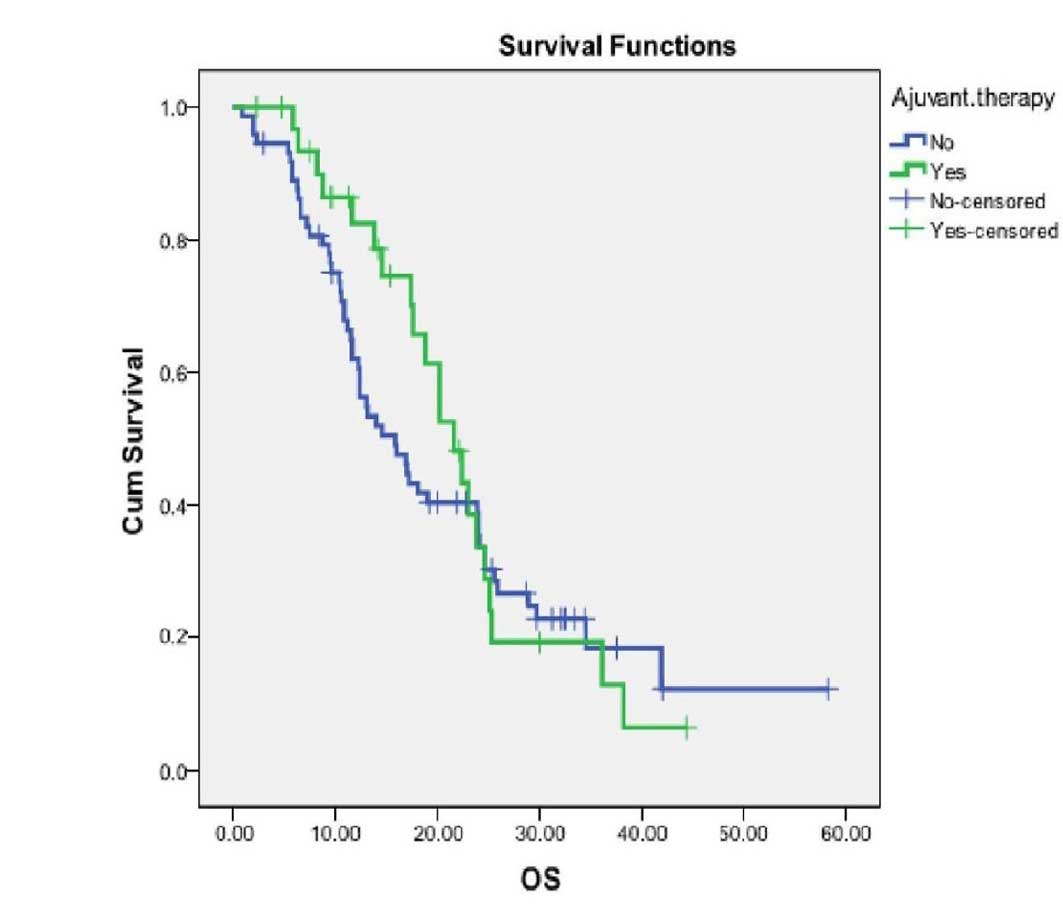
associated with improved OS (HR=0.87, P=0.57). The survival curves
were not significantly different between the AT and non-AT groups
for the entire cohort of patients (Fig. 1).
Baseline characteristics of patient
demographics and tumor characteristics between AT and non-AT
groups
We used Chi-square tests for the categorical
comparisons of patient baseline characteristics between the AT and
non-AT groups (Table II).
Significant differences were found in baseline characteristics
between the two groups. The patients who received AT (n=32) were
younger compared to the non-AT patients (n=73) (90.6 vs. 69.9%,
respectively, were aged <70 years; P=0.02). In addition, the
patients who received AT exhibited a higher rate of stage 3/4
disease (46.9 vs. 24.7%, respectively; P=0.02), lymphatic
metastasis (62.5 vs. 27.4%, respectively; P=0.001) and positive
resection margins (56.3 vs. 34.2%, respectively; P=0.035). As a
result, the survival outcomes between the two groups cannot be
directly compared due to the significantly different baseline
characteristics.
 | Table IIComparison of baseline
characteristics between treatment groups with the Chi-square
test. |
Table II
Comparison of baseline
characteristics between treatment groups with the Chi-square
test.
| Clinical
factors | Non-AT
n=73 (%) | AT
n=32 (%) | P-value |
|---|
| Age at surgery
(years) |
| <70 | 51 (69.9) | 29 (90.6) | 0.02a |
| ≥70 | 22 (30.1) | 3 (9.4) | |
| Gender |
| Male no. | 39 (53.4) | 11 (34.4) | 0.07 |
| Tumor
characteristics |
| T stage |
| 1/2 | 55 (75.3) | 17 (53.1) | 0.02a |
| 3/4 | 18 (24.7) | 15 (46.9) | |
| Lymphatic
metastasis |
| No | 53 (72.6) | 12 (37.5) | 0.001a |
| Yes | 20 (27.4) | 20 (62.5) | |
| Histology
grade |
| 1/2 | 48 (65.7) | 20 (62.5) | 0.75 |
| 3 | 25 (34.3) | 12 (37.5) | |
| Surgical
margins |
| Non-R0b | 25 (34.2) | 18 (56.3) | 0.035a |
| R0 | 48 (65.8) | 14 (43.7) | |
| Preoperative
cholangitis |
| Yes | 14 (19.2) | 8 (25.0) | 0.50 |
| No | 59 (80.8) | 24 (75.0) | |
Subgroup survival analysis by the
Kaplan-Meier method
The patients were stratified into seven risk
subgroups according to clinical factors and the survival rate was
compared within each subgroup between patients who received AT and
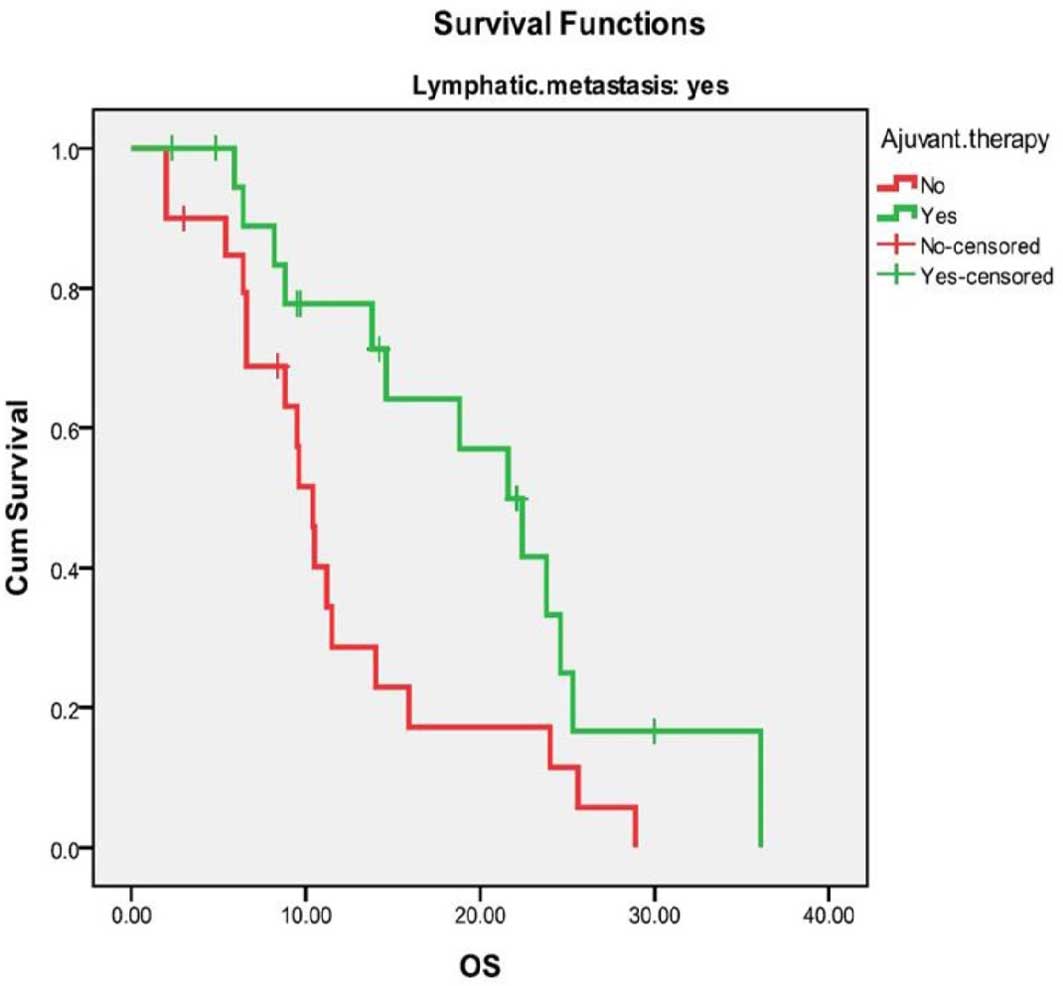
those who did not. Only patients with pathological lymphatic
metastasis exhibited a significant difference in the 3-year
survival rate between the AT and non-AT groups (median OS, 21.6 vs.
10.4 months; and 3-year OS, 16.6 vs. 0%, respectively; P=0.02)
(Table III). The survival curves
of the AT and non-AT groups for node-positive patients were
significantly different (Fig. 2).
The remaining patients did not achieve a significant improvement in
OS with AT (P>0.05) (Table
III).
 | Table IIISubgroup survival analysis comparing
patients who received AT to those who did not using the
Kaplan-Meier method. |
Table III
Subgroup survival analysis comparing
patients who received AT to those who did not using the
Kaplan-Meier method.
| No. of
patients | Median overall
survival (months) | 3-year survival
(%) |
|---|
|
|
|
|
|---|
| Clinical
factors | Non-AT | AT | Non-AT | AT | P-value | Non-AT | AT |
|---|
| All patients
(%) | 73 (69.5) | 32 (30.5) | 15.9 | 21.6 | 0.57 | 18.3 | 19.3 |
| Age (years) |
| ≤70 | 51 | 29 | 12.3 | 21.6 | 0.23 | 15.3 | 21.2 |
| >70 | 22 | 3 | 24.6 | 25.1 | 0.57 | 19.7 | 33.3 |
| Gender |
| Female | 34 | 21 | 15.9 | 22.4 | 0.76 | 22.3 | 20.8 |
| Male | 39 | 11 | 13.1 | 20.2 | 0.63 | 15.8 | 14.8 |
| Histological
grade |
| 1/2 | 48 | 20 | 17.0 | 23.8 | 0.30 | 21.7 | 24.2 |
| 3 | 25 | 12 | 12.3 | 14.6 | 0.93 | 0 | 11.1 |
| Lymphatic
metastasis |
| No | 53 | 12 | 19.0 | 20.2 | 0.80 | 25.3 | 22.5 |
| Yes | 20 | 20 | 10.4 | 21.6 | 0.02a | 0 | 16.6 |
| AJCC stage |
| 1/2 | 55 | 17 | 23.9 | 24.6 | 0.30 | 24.5 | 37.5 |
| 3/4 | 18 | 15 | 10.5 | 14.6 | 0.10 | 0 | 0 |
| Surgical
margins |
| R0 | 48 | 14 | 24.0 | 20.2 | 0.89 | 22.3 | 20.0 |
| Non-R0b | 25 | 18 | 9.6 | 22.4 | 0.07 | 8.9 | 18.6 |
| Preoperative
cholangitis |
| Yes | 14 | 8 | 12.3 | 12.8 | 0.5 | 0 | 14.3 |
| No | 59 | 24 | 17.0 | 21.6 | 0.57 | 23.7 | 20.9 |
Multivariate analysis of prognostic
factors
The prognostic factors considered significant on
univariate analysis were subjected to multivariate analysis using a
Cox proportional hazards model. These factors included surgical
margins, lymphatic metastasis, histological grade, preoperative
cholangitis and AT. Our data demonstrated that lymph node
metastasis (HR=2.185, P=0.009), surgical margin positivity
(HR=1.893, P=0.015) and AT (HR=0.451, P=0.011) remained
independently associated with OS (Table IV).
 | Table IVMultivariate analysis of overall
survival following surgical resection for ECC (n=105). |
Table IV
Multivariate analysis of overall
survival following surgical resection for ECC (n=105).
| | | | | | | 95% CI for
Exp(B) |
|---|
| | | | | | |
|
|---|
| Clinical
factors | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
|---|
| Lymphatic
metastasis | 0.782 | 0.300 | 6.814 | 1 | 0.009a | 2.185 | 1.215 | 3.931 |
| Surgical
margins | 0.638 | 0.263 | 5.889 | 1 | 0.015a | 1.893 | 1.131 | 3.171 |
| Adjuvant
therapy | −0.796 | 0.314 | 6.441 | 1 | 0.011a | 0.451 | 0.244 | 0.834 |
| Histological
grade | 0.039 | 0.274 | 0.020 | 1 | 0.887 | 1.040 | 0.607 | 1.781 |
| Preoperative
cholangitis | 0.425 | 0.294 | 2.098 | 1 | 0.147 | 1.530 | 0.861 | 2.720 |
Discussion
The documentation of ECC outcomes is sparse and its
prognosis remains unsatisfactory, even after radical surgical
resection. The overall 1- and 3-year survival rates in our series
were consistent with previous findings (5). The median survival time in this study
(17.6 months) is quite similar to the median survival time of 17
months reported by Fuller et al (18). However, our results differ from the
higher survival rates reported by previous studies (19–21).
These differences may be attributed to patient demographics and
tumor characteristics, duration of follow-up and treatment
modalities. In addition, there was a considerable number of ECC
patients with positive lymph nodes (40/105, 38.1%), residual
margins (43/105, 40.96%), preoperative cholangitis (22/105,
20.95%), poor histological differentiation (37/105, 35.24%) and
stage 3/4 disease (33/105, 31.43%) in our study, which were adverse
prognostic factors and may result in lower survival rates and a
shorter median survival time.
The effect of preoperative inflammation on the
prognosis of patients with ECC has not been extensively
investigated (22). Previous
findings verified that preoperative cholangitis in ECC patients was
associated with a 2.2-fold higher mortality rate compared to that
in patients without preoperative cholangitis (22). However, Liu et al (23) observed that the presence of
inflammation was associated with improved postoperative survival in
ICC patients. Luo et al (24) concluded that preoperative chronic
proliferative cholangitis, possibly caused by hepatolithiasis, was
unrelated to the OS of ICC. In our study, the univariate analysis
identified preoperative cholangitis as a disadvantageous factor
associated with the OS of ECC patients, while the multivariate
analysis with a Cox proportional hazards model did not yield
similar results. Further investigation on whether inflammation is a
prognostic factor for ECC is required.
The role of postoperative AT remains controversial
for ECC (11). Several experts
recommended postoperative adjuvant radiotherapy or chemotherapy for
ECC patients, based mainly on institutional small-sampled evidence
(11). However, the findings have
been inconsistent. Certain retrospective studies reported a
positive effect of adjuvant chemotherapy or radiotherapy on
patients with resectable ECC (19,25)
whereas others reported no such effect (4,26).
In one randomized controlled trial investigating adjuvant
chemotherapy for biliary carcinoma, Takada et al (14) reported the efficacy of adjuvant
chemotherapy with mitomycin C and 5-FU in gallbladder carcinoma,
but not in bile duct carcinoma patients. Additionally, Pitt et
al (27) reported no
improvement in OS with adjuvant radiation in the only randomized
controlled trial on postoperative adjuvant radiotherapy for
perihilar cholangiocarcinoma. These two large-sampled controlled
clinical trails demonstrated that neither adjuvant chemotherapy nor
radiotherapy improved the survival of ECC patients. Thus far, the
findings of AT have been quite discouraging for oncologists.
However, the high rates of relapse and metastasis
following surgical resection have prompted further investigation of
AT for ‘high-risk’ ECC, although the literature in this area
remains sparse. The combined administration of gemcitabine and
cisplatin has shown convincing efficacy regarding survival in
advanced biliary tract carcinoma and has become a standard therapy
(28). Consequently, certain
investigators attempted to treat postoperative ECC patients using
gemcitabine-based chemotherapeutic regimens. A systematic review
and meta-analysis demonstrated beneficial effects of AT on
cholangiocarcinoma patients, with significant prolongation of the
OS in the lymphatic metastasis and surgical margin-positive
subgroups (11). The latest
results from several small-sampled retrospective studies indicated
that gemcitabine-based chemotherapy may improve OS outcomes for ECC
following surgical resection, as compared to the outcomes reported
in previous studies (3,25,29–31).
In addition, an open-label, phase 3, randomized controlled trial
investigating adjuvant chemotherapy for periampullary
adenocarcinoma, including 297 cases of ampullary cancer, 96 cases
of bile duct cancer and 35 cases of other cancers, reported that
adjuvant chemotherapy was associated with significant survival
benefits in the entire patient cohort (HR=0.75), although this
effect requires further improvement (32).
Similarly, certain researchers treated ECC patients
with radiotherapy following surgical resection. A bulk of
retrospective data suggested that improved survival may be achieved
with the use of adjuvant radiation following surgical treatment,
particularly with dose escalation (10). In addition, previous studies
reported that adjuvant concurrent chemoradiotherapy using
three-dimensional conformal radiotherapy improved locoregional
control and survival in ECC patients with R1 resection or positive
lymph nodes (33–38). In the present study, postoperative
AT did not appear to exert a positive effect on ECC patients as a
whole. However, the survival time of node-positive ECC patients was
significantly prolonged when compared to that of non-AT patients.
Following adjustment for lymph node metastasis, histological type,
surgical margins, preoperative cholangitis and stage, AT remained a
statistically significant prognostic factor for postoperative
survival. Postoperative AT contributed to a 0.45-fold mortality
rate compared to non-AT ECC patients.
There were certain limitations to this study. First,
the study design was non-randomized and retrospective, which may be
the source of uncontrolled bias. Second, the interval time of
follow-up was not equally controlled for each patient; therefore,
the relapse-free survival time of patients could not be presented.
Additionally, the ATs involved in this study have not been
separately analyzed due to the limited samples.
In conclusion, negative surgical margins and
negative lymph node status, together with AT, were identified as
independent favorable prognostic factors in ECC patients following
surgical resection. Therefore, AT may prolong the OS of lymph
node-positive EEC patients following surgical resection. The
findings of the present study suggest that patients with
node-positive disease may benefit from postoperative adjuvant
chemotherapy or radiotherapy. However, a prospective clinical trial
of AT in ECC is required to confirm these results.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant no. 81172169).
References
|
1
|
Castro FA, Koshiol J, Hsing AW and Devesa
SS: Biliary tract cancer incidence in the United States-Demographic
and temporal variations by anatomic site. Int J Cancer.
133:1664–1671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gwak HK, Kim WC, Kim HJ and Park JH:
Extrahepatic bile duct cancers: surgery alone versus surgery plus
postoperative radiation therapy. Int J Radiat Oncol Biol Phys.
78:194–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murakami Y, Uemura K, Sudo T, et al:
Adjuvant gemcitabine plus S-1 chemotherapy improves survival after
aggressive surgical resection for advanced biliary carcinoma. Ann
Surg. 250:950–956. 2009. View Article : Google Scholar
|
|
4
|
Aljiffry M, Walsh MJ and Molinari M:
Advances in diagnosis, treatment and palliation of
cholangiocarcinoma: 1990–2009. World J Gastroenterol. 15:4240–4262.
2009.PubMed/NCBI
|
|
5
|
Pattanathien P, Khuntikeo N, Promthet S
and Kamsa-Ard S: Survival rate of extrahepatic cholangiocarcinoma
patients after surgical treatment in Thailand. Asian Pac J Cancer
Prev. 14:321–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagorney DM, Donohue JH, Farnell MB,
Schleck CD and Ilstrup DM: Outcomes after curative resections of
cholangiocarcinoma. Arch Surg. 128:871–879. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JW, Jo S, Moon HJ, Heo JS, Choi SH,
Joh JW, Choi DW, Chung JC and Kim YI: Prognostic factors after
major resection for distal extrahepatic cholangiocarcinoma. Korean
J Gastroenterol. 47:144–152. 2006.(In Korean).
|
|
8
|
Woo SM, Ryu JK, Lee SH, et al: Recurrence
and prognostic factors of ampullary carcinoma after radical
resection: comparison with distal extrahepatic cholangiocarcinoma.
Ann Surg Oncol. 14:3195–3201. 2007. View Article : Google Scholar
|
|
9
|
Unno M, Katayose Y, Rikiyama T, et al:
Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary
Pancreat Sci. 17:463–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anderson C and Kim R: Adjuvant therapy for
resected extrahepatic cholangiocarcinoma: a review of the
literature and future directions. Cancer Treat Rev. 35:322–327.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: a
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimoda M and Kubota K: Multi-disciplinary
treatment for cholangiocellular carcinoma. World J Gastroenterol.
13:1500–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takada T, Amano H, Yasuda H, et al: Is
postoperative adjuvant chemotherapy useful for gallbladder
carcinoma? A phase III multicenter prospective randomized
controlled trial in patients with resected pancreaticobiliary
carcinoma. Cancer. 95:1685–1695. 2002. View Article : Google Scholar
|
|
15
|
Sasaki R, Takeda Y, Funato O, Nitta H,
Kawamura H, Uesugi N, Sugai T, Wakabayashi G and Ohkohchi N:
Significance of ductal margin status in patients undergoing
surgical resection for extrahepatic cholangiocarcinoma. World J
Surg. 9:1788–1796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th Edition.
New York: Springer; pp. 66–71. 2010
|
|
17
|
Kiriyama S, Takada T, Strasberg SM, et al;
Tokyo Guidelines Revision Committee. New diagnostic criteria and
severity assessment of acute cholangitis in revised Tokyo
Guidelines. J Hepatobiliary Pancreat Sci. 19:548–556. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuller CD, Wang SJ, Choi M, et al:
Multimodality therapy for locoregional extrahepatic
cholangiocarcinoma: a population-based analysis. Cancer.
115:5175–5183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng Q, Luo X, Zhang B, Jiang X, Yi B and
Wu M: Predictive factors for prognosis of hilar cholangiocarcinoma:
postresection radiotherapy improves survival. Eur J Surg Oncol.
33:202–207. 2007. View Article : Google Scholar
|
|
20
|
Khuntikeo N, Pugkhem A, Bhudhisawasdi V
and Uttaravichien T: Major hepatic resection for hilar
cholangiocarcinoma without preoperative biliary drainage. Asian Pac
J Cancer Prev. 9:83–85. 2008.PubMed/NCBI
|
|
21
|
Li H, Qin Y, Cui Y, Chen H, Hao X and Li
Q: Analysis of the surgical outcome and prognostic factors for
hilar cholangiocarcinoma: a Chinese experience. Dig Surg.
28:226–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho JY, Han HS, Yoon YS, et al:
Preoperative cholangitis and metastatic lymph node have a negative
impact on survival after resection of extrahepatic bile duct
cancer. World J Surg. 36:1842–1847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu RQ, Shen SJ, Hu XF, Liu J, Chen LJ and
Li XY: Prognosis of the intrahepatic cholangiocarcinoma after
resection: hepatitis B virus infection and adjuvant chemotherapy
are favorable prognosis factors. Cancer Cell Int. 13:992013.
View Article : Google Scholar
|
|
24
|
Luo X, Yuan L, Wang Y, Ge R, Sun Y and Wei
G: Survival outcomes and prognostic factors of surgical therapy for
all potentially resectable intrahepatic cholangiocarcinoma: a large
single-center cohort study. J Gastrointest Surg. 18:562–572. 2014.
View Article : Google Scholar
|
|
25
|
Wirasorn K, Ngamprasertchai T, Khuntikeo
N, et al: Adjuvant chemotherapy in resectable cholangiocarcinoma
patients. J Gastroenterol Hepatol. 28:1885–1891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vern-Gross TZ, Shivnani AT, Chen K, et al:
Survival outcomes in resected extrahepatic cholangiocarcinoma:
effect of adjuvant radiotherapy in a surveillance, epidemiology,
and end results analysis. Int J Radiat Oncol Biol Phys. 81:189–198.
2011. View Article : Google Scholar
|
|
27
|
Pitt HA, Nakeeb A, Abrams RA, Coleman J,
Piantadosi S, Yeo CJ, Lillemore KD and Cameron JL: Perihilar
cholangiocarcinoma. Postoperative radiotherapy does not improve
survival. Ann Surg. 221:788–798. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valle J, Wasan H, Palmer DH, et al; ABC-02
Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine
for biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murakami Y, Uemura K, Sudo T, et al:
Gemcitabine-based adjuvant chemotherapy improves survival after
aggressive surgery for hilar cholangiocarcinoma. J Gastrointest
Surg. 13:1470–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murakami Y, Uemura K, Sudo T, et al:
Adjuvant chemotherapy with gemcitabine and S-1 after surgical
resection for advanced biliary carcinoma: outcomes and prognostic
factors. J Hepatobiliary Pancreat Sci. 19:306–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamanaka K, Hatano E, Kanai M, et al: A
single-center analysis of the survival benefits of adjuvant
gemcitabine chemotherapy for biliary tract cancer. Int J Clin
Oncol. 19:485–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neoptolemos JP, Moore MJ, Cox TF, et al;
European Study Group for Pancreatic Cancer. Effect of adjuvant
chemotherapy with fluorouracil plus folinic acid or gemcitabine vs
observation on survival in patients with resected periampullary
adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial.
JAMA. 308:147–156. 2012. View Article : Google Scholar
|
|
33
|
Hughes MA, Frassica DA, Yeo CJ, et al:
Adjuvant concurrent chemoradiation for adenocarcinoma of the distal
common bile duct. Int J Radiat Oncol Biol Phys. 68:178–182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borghero Y, Crane CH, Szklaruk J, et al:
Extrahepatic bile duct adenocarcinoma: patients at high-risk for
local recurrence treated with surgery and adjuvant chemoradiation
have an equivalent overall survival to patients with standard-risk
treated with surgery alone. Ann Surg Oncol. 15:3147–3156. 2008.
View Article : Google Scholar
|
|
35
|
Bonet Beltran M, Roth AD, Mentha G and
Allal AS: Adjuvant radio-chemotherapy for extrahepatic biliary
tract cancers. BMC Cancer. 11:2672011.
|
|
36
|
Narang AK, Miller RC, Hsu CC, et al:
Evaluation of adjuvant chemoradiation therapy for ampullary
adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic
collaborative study. Radiat Oncol. 6:1262011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JH, Choi EK, Ahn SD, et al:
Postoperative chemoradiotherapy for extrahepatic bile duct cancer.
Int J Radiat Oncol Biol Phys. 79:696–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Habermehl D, Lindel K, Rieken S, et al:
Chemoradiation in patients with unresectable extrahepatic and hilar
cholangiocarcinoma or at high risk for disease recurrence after
resection: Analysis of treatment efficacy and failure in patients
receiving postoperative or primary chemoradiation. Strahlenther
Onkol. 188:795–801. 2012. View Article : Google Scholar
|