Introduction
Esophageal squamous cell carcinoma (ESCC) is a
highly malignant tumor and its prognosis is generally poor. Recent
epidemiological studies demonstrated that the incidence of ESCC is
on the increase (1). Neoadjuvant
chemoradiotherapy (CRT) followed by surgery may improve long-term
survival and reduce local recurrence in patients with esophageal
cancer. However, the overall cure rate of esophageal cancer remains
<20% (2–4).
Fas and Fas ligand (FasL) are transmembrane proteins
that belong to the tumor necrosis factor family and their signaling
pathway is a key regulator of apoptotic cell death, i.e., Fas
binding of FasL induces an apoptotic cascade. Furthermore, the
Fas/FasL pathway regulates the tumor microenvironment, including
the host immune system and extracellular matrix (5–12).
Several authors have reported that Fas/FasL expression is
correlated with tumor progression and poor prognosis in esophageal
cancer (6,13,14).
By contrast, Takikita et al (15) reported that the Fas/FasL apoptotic
pathway, including Fas-associated death domain protein and caspases
8 and 10 were not prognostic factors in ESCC.
In the present study, we evaluated the expression of
Fas, FasL and Ki67 (a proliferation marker) in ESCC following
neoadjuvant CRT and analyzed the correlation of their expression
with clinical outcome. Additionally, the association of Fas/FasL
expression with peritumoral immune cells was investigated.
Materials and methods
Patients and specimens
A total of 20 patients who had received neoadjuvant
CRT followed by surgery were enrolled in this study. All the
formalin-fixed, paraffin-embedded (FFPE) specimens of the patients
were available for evaluation. The study protocol was approved by
the Ethics Review Board of Mie University Hospital and all the
included patients provided written informed consent for their
tissues to be used in this study.
5-Fluorouracil (5-FU) and cisplatin
(CDDP)-based CRT
All the patients received systemic 5-FU and CDDP
chemotherapy with concurrent radiotherapy. The regimen included 4
cycles of 5-FU (600 mg/m2 administered intravenously
over 24 h), plus tegafur and uracil (400 mg/kg of body weight
administered orally for 5 days) and CDDP (4 mg/day administered
intravenously for 5 days) with concurrent 40 Gy radiation followed
by surgery. Preoperative radiotherapy was delivered to the primary
tumor as well as the peritumoral area at a dose of 40 Gy in 20
fractions within 4 weeks. The time interval between neoadjuvant CRT
and surgery was 2–3 weeks.
Clinical response and
histopathological analysis of tumor regression following CRT
The clinical response following preoperative CRT was
evaluated by barium esophagography, endoscopy and computed
tomography. The results were graded as complete response (CR),
partial response (PR), no change (NC) or progressive disease. The
pathological response to CRT was evaluated using the Mandard tumor
regression grade (TRG) (16). The
tumors were classified according to the Mandard system into 5
grades as follows: i) TRG1, CR with absence of residual cancer and
fibrosis extending through the wall; ii) TRG2, presence of residual
tumor cells scattered through the fibrotic area; iii) TRG3,
increased numbers of residual cancer cells, with predominant
fibrosis; iv) TRG4, residual cancer outgrowing the fibrotic area;
v) TRG5, absence of regressive changes. We categorized patients in
categories TRG1 and 2 as responders and those in categories TRG3-5
as non-responders.
Immunohistochemistry (IHC)
The FFPE specimens were cut into 2- to 3-µm
sections. Following deparaffinization and dehydration, the sections
were placed in a 10 mmol/l sodium citrate buffer (pH 6.0) and
autoclaved at 121°C for 10 min for antigen retrieval. The sections
were incubated in 3% hydrogen peroxide for 10 min, blocked and
incubated with a primary antibody overnight at 4°C. Monoclonal
mouse anti-human Fas antibody (B-10, catalog no. sc-8009; Santa
Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:100),
polyclonal rabbit anti-human FasL antibody (C-20, catalog no.
sc-957; Santa Cruz Biotechnology; dilution 1:100) and monoclonal
mouse anti-human Ki67 antibody (MIB-1, code M7240; Dako Cytomation,
Glostrup, Denmark; dilution 1:100) were the primary antibodies used
in a labeled streptavidin-biotin system (EnVision™ + Dual Link
System-HRP; Dako Cytomation). Antibody binding was visualized using
3,3′-diaminobenzidine (Dako Cytomation). All the sections were
counterstained with hematoxylin prior to being dehydrated and
mounted. At least 2 sections per specimen were stained to confirm
reproducibility. Negative controls were prepared simultaneously
with pre-immune immunoglobulin.
IHC evaluation
The sections were observed under a light microscope
(BX50, Olympus, Tokyo, Japan). We calculated IHC scores by
multiplying the percentage of positive epithelial cells (0–100%) by
the staining intensity, as previously described (17). The staining intensities were scored
as follows: 0, negative; 1, weak; and 2, strong for Fas and Ki67;
and 0, negative; 1, weak; 2, moderate; and 3, strong for FasL. The
IHC scores ranged between 0 and 200 for Fas and Ki67; and between 0
and 300 for FasL. Each sample was scored by two investigators (K.T.
and Y.O.) who were blinded to the clinicopathological information
regarding the origin of the samples.
Enumeration of peritumoral CD8- and
Foxp3-positive cells
We previously recorded the numbers of peritumoral
CD8- and Foxp3-positive cells using light microscopy and the median
count was calculated for each sample. The median values for the
number of CD8- and Foxp3-positive cells were 70 (range, 18–250) at
a magnification x200 and 13 (range, 1–57) at a magnification x100
(18). We analyzed the correlation
of Fas/FasL expression with peritumoral CD8- and Foxp3-positive
cells.
Statistical analysis
Statistical analysis was performed using StatView
v5.0 software (SAS Institute Inc., Cary, NC, USA). Significant
differences were analyzed using the Chi-square test. The Pearson's
correlation test was used to determine statistical correlations.
Recurrence-free and overall survival probabilities were calculated
from the date of surgery to the date of recurrence or death, using
the Kaplan-Meier product limit method; intergroup differences were
determined using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median age of the
patients was 68 years (range, 52–77 years) and the male:female
ratio was 9:1. The median follow-up period was 16 months (range,
4–91 months). Of the 20 tumors, 12 were located in the lower, 7 in
the middle and 1 in the upper part of the esophagus. The mean size
of the tumors was 40 mm (range, 20–82 mm). The post-CRT
pathological T stages were as follows: pT1 (n=1), pT2 (n=9), pT3
(n=9) and pT4 (n=1). A total of 10 patients (50%) presented with
lymph node metastases. The median number of total dissected lymph
nodes was 14 (range, 0–39) and the median value of lymph node
density was 5.45% (range, 0–55.6%). A total of 17 tumors (85%)
exhibited well or moderately differentiated squamous cell carcinoma
histology. R0 resection was performed in 80% of the cases. The
clinical response was classified as follows: NC, 9 patients; PR, 10
patients; and CR, 1 patient. The Mandard TRG grades were as
follows: TRG1, no patients; TRG2, 9 patients; TRG3, 7 patients;
TRG4, 4 patients; and TRG5, no patients (non-responders, n=11;
responders, n=9).
 | Table IPatient characteristics (n=20). |
Table I
Patient characteristics (n=20).
| Characteristics | Values (%) |
|---|
| Age, years | |
|
Mediam | 69 |
| Range | 52–77 |
| Gender | |
| Male | 18 (90) |
|
Female |
2(10) |
| Location | |
|
Upper | 1
(5) |
|
Middle | 7
(35) |
|
Lower | 12 (60) |
| ypT | |
| T1/2 | 10 (50) |
| T3/4 | 10 (50) |
| ypN | |
|
Absent | 10 (50) |
|
Present | 10 (50) |
| Postoperative
stage | |
| I | 1
(5) |
| II | 9
(45) |
| III | 7
(35) |
| IV | 3
(15) |
| Lymphatic
invasion | |
|
Absent | 4
(20) |
|
Present | 16 (80) |
| Vascular
invasion | |
|
Absent | 12
(60) |
|
Present | 8
(40) |
| Histological
differentiation | |
|
High/moderate | 17
(85) |
| Poor | 3
(15) |
| R0 resection | |
| Yes | 16
(80) |
| No | 4
(20) |
| Mandard TRG | |
| 5 | 0
(0) |
| 4 | 4
(20) |
| 3 | 7
(35) |
| 2 | 9
(45) |
| 1 | 0
(0) |
| Recurrence(R0,
n=16) | |
|
Absent | 10 (62.5) |
|
Present | 6
(37.5) |
IHC findings for Fas, FasL and Ki67 in
ESCC following neoadjuvant CRT
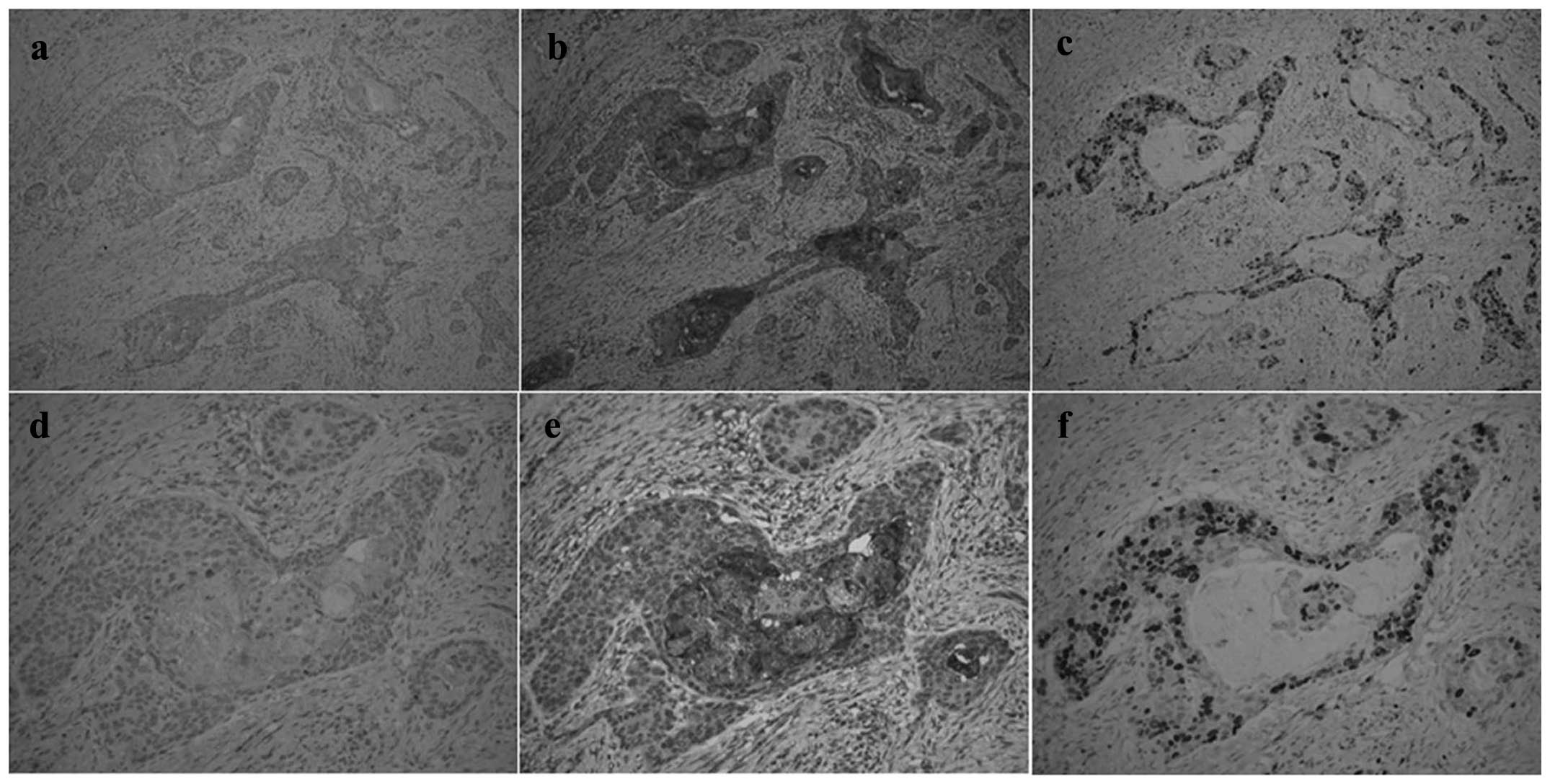
Fig. 1 shows the
IHC findings for Fas, FasL and Ki67 in ESCC following neoadjuvant
CRT. Fas and FasL were expressed in the cytoplasm and nuclei of
cancer cells. Additionally, FasL expression was diffusely detected
in stromal cells. Ki67 expression was detected in cancer cell
nuclei. There was no correlation between the IHC findings for
Fas/FasL and Ki67. The median values of the IHC scores for Fas,
FasL and Ki67 were 80 (range, 1–200), 190 (range, 30–300) and 10
(range, 1–180), respectively. We classified those cases with values
above the median IHC score as the high-expression group and the
remainder as the low-expression group.
Correlations of Fas, FasL and Ki67
expression with clinicopathological variables
High FasL expression was significantly correlated
with disease recurrence in patients treated with curative intent
(P=0.0134). However, there were no correlations between Fas/FasL
expression and other clinicopathological characteristics (Table II). High Ki67 expression was
significantly correlated with lymph node metastasis and lymphatic
invasion (P=0.007 and 0.025, respectively) (data not shown).
 | Table IICorrelations of Fas, Fas ligand (FasL)
and Ki67 expression with clinicopathological variables. |
Table II
Correlations of Fas, Fas ligand (FasL)
and Ki67 expression with clinicopathological variables.
| Variables | Fas-low | Fas-high | P-valuea | FasL-low | FasL-high | P-valuea |
|---|
| Median age,
years | | | | | | |
| ≤69 | 6 | 4 | 0.371 | 4 | 6 | 0.371 |
|
>69 | 4 | 6 | | 6 | 4 | |
| Gender | | | | | | |
| Male | 8 | 10 | 0.136 | 9 | 9 | 0.999 |
|
Female | 2 | 0 | | 1 | 1 | |
| Location | | | | | | |
| Upper | 1 | 0 | 0.270 | 1 | 0 | 0.565 |
| Middle | 2 | 5 | | 3 | 4 | |
| Lower | 7 | 5 | | 6 | 6 | |
| Size, mm | | | | | | |
|
<40 | 6 | 8 | 0.329 | 7 | 7 | 0.999 |
|
≥40 | 4 | 2 | | 3 | 3 | |
| ypT | | | | | | |
|
T0/1/2 | 7 | 3 | 0.074 | 4 | 6 | 0.371 |
|
T3/4 | 3 | 7 | | 6 | 4 | |
| Lymph node
metastasis | | | | | | |
|
Absent | 3 | 7 | 0.0746 | 6 | 4 | 0.371 |
|
Present | 7 | 3 | | 4 | 6 | |
| Postoperative
stage | | | | | | |
|
0/I/II | 5 | 5 | 0.999 | 6 | 4 | 0.371 |
|
III/IV | 5 | 5 | | 4 | 6 | |
| Lymphatic
invasion | | | | | | |
|
Absent | 1 | 3 | 0.264 | 3 | 1 | 0.264 |
|
Present | 9 | 7 | | 7 | 9 | |
| Vascular
invasion | | | | | | |
|
Absent | 6 | 6 | 0.999 | 6 | 6 | 0.999 |
|
Present | 4 | 4 | | 4 | 4 | |
| Histological
differentiation | | | | | | |
|
High/moderate | 9 | 8 | 0.531 | 9 | 8 | 0.531 |
|
Poor | 1 | 2 | | 1 | 2 | |
| R0 resection | | | | | | |
|
Yes | 9 | 7 | 0.264 | 9 | 7 | 0.264 |
| No | 1 | 3 | | 1 | 3 | |
| Pathological
response | | | | | | |
|
Non-responders | 6 | 5 | 0.653 | 5 | 6 | 0.653 |
|
Responders | 4 | 5 | | 5 | 4 | |
| Recurrence (R0,
n=16) | | | 0.0907 | | | 0.0134 |
|
Absent | 4 | 6 | | 8 | 2 | |
|
Present | 5 | 1 | | 1 | 5 | |
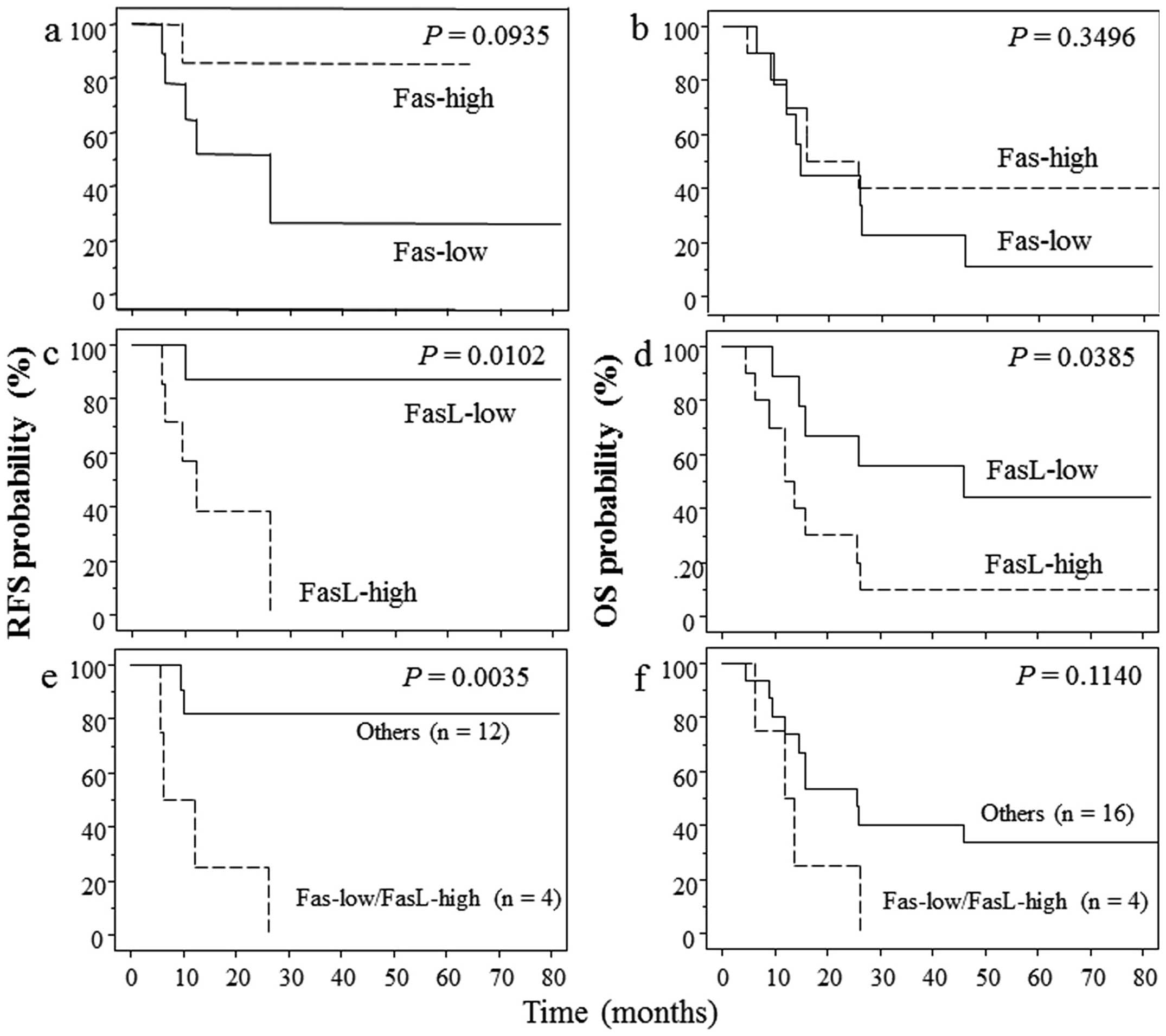
Survival analysis based on Fas and
FasL expression
The recurrence-free survival and overall survival
according to Fas and FasL expression using the Kaplan-Meier product
limit method are shown in Fig. 2.
High expression of FasL was found to be significantly associated
with poor recurrence-free and overall survival (P=0.0102 and
0.0385, respectively). Patients with low Fas and high FasL
expression exhibited a poorer recurrence-free survival
(P=0.0035).
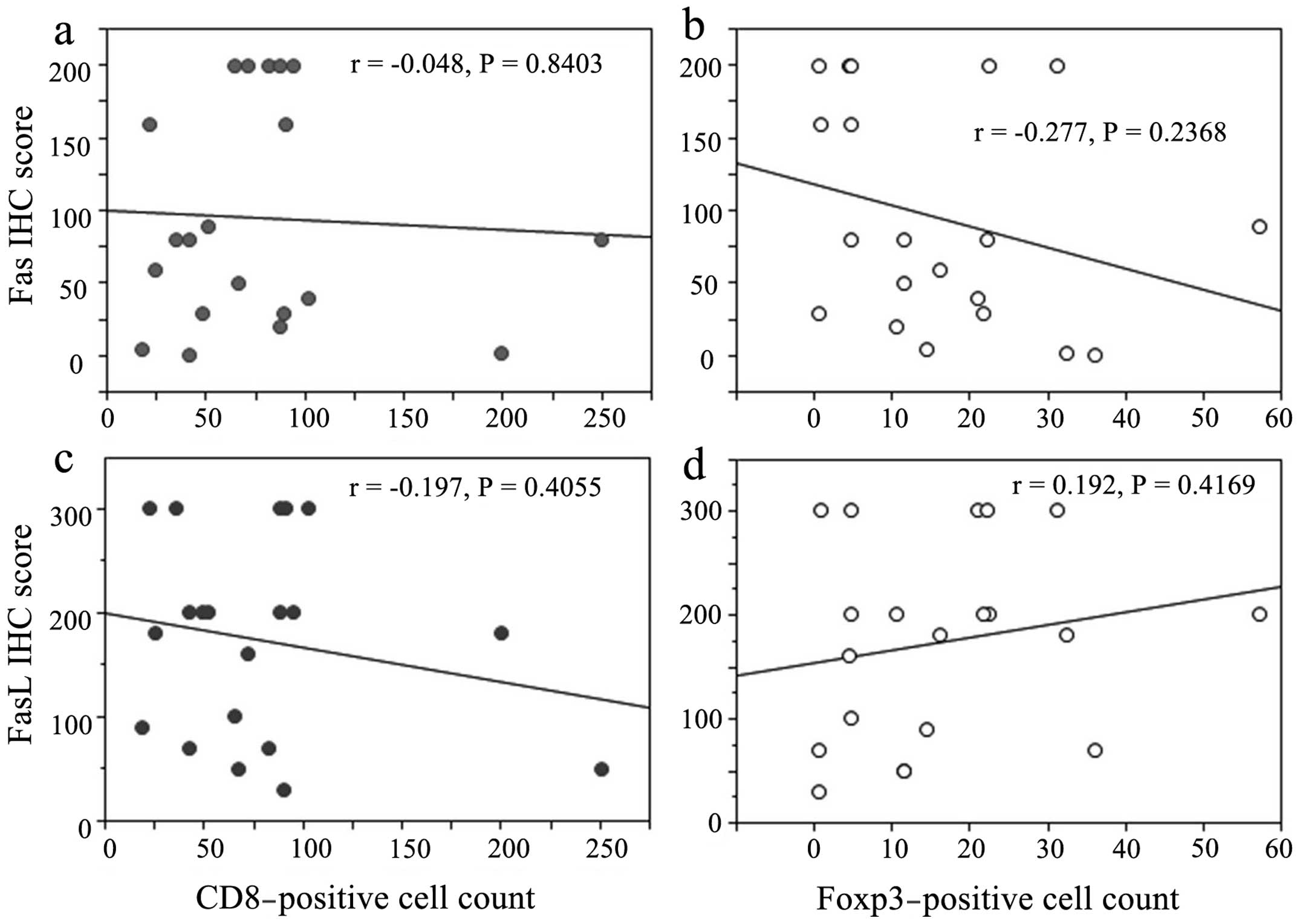
Correlation of Fas/FasL expression
with peritumoral CD8- and Foxp3-positive cells
Although the differences did not achieve statistical
significance, Fas expression was inversely correlated with
peritumoral Foxp3-positive cells, whereas FasL expression appeared
to be inversely correlated with peritumoral CD8-positive cells and
directly correlated with Foxp3-positive cells (Fig. 3).
Discussion
In esophageal cancer, upregulation of FasL and
downregulation of Fas have been reported (19). In the present study, the median
value of the FasL IHC score was significantly higher compared to
that of the Fas IHC score in ESCC following preoperative CRT
(P=0.006). Gratas et al (19) demonstrated that FasL expression was
found in over half of the examined cancer cells, whereas Fas
expression was rarely observed in tumor cells in ESCC. We observed
that Fas expression was present in all the cases (median percentage
of Fas-positive cancer cells, 80%; range, 1–100%). To explain this
difference, we surmised that Fas expression may have been
upregulated by radiation based on a previous study reporting that
Fas expression in ESCC was increased by irradiation in vitro
in a dose-dependent manner (20).
In the present study, we did not compare the expression of Fas pre-
and post-CRT.
We observed that a high FasL expression was
correlated with tumor relapse. Moreover, patients with high FasL
expression exhibited poorer recurrence-free and overall survival
among ESCC patients treated with preoperative CRT. Shibakita et
al (6) reported that FasL
expression did not affect survival, whereas Fas expression was an
independent favorable prognostic factor for patient survival in
ESCC. In our data, patients with low Fas expression tended to have
poor recurrence-free survival compared to those with high Fas
expression.
One possible mechanism explaining the involvement of
Fas/FasL expression in the prognosis of ESCC is evasion of the host
immune response. Tumor-derived FasL counteracts the host immune
system by eliminating Fas-sensitive cytotoxic T cells, such as
CD8-positive cells (6,19). In the present study, FasL
expression was diffusely observed in stromal cells in ESCC
following CRT. Although significant differences were not observed,
Fas appeared to be negatively correlated with peritumoral
Foxp3-positive cells (regulatory T cells) and FasL appeared to be
positively correlated with peritumoral CD8-positive cells. Rigberg
et al (20) hypothesized
that Fas/FasL proteins may provide certain tumors with an immune
privilege and increase their resistance to radiotherapy. Moreover,
several authors have reported that Fas/FasL signaling plays a role
in chemoresistance to doxorubicin and oxaliplatin and immune
responses by interaction with matrix metalloproteinase-7 (10–12).
Although there were no correlations between Fas/FasL expression and
TRG in this study, pharmacological control of Fas/FasL signaling
may result in improved therapeutic efficacy and outcome in ESCC
patients who receive preoperative CRT.
Rigberg et al (20) demonstrated that neither anti-Fas
monoclonal antibody nor transduction of FasL directly inhibited
tumor cell growth in an in vitro study. Thus, the main role
of Fas/FasL signaling in tumor progression is likely the regulation
of the tumor microenvironment. Sun et al (21) reported that polymorphisms of
Fas/FasL genes appeared to be associated with an increased risk of
developing ESCC. Taken together, the Fas/FasL pathway may be
considered to be a candidate therapeutic target in ESCC, as the
Fas/FasL signaling pathway plays an important role in tumorigenesis
and tumor progression (22,23).
In conclusion, high FasL expression was associated
with poor prognosis and the evaluation of FasL expression may
provide clinically useful prognostic information for ESCC patients
receiving neoadjuvant CRT. Moreover, the control of Fas/FasL
signaling may lead to improved therapeutic efficacy and outcome in
ESCC patients following neoadjuvant CRT. However, the data in this
study should be interpreted with caution. The major limitations
were the limited patient sample and the retrospective nature of the
study. A larger study population is required to validate our
conclusions.
References
|
1
|
Stahl M, Stuschke M, Lehmann N, et al:
Chemoradiation with and without surgery in patients with locally
advanced squamous cell carcinoma of the esophagus. J Clin Oncol.
23:2310–2317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lv J, Cao XF, Zhu B, Ji L, Tao L and Wang
DD: Effect of neoadjuvant chemoradiotherapy on prognosis and
surgery for esophageal carcinoma. World J Gastroenterol.
15:4962–4968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sjoquist KM, Burmeister BH, Smithers BM,
et al: Survival after neoadjuvant chemotherapy or chemoradiotherapy
for resectable oesophageal carcinoma: an updated meta-analysis.
Lancet Oncol. 12:681–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Hagen P, Hulshof MC, van Lanschot JJ,
et al: Preoperative chemoradiotherapy for esophageal or junctional
cancer. N Engl J Med. 366:2074–2084. 2012.
|
|
5
|
Igney FH and Krammer PH: Tumor
counterattack: fact or fiction? Cancer Immunol Immunother.
54:1127–1136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibakita M, Tachibana M, Dhar DK, et al:
Prognostic significance of Fas and Fas ligand expressions in human
esophageal cancer. Clin Cancer Res. 5:2464–2469. 1999.PubMed/NCBI
|
|
7
|
Green DR and Ferguson TA: The role of Fas
ligand in immune privilege. Nat Rev Mol Cell Biol. 2:917–924. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abrahams VM, Kamsteeg M and Mor G: The
Fas/Fas ligand system and cancer: immune privilege and apoptosis.
Mol Biotechnol. 25:19–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholz M and Cinatl J: Fas/FasL
interaction: a novel immune therapy approach with immobilized
biologicals. Med Res Rev. 25:331–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitsiades N, Yu WH, Poulaki V, Tsokos M
and Stamenkovic I: Matrix metalloproteinase-7-mediated cleavage of
Fas ligand protects tumor cells from chemotherapeutic drug
cytotoxicity. Cancer Res. 61:577–581. 2001.PubMed/NCBI
|
|
11
|
Wang WS, Chen PM, Wang HS, Liang WY and Su
Y: Matrix metalloproteinase-7 increases resistance to Fas-mediated
apoptosis and is a poor prognostic factor of patients with
colorectal carcinoma. Carcinogenesis. 27:1113–1120. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Almendro V, Ametller E, Garcia-Recio S, et
al: The role of MMP7 and its cross-talk with the FAS/FASL system
during the acquisition of chemoresistance to oxaliplatin. PLoS One.
4:e47282009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Younes M, Schwartz MR, Ertan A, Finnie D
and Younes A: Fas ligand expression in esophageal carcinomas and
their lymph node metastases. Cancer. 88:524–528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kase S, Osaki M, Adachi H, Kaibara N and
Ito H: Expression of Fas and Fas ligand in esophageal tissue mucosa
and carcinomas. Int J Oncol. 20:291–297. 2002.PubMed/NCBI
|
|
15
|
Takikita M, Hu N, Shou JZ, et al:
Biomarkers of apoptosis and survival in esophageal squamous cell
carcinoma. BMC Cancer. 9(310)2009. View Article : Google Scholar
|
|
16
|
Mandard AM, Dalibard F, Mandard JC, et al:
Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar
|
|
17
|
Wong SC, Lo SF, Lee KC, Yam JW, Chan JK
and Wendy Hsiao WL: Expression of frizzled-related protein and
Wnt-signalling molecules in invasive human breast tumours. J
Pathol. 196:145–153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saigusa S, Tanaka K, Ohi M, et al:
Clinical significance of peritumoral mast cells in esophageal
squamous cell carcinoma with neoadjuvant chemoradiotherapy.
Esophagus. 10:12–19. 2013. View Article : Google Scholar
|
|
19
|
Gratas C, Tohma Y, Barnas C, Taniere P,
Hainaut P and Ohgaki H: Up-regulation of Fas (APO-1/CD95) ligand
and down-regulation of Fas expression in human esophageal cancer.
Cancer Res. 58:2057–2062. 1998.PubMed/NCBI
|
|
20
|
Rigberg DA, Centeno J, Kim FS, et al:
Irradiation-induced up-regulation of Fas in esophageal squamous
cell carcinoma is not accompanied by Fas ligand-mediated apoptosis.
J Surg Oncol. 71:91–96. 1999. View Article : Google Scholar
|
|
21
|
Sun T, Miao X, Zhang X, Tan W, Xiong P and
Lin D: Polymorphisms of death pathway genes FAS and FASL in
esophageal squamous-cell carcinoma. J Natl Cancer Inst.
96:1030–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Brien DI, Nally K, Kelly RG, O'Connor
TM, Shanahan F and O'Connell J: Targeting the Fas/Fas ligand
pathway in cancer. Expert Opin Ther Targets. 9:1031–1044. 2005.
|
|
23
|
Villa-Morales M and Fernandez-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Expert
Opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar : PubMed/NCBI
|