Introduction
Chondrosarcoma is a highly aggressive bone cancer of
mesenchymal origin, which is resistant to chemo- and radiation
therapies, leaving surgical resection as the only option.
Metastatic spread of malignant cells eventually develops following
surgical resection. The malignant progression to chondrosarcoma has
been suggested to involve a degree of mesenchymal-to-epithelial
transition (MET) and it has been demonstrated in an in vitro
model that chondrosarcoma cells may transition to a more
epithelial-like phenotype under certain conditions, with the
acquisition of epithelial markers, such as E-cadherin, desmocollin
3, maspin and 14-3-3σ due to the epigenetic switch (1). The epithelial-to-mesenchymal
transition (EMT) and its reverse process, MET, play central roles
in embryogenesis. EMT is an important process that permits
epithelial cells to dissolve intercellular connections and acquire
mesenchymal properties, exhibiting augmented migratory ability. The
hallmarks of EMT include loss of structural adhesion components,
such as E-cadherin, cell-cell adhesion glycoprotein, and gain of
mesenchymal cell markers, such as vimentin. E-cadherin expression
is lost in carcinomas during EMT and restored during MET, providing
a survival advantage for these cells in a challenging ectopic
environment. Aberrant activation of EMT has been reported to
contribute to tumour progression/metastasis. During
metastasis-associated MET, the epithelial tumour cells, which have
undergone EMT and are present in the blood circulation, reverse the
EMT process to grow at distant sites as epithelial tumour cells
(2,3). Sarcoma metastasis is not well
understood (1). Sarcomas are
considered to arise from mesenchymal cells and, thus, do not have a
baseline epithelial differentiation state that is seen in several
carcinomas. This fact excludes sarcomas from the EMT-MET metastasis
paradigm, whereby tumor cells in carcinomas must lose their
epithelial characteristics to escape the primary tumor, but regain
them to colonize the secondary site. Undifferentiated Ewing's
sarcoma cells, for example, may innately have the right balance of
epithelial and mesenchymal characteristics that allow for
successful growth at the primary and secondary sites, as well as a
high propensity for metastasis. Providing support for this
intrinsic ability, micrometastatic spread is ubiquitous in Ewing's
sarcoma patients and occurs during the early stages of the disease,
suggesting a parallel progression of tumor and metastases. This is
in contrast to certain carcinomas, such as colorectal cancer, where
a slow progression and accumulation of mutations eventually leads
to an invasive malignancy. A ‘passive metastasis’ model was
recently proposed for Ewing's sarcoma dissemination to contrast the
deliberate steps required in carcinoma metastasis (4). MET in sarcoma is represented by
expression of epithelial-like markers, such as E-cadherin, whereas
mesenchymal markers predominate in tumor cells. Disruption of
cell-cell junctions and the concomitant loss of polarity represent
hallmark phenotypes for a number of different cancer cells.
Moreover, a variety of evidence supports the argument that these
two common phenotypes of cancer cells directly contribute to
tumorigenesis, rather than simply representing indirect
consequences of the transformed state of these cells (5). Intercellular junctions are of
paramount importance in EMT and MET. A disruption of this cell-cell
communication, through multiple mechanisms, may in the short term
be a protective mechanism to limit the spread of toxicity in a
tissue following chemical or radiation damage. However, sustained
downregulation confers a loss of tumor-suppressive action (6).
Adherens junctions, also referred to as belle
desmosomes, are protein complexes that are found in cell-cell
junctions and their cytoplasmic face is linked to the actin
cytoskeleton. Three main protein families constitute traditional
adherens junction complexes: Transmembrane cadherins, armadillo
proteins and cytoskeletal adaptors. The cytoplasmic tails of
cadherins bind members of the armadillo protein family, such as
β-catenin. Cadherins communicate with the actin cytoskeleton
through contacts with β-catenin (7).
Similar to adherens junctions, desmosomes comprise
three main protein families, namely cadherins, armadillo proteins
and plakins. The two types of desmosomal cadherins, namely the
desmogleins (DSG) 1–4 and the desmocollins (DSC) 1–3, mediate
adhesion between apposing cells through interactions of their
ectodomains. Intracellularly, desmosomal cadherins bind to the
armadillo proteins junction plakoglobin (JUP, γ-catenin) and
plakophilins (PKP) 1–3, which help bridge the cadherins to the
intermediate filament cytoskeleton. The loss or reduction of one or
more desmosome components, including DSG 1–3, DSC 2, DSC 3, JUP,
PKP 1–3 and DSP, is observed during development and/or the
progression of various human cancers. Adherens junctions and
desmosomes are known to be downregulated in cancer, as they exert
tumor-suppressive effects (8–11).
The other main types of vertebrate intercellular
junctions may be broadly categorized as ‘communicating’ and
‘occluding’ junctions. The latter, also known as tight junctions,
seal adjacent membranes through multiple spanning transmembrane
proteins, such as occludins and claudin (5).
Finally, there are also gap junctions, some of which
may be downregulated in cancer (12), or may not depend on cellular
context. Gap junctions are intercellular membrane channels that
allow the direct exchange of small molecules (<1.2 kD) between
adjacent cells and are composed of two hemichannels (connexons),
which are in turn formed by the oligomerization of six protein
subunits, termed connexins.
The focus of the present study was the investigation
of intercellular junction protein expression in human malignant
chondrosarcoma and the effect of the antiproliferative cytostatic,
proline-rich polypeptide-1 (PRP-1) (13–18)
on their expression. PRP-1 was originally isolated from the
neurosecretory granules of bovine neurohypophysis. This compound is
a cytokine immunomodulator (17),
with a wide spectrum of properties (18).
Materials and methods
Cell lines and tissue culture
JJ012 chondrosarcoma cells were cultured in a
monolayer, as previously described (13–16),
in a medium consisting of Dulbecco's modified Eagle's medium,
supplemented with F12, 10% fetal bovine serum, 25 µg/ml ascorbic
acid, 100 ng/ml insulin, 100 nM hydrocortisone and 1%
penicillin/streptomycin. Upon confluency, the cells were
trypsinized and seeded in 6-well cluster dishes at a concentration
of 1×106 cells/ml and incubated for 24 h in a 5%
CO2 incubator. The experiments were performed in
biological triplicates. The cells were treated with PRP-1 peptide
in the experimental samples, while no peptide was added to the
control wells.
Gel electrophoresis and western
blotting
Upon confluency, the cells were trypsinized and
seeded in 6-well cluster dishes at a concentration of
1×106 cells/ml. The experimental samples were treated
with PRP-1 in corresponding concentrations, whereas control samples
were not treated with the peptide. The cells were incubated for 24
h in a 5% CO2 incubator at 37°C. On the following day,
the cells were washed with ice-cold phosphate-buffered saline. A
protease inhibitor was added to the cell lysis buffer (C2978;
Sigma-Aldrich, St. Louis, MO, USA) in a 1:100 ratio. The cells were
collected with a scraper and centrifuged at 15,000 × g at 4°C. The
supernatant was collected and the protein concentration was
measured. The pellets were frozen at −80°C until loading on the gel
(20 µg/lane). Polyacrylamide gel electrophoresis and western
blotting reagents were supplied by Lonza, Inc., (Allendale, NJ,
USA) and all the related procedures followed the company's
protocol. The catalog numbers for the reagents and the suppliers
are listed below.
Pager Gold Precast Gels (59502; 10% Tris-Glycine;
Lonza, Inc.); ECL reagent (RPN2109; GE Healthcare, Little Chalfont,
UK); Western Blocker solution (W0138; Sigma-Aldrich); ProSieve Quad
Color Protein marker (4.6–300 kD, 00193837; Lonza, Inc.); 20X
reducing agent for ProSieve ProTrack Dual Color Loading buffer
(00193861; Lonza, Inc.); ProTrack Loading buffer (00193861; Lonza,
Inc.); ProSieve ProTrack Dual Color Loading buffer EX running
buffer (00200307; Lonza, Inc.); ProSieve EX Western Blot Transfer
buffer (00200309; Lonza, Inc.); Immobilon®-P Polyvinylidene
difluoride membranes (P4188; Sigma-Aldrich).
Antibodies
Primary
Rabbit anti-desmoplakin antibody (ab71690; Abcam,
Cambridge, MA, USA); rabbit anti-JUP antibody (2309S; Cell
Signaling Technology, Inc., Beverly, MA, USA); mouse anti-DSG 2
antibody (ab14415; Abcam); rabbit anti-claudin 11 antibody
(LS-C6168; LifeSpan Biosciences, Inc., Seattle, WA, USA); mouse
anti-E-cadherin (32A8) (5296; Cell Signaling Technology, Inc.); and
mouse monoclonal anti-α tubulin antibody (T5168;
Sigma-Aldrich).
Secondary
Anti-mouse IgG (A9044; Sigma-Aldrich); and goat
anti-rabbit IgG peroxidase conjugate (A0545; Sigma-Aldrich).
RNA isolation
The cells were spun down, the medium was aspirated,
the pellets were snap-frozen and shipped to the Qiagen Service Core
for Genomics and Gene Expression.
RNA was isolated using RNEasy Mini kit (74104;
Qiagen, Hilden, Germany) following the manufacturer's protocol for
cells. RNA quality was determined using the Agilent BioAnalyzer
2100 (Agilent Technologies, Santa Clara, CA, USA) with RNA 6000
Nano kits (5067-1511; Agilent Technologies). The total RNA yield,
260/280 and 260/230 ratios were measured using a NanoDrop
spectrophotometer 2000 (Thermo Scientific, Waltham, MA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The following methods were implemented to assess
gene expression. RNA was isolated using the Qiagen RNEasy Mini kit
(74101; Qiagen). RNA concentration was determined with the NanoDrop
2000 spectrophotometer. An RNA quality check was performed using
BioAnalyzer 2100 (Agilent Technologies). The RT reaction was
performed using RT2 First Strand kit (330401; Qiagen) with 100 ng
RNA input per sample. RT-PCR was performed using the ABI 7900 PCR
system (Applied Biosystems, Foster City, CA, USA) in a 384-well
format. Ct values were normalized using the Ct value of
glyceraldehyde 3-phosphate dehydrogenase. Data analysis was
performed using the data analysis tool at http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php.
Cell junction pathway
This array analysis was performed by Qiagen
(SABiosciences) using PAHS-213Z cell junction finder arrays.
Dispase assay
Dispase assay was performed as previously described
(19). Dispase II was supplied by
Roche Life Science, Indianapolis, IN (04942078001).
Nuclear extraction
The extraction procedure was performed with the
EpiSeeker Nuclear Extraction kit (ab113474; Abcam).
H3K9 demethylase activity assay
The quantification of H3K9-specific histone
demethylase activity was performed using the EpiSeeker Histone H3
(K9) Demethylase Activity Quantification Assay kit (113458;
Abcam).
Results
Downregulation of desmosomal and tight
junction proteins in the JJ012 human chondrosarcoma cell line
We investigated the status of intercellular junction
protein expression in JJ012 human malignant chondrosarcoma cells
and investigated the effect of the antitumorigenic cytokine, PRP-1,
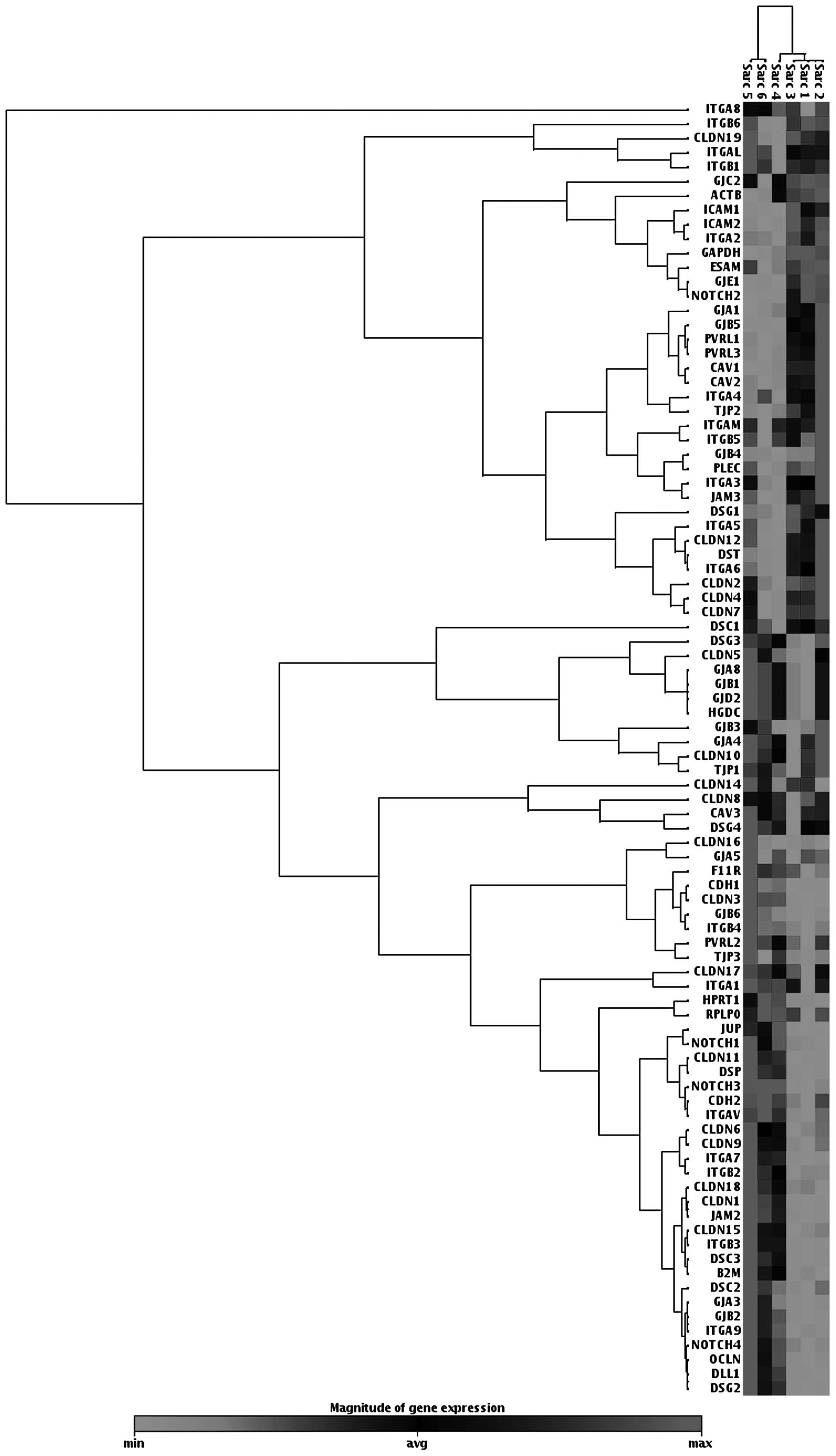
on their expression. The cell junction pathway array data indicated
downregulation of desmosomal proteins, such as DSG (1,428-fold),
desmoplakin (620-fold) and JUP (442-fold). The tight junction
proteins claudin 11, claudin I and E-cadherin were also
downregulated (399-, 206- and 52-fold, respectively) (Table I, Fig.1).
 | Table IDownregulated proteins in the JJ012
human chondrosarcoma cell line detected by cell junction pathway
arrays. |
Table I
Downregulated proteins in the JJ012
human chondrosarcoma cell line detected by cell junction pathway
arrays.
| Cellular
localization | Gene symbol | Gene name | Fold
downregulationa |
|---|
| Desmosomes | DSG2 | Desmoglein-2 | 1,428 |
| Desmosomes | DSP | Desmoplakin | 620 |
| Desmosomes | JUP | Junction
plakoglobin | 442 |
| Tight junctions | CLDN11 | Claudin 11 | 399 |
| Tight junctions | CLDN1 | Claudin 1 | 206 |
| Tight junctions | CDH1 | E-cadherin | 52 |
| Gap junctions | GJB2 | Connexin 26; gap
junction β-2 protein | 49 |
| Desmosomes | DSC3 | Desmocollin 3 | 49 |
| Focal adhesions | ITGB3 | Integrin, β 3;
platelet glycoprotein IIIa; antigen CD61 | 22 |
| Focal adhesions | ITGA7 | α 7 integrin
gene | 20 |
| Tight
junctions | JAM2 | Junctional adhesion
molecule B | 12 |
| Gap junctions | GJA3 | GJA3 gap junction
protein, α 3 | 15 |
| Tight
junctions | CLDN9 | Claudin 9 | 10 |
| Focal
adhesions | ITGA9 | Integrin, α 9 | 8 |
| Tight
junctions | OCLN | Occludin | 5 |
| Tight
junctions | CLDN6 | Claudin 6 | 4 |
By contrast, the cell junction pathway array
indicated the upregulation of gap junction β-5 protein (GJB5,
connexin 31.1) and the pro-inflammatory pathway protein
intercellular adhesion molecule (ICAM), which are both common in
tumors and pro-inflammatory pathways (Table II, Fig. 1).
 | Table IIUpregulated proteins in the JJ012
human chondrosarcoma cell line detected by cell junction pathway
arrays. |
Table II
Upregulated proteins in the JJ012
human chondrosarcoma cell line detected by cell junction pathway
arrays.
| Cellular
localization | Gene symbol | Gene name | Fold
upregulationa |
|---|
| Gap junctions | GJB5 | Gap junction β-5
protein | 129 |
| Tight
junctions | ICAM1 | Intercellular
adhesion molecule 1; CD54; cluster of differentiation 54 | 43 |
| Gap junctions | GJE1 | Gap junction
protein, ε 1 | 34 |
| Tight
junctions | ICAM2 | Intercellular
adhesion molecule 2 | 23 |
| Focal
adhesions | CAV1 | Caveolin-1 | 15 |
| Adherens
junctions | PVRL1 | Poliovirus
receptor-related 1 herpes virus entry mediator C; nectin-1;
CD111 | 8 |
| Focal
adhesions | ITGA2 | Integrin, α 2
(CD49B, α 2 subunit of VLA-2 receptor) | 8 |
| Tight
junctions | ESAM | Endothelial
cell-selective adhesion molecule | 7 |
| Hemidesmosomes | DST | Dystonin | 5 |
| Focal
adhesions | ITGA6 | Integrin, α 6 | 6 |
| Focal
adhesions | ITGA5 | Integrin, α 5 | 6 |
| Focal
adhesions | ITGA4 | Integrin, α 4 | 4 |
| Focal
adhesions | ITGA3 | Integrin, α 3 | 3 |
| Tight
junctions | CLDN4 | Claudin 4 | 3 |
| Tight
junctions | CLDN12 | Claudin 12 | 3 |
PRP-1 restores desmosomal protein
expression in the JJ012 human chondrosarcoma cell line
The selection of the proteins for western blot
analysis was based on the cell junction pathway arrays data. The
majority of the downregulated desmosomal and tight junction
proteins were included in these series. The western blot
experiments indicated the restoration of desmosomal proteins JUP,
DSG and desmoplakin following PRP-1 treatment (10 µg/ml) in a
dose-dependent manner. PRP-1 did not exert any effect on tight
junction protein expression, including E-cadherin (Fig. 2).
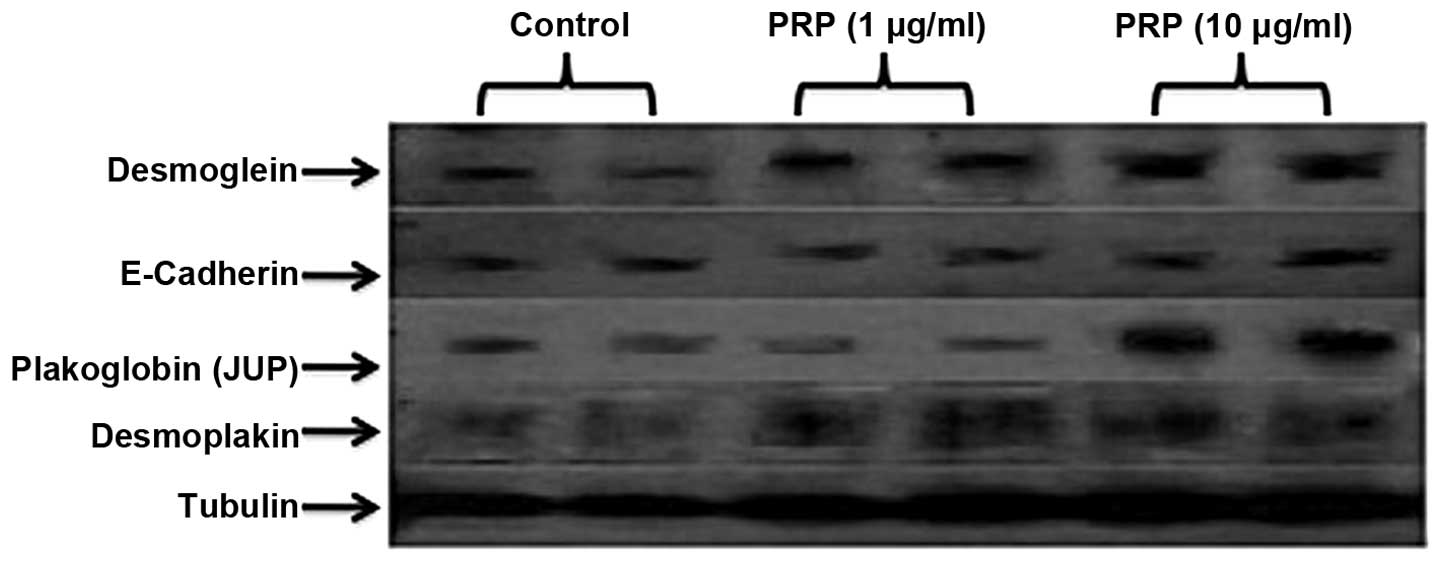 | Figure 2Restoration of desmosomal protein gene
expression following treatment with proline-rich polypeptide-1
(PRP-1). PRP-1 restored desmosomal protein expression, including
desmoglein 2, plakoglobin (JUP), desmoplakin expression levels, in
a dose-dependent manner. For JUP, the restorative effect of PRP-1
appeared at 10 µg/ml, whereas for desmoglein 2, it gradually
increased upon treatment with 1–10 µg/ml PRP-1. For desmoplakin, a
strong effect was already present at 1 µg/ml PRP-1 and continued at
10 µg/ml. However, PRP-1 did not affect E-cadherin protein levels,
which, despite downregulation in chondrosarcoma, was weakly
expressed due to mesenchymal-to-epithelial transition,
characteristic to sarcomas. PRP-1 did not exert any effect on the
expression of claudins (data not shown). α tubulin was used as a
control. |
Restoration of tumor suppressor
proteins with PRP-1 was cell-cell adhesion-independent
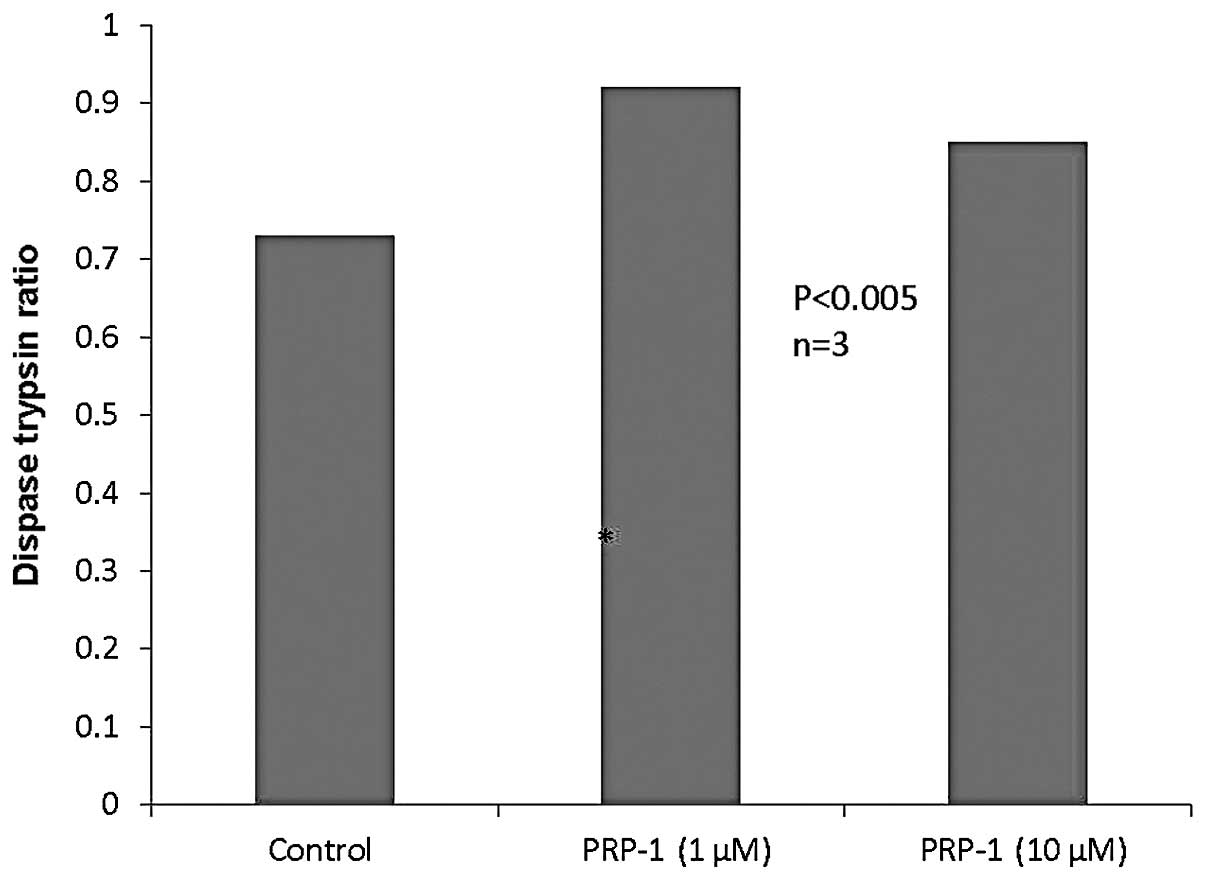
To determine whether the restoration of the
desmosomal proteins with PRP-1 was adhesion-dependent, we performed
a cell-cell adhesion dispase assay. By dividing the number of cells
disassociated by the dispase by the total number of cells in the
trypsin, we obtained a percent disassociation. The higher the
number, the less adhesive the cells. As seen in Fig. 3, PRP-1 in different concentrations
did not exert any effect on dispase activity.
PRP-1 inhibited H3K9 demethylase
activity in the JJ012 human chondrosarcoma cell line
We conducted experiments to determine H3K9-specific
histone demethylase activity in JJ012 human chondrosarcoma cells
and investigated the effect of PRP-1 on the enzymes activity, which
is known to be cellular context-dependent. Lysine histone
methylation is one of the most important epigenetic marks and is
essential for the regulation of multiple cellular processes. The
methylation of H3K9 is of particular importance, as it is
associated with repression regions of the genome. H3K9 demethylase
(including JMJD1 and JMJD2) families have been found to be involved
in cancer progression. The total H3K9 demethylase activity in
chondrosarcoma cells was detected as 421 ng/h/µg and was inhibited
by PRP-1 in a dose-dependent manner: 0.5 µg/ml, 63%f; 1 µg/ml, 74%;
and 10 µg/ml, 91% inhibition (Table
III).
 | Table IIIH3K9-specific histone demethylase
(HDM) activity in the JJ012 cell line and inhibition with different
concentrations of proline-rich polypeptide-1 (PRP-1). |
Table III
H3K9-specific histone demethylase
(HDM) activity in the JJ012 cell line and inhibition with different
concentrations of proline-rich polypeptide-1 (PRP-1).
| H3K9 HDM activity
(RFU/h/μg) | H3K9 HDM activity
(ng/h/μg) | PRP-1 (μg/ml) | Inhibition (%) |
|---|
| 843 | 421 | 0.5 | 63 |
| | 1 | 74 |
| | 10 | 91 |
Discussion
We previously described the antitumorigenic effect
of cytostatic PRP-1 in the JJ012 human chondrosarcoma cell line
(13–16). In our attempt to further elucidate
the antiproliferative effect of this peptide, we conducted
experiments to determine its possible role in the regulation of
intercellular junctions. Intercellular junctions are crucial for
cell function and enable cell interactions and contact between the
plasma membranes of adjacent cells. Most importantly, the
intercellular junction desmosomal proteins, unlike the majority of
gap junction proteins, manifest tumor suppressor functions, which
are lost due to the downregulation of these proteins in tumors. To
the best of our knowledge, this is the first attempt to
characterize the status of intercellular adhesion junction proteins
in the JJ012 human chondrosarcoma cell line and investigate the
effect of the cytostatic PRP-1 peptide on the expression of these
proteins. Cell junction pathway array profiling was performed to
compare the expression of intercellular junction proteins in JJ012
human chondrosarcoma cells to that in control C-28
chondrocytes.
The array data indicated the upregulation of GJB5
(connexin 31.1) and the pro-inflammatory pathway protein ICAM,
which are both common in tumors (Table II, Fig. 1). Upregulated GJB5 protein was
found in colon cancer, which regulates cell adhesion, motility,
proliferation and metastasis. Gap junction proteins promote
stromal-epithelial interactions in tumors, play a role in tumor
progression and regulate the survival of certain stem cells
(20). ICAM was reported to play
an important role in cancer invasion (21). Strong downregulation of desmosomal
proteins, such as DSG, desmoplakin and JUP was observed. The tight
junction proteins claudin 11 and E-cadherin were also downregulated
(Table I, Fig. 1). Currently available evidence
indicates that desmosomes play an important role in the regulation
of cell proliferation, differentiation and signal transduction. A
recent study using mouse genetic approaches uncovered tumor
suppressor properties and demonstrated that desmosome
downregulation occurs prior to that of adherens junctions (22). Desmosome loss promotes cancer as a
specific mechanism, rather than by a general change in the
differentiation status, such as EMT (23,24).
The fact that some experiments support a tumour-suppressive role
for desmosomes in cancer and others provide evidence for an
oncogenic function, may reflect real context-dependent differences
in the contribution of desmosomes to cancer (25,26).
The selection of the proteins to be subjected to western blotting
analysis was based on the level of downregulation of the proteins
in JJ012 human chondrosarcoma cells compared to control C-28
chondrocytes in cell junction pathway arrays data. We did not
investigate the effect of PRP-1 on the upregulated proteins, as we
focused on underexpressed junctional proteins in tumorigenesis and
the effect of antitumorigenic PRP-1 on their restoration in
chondrosarcoma. In our western blot experiments, we were able to
demonstrate the restoration of the expression of the desmosomal
proteins JUP, desmoplakin and DSG following treatment with PRP-1,
compared to untreated controls, in chondrosarcoma cells (Fig. 2). JUP encodes for the cell-adhesion
protein γ-catenin. In conjunction with β-catenin, γ-catenin links
E-cadherin to the actin cytoskeleton, forming intercellular
cadherin-catenin complexes, a key part of the extracellular matrix.
Loss of JUP as a tumor suppressor is associated with increased
cancer invasion. JUP may efficiently suppress the tumorigenicity of
cells in the presence of, or independently of the cadherin-catenin
complex (27,28). Several groups have observed the
loss of JUP expression in a wide range of tumors. The majority of
those studies investigated JUP in conjunction with other adhesive
junctional proteins and demonstrated that loss of JUP expression,
which is an early event in tumorigenesis, in conjunction with the
lack of expression of other cell-cell adhesion proteins, such as
E-cadherin, α-catenin, β-catenin, DSG, or desmoplakin, resulted in
increased tumor formation and size and was correlated with advanced
tumor stage, poor patient survival and increased metastasis
(29). Decreased expression/loss
of desmoplakin and DSG as tumor suppressors was reportedly
attributed to invasive tumorigenic potential (9,11).
PRP-1 did not exert any effect on occluding/tight junction
proteins, such as claudins (data not shown) and E-cadherin
(Fig. 2). The latter was weakly
expressed in chondrosarcoma cells due to MET, as was previously
discussed. Of note, the dispase assay results did not indicate any
change in cell-cell adhesion in human chondrosarcoma cells
following PRP-1 treatment (Fig.
3). Thus, PRP-1 restored cell adhesion intercellular junction
desmosomal protein expression without restoring cell-cell adhesion.
This fact supports the hypothesis that the tumor-suppressive
function of desmosomal proteins is independent from their cell-cell
adhesive properties in certain cases and is possibly
tissue-specific. JUP, for example, suppressed keratinocyte motility
through both cell-cell adhesion-dependent and -independent
mechanisms (30). An analysis of
the JUP promoter described CpG islands within the promoter and it
was observed that inhibition of DNA methylation may result in
increased JUP expression (29).
The formation of the H3K9me3 mark in promoter-associated chromatin
has important consequences on long-term gene silencing, as this
modification is considered to be a prelude to the recruitment of
DNA methyltransferases that catalyze DNA methylation. Methylation
at the CpG dinucleotides near gene promoters is associated with
highly stable gene silencing that may be inherited with high
fidelity over the course of multiple successive cell divisions
(31–33).
Based on this information, one may expect activation
of H3K9 demethylase following PRP-1 treatment, since PRP-1 restored
the expression of desmosomal proteins. The subsequent set of
experiments was focused on H3K9 demethylase activity estimation in
chondrosarcoma cells and the effect of PRP-1 on this epigenetic
enzymes activity.
Our experimental results, however, revealed a strong
dose-dependent inhibition of H3K9 demethylase activity in JJ012
cells upon PRP-1 treatment (Table
III). This fact may be explained by other literature results,
demonstrating that JMJD2A, a H3K9me3 demethylase, transcriptionally
repressed tumor suppressor genes and contributed to cancer
progression (34). Moreover, a
previous study reported the G1/S arrest of cancer cells following
knockdown of JMJD2A (35). This is
in accord with our previous observations, where PRP-1 exerts its
cytostatic effect by causing G1/S arrest (15). To the best of our knowledge, this
is the first report on intercellular junction protein expression in
JJ012 human chondrosarcoma cells, providing new experimental
evidence on desmosomal protein expression restoration by PRP-1 in a
cell-cell adhesion-independent manner, accompanied by inhibition of
H3K9-specific histone demethylase activity, leading to suppression
of the tumorigenic potential in chondrosarcoma cells.
Acknowledgements
We would like to thank the scientific team of
SABiosciences for their help with the cell junction pathway array
data. This study was supported by the University of Miami Tissue
Bank Research account.
References
|
1
|
Fitzgerald MP, Gourronc F, Teoh ML, et al:
Human chondrosarcoma cells acquire an epithelial-like gene
expression pattern via an epigenetic switch: evidence for
mesenchymal-epithelial transition during sarcomagenesis. Sarcoma.
2011(598218)2011. View Article : Google Scholar
|
|
2
|
Yang J, Du X, Wang G, et al: Mesenchymal
to epithelial transition in sarcomas. Eur J Cancer. 50:593–601.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiles ET, Bell R, Thomas D, Beckerle M and
Lessnick SL: ZEB2 represses the epithelial phenotype and
facilitates metastasis in Ewing sarcoma. Genes Cancer. 4:486–500.
2013. View Article : Google Scholar
|
|
5
|
Latorre IJ, Frese KK and Javier RT: Tigh.
junction proteins and cancer. Tight Junctions. Gonzalez-Mariscal L:
Springer US; New York, NY: pp. 116–134. 2006, View Article : Google Scholar
|
|
6
|
Chipman JK, Mally A and Edwards GO:
Disruption of gap junctions in toxicity and carcinogenicity.
Toxicol Sci. 71:146–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gill GA, Buda A, Moorghen M, et al:
Characterisation of adherens and tight junctional molecules in
normal animal larynx; determining a suitable model for studying
molecular abnormalities in human laryngopharyngeal reflux. J Clin
Pathol. 58:1265–1270. 2005. View Article : Google Scholar
|
|
8
|
Kolegraff K, Nava P, Helms MN, et al: Loss
of desmocollin-2 confers a tumorigenic phenotype to colonic
epithelial cells through activation of Akt/β-catenin signaling. Mol
Biol Cell. 22:1121–1134. 2011.PubMed/NCBI
|
|
9
|
Yang L, Chen Y, Cui T, et al: Desmoplakin
acts as a tumor suppressor by inhibition of the Wnt/β-catenin
signaling pathway in human lung cancer. Carcinogenesis.
33:1863–1870. 2012.PubMed/NCBI
|
|
10
|
Holen I, Whitworth J, Nutter F, et al:
Loss of plakoglobin promotes decreased cell-cell contact, increased
invasion and breast cancer cell dissemination in vivo. Breast
Cancer Res. 14:R862012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yashiro M, Nishioka N and Hirakawa K:
Decreased expression of the adhesion molecule desmoglein-2 is
associated with diffuse-type gastric carcinoma. Eur J Cancer.
42:2397–2403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leithe E, Sirnes S, Omori Y, et al:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galoian K, Scully S, McNamara G, et al:
Antitumorigenic effect of brain proline rich polypeptide-1 in human
chondrosarcoma. Neurochem Res. 34:2117–2121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galoian K, Temple TH and Galoyan A:
Cytostatic effect of the hypothalamic cytokine PRP-1 is mediated by
mTOR and cMyc inhibition in high grade chondrosarcoma. Neurochem
Res. 36:812–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galoian KA, Temple TH and Galoyan A:
Cytostatic effect of novel mTOR inhibitor, PRP-1 (galarmin) in MDA
231 (ER-) breast carcinoma cell line. PRP-1 inhibits mesenchymal
tumors. Tumour Biol. 32:745–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galoian K, Temple TH and Galoyan A: mTORC1
inhibition and ECM-cell adhesion-independent drug resistance via
PI3K-AKT and PI3K-RAS-MAPK feedback loops. Tumour Biol. 33:885–890.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galoyan AA and Aprikyan VS: A new
hypothalamic polypeptide is a regulator of myelopoiesis. Neurochem
Res. 27:305–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galoyan A: Neurochemistry of brain
neuroendocrine immune system: signal molecules. Neurochem Res.
25:1343–1355. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silberberg M, Charron AJ, Bacallao R, et
al: Mispolarization of desmosomal proteins and altered
intercellular adhesion in autosomal dominant polycystic kidney
disease. Am J Physiol Renal Physiol. 288:F1153–F1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delker DA, McGettigan BM, Kanth P, Pop S,
Neklason DW, Bronner MP, Burt RW and Hagedorn CH: RN. sequencing of
sessile serrated colon polyps identifies differentially expressed
genes and immunohistochemical markers. PLoS One. 9:e883672014.
View Article : Google Scholar
|
|
21
|
Rosette C, Roth RB, Oeth P, et al: Role of
ICAM1 in invasion of human breast cancer cells. Carcinogenesis.
26:943–950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chun MG and Hanahan D: Genetic deletion of
the desmosomal component desmoplakin promotes tumor microinvasion
in a mouse model of pancreatic neuroendocrine carcinogenesis. PLoS
Genet. 6:e10011202010. View Article : Google Scholar
|
|
23
|
Dusek RL and Attardi LD: Desmosomes: new
perpetrators in tumour suppression. Nat Rev Cancer. 11:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chidgey M and Dawson C: Desmosomes: a role
in cancer? Br J Cancer. 96:1783–1787. 2007. View Article : Google Scholar
|
|
25
|
Delva E, Tucker DK and Kowalczyk AP: The
desmosome. Cold Spring Harb Perspect Biol. 1:a0025432009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Green KJ and Gaudry CA: Are desmosomes
more than tethers for intermediate filaments? Nat Rev Mol Cell
Biol. 1:208–216. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rieger-Christ KM, Ng L, Hanley RS, et al:
Restoration of plakoglobin expression in bladder carcinoma cell
lines suppresses cell migration and tumorigenic potential. Br J
Cancer. 92:2153–2159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simcha I, Geiger B, Yehuda-Levenberg S, et
al: Suppression of tumorigenicity by plakoglobin: an augmenting
effect of N-cadherin. J Cell Biol. 133:199–209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aktary Z and Pasdar M: Plakoglobin: role
in tumorigenesis and metastasis. Int J Cell Biol. 2012(189521)2012.
View Article : Google Scholar
|
|
30
|
Yin T, Getsios S, Caldelari R, et al:
Plakoglobin supresses keratinocyte motility through both cell-cell
adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci
USA. 102:5420–5425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tam LW and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi L, Sun L, Li Q, et al: Histone
demethylase JMJD2B coordinates H3K4/H3K9 methylation and promotes
hormonally responsive breast carcinogenesis. Proc Natl Acad Sci
USA. 108:7541–7546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirmizis A, Bartley SM, Kuzmichev A,
Margueron R, et al: Silencing of human polycomb target genes is
associated with methylation of histone H3 Lys 27. Genes Dev.
18:1592–1605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li LL, Xue AM, Li BX, et al: JMJD2A
contributes to breast cancer progression through transcriptional
repression of the tumor suppressor ARHI. Breast Cancer Res.
16:R562014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kogure M, Takawa M, Cho HS, et al:
Deregulation of the histone demethylase JMJD2A is involved in human
carcinogenesis through regulation of the G(1)/S transition. Cancer
Lett. 336:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|