Introduction
Over the last few years, with the advances in
multidisciplinary therapy, including neoadjuvant chemotherapy
(1, 2), radiation therapy (3) and limb-preserving surgery (4, 5),
the prognosis of high-grade bone and soft tissue tumors has
markedly improved, with a corresponding increase in the number of
long-term survivors. This increase has generated new problems in
the form of latent treatment-related adverse effects (6). Although several types of cancer occur
predominantly in the elderly, a number of cancers arise prior to or
during the reproductive age. For example, high-grade bone and soft
tissue tumors, such as osteosarcoma, Ewing's sarcoma and synovial
sarcoma, most commonly occur in adolescents and young adults.
Similar to healthy individuals, young survivors of such cancers
would expect to get married and parent their own offspring
(7).
Amputation or limb-preserving surgery are now
routinely performed on patients with high-grade bone and soft
tissue tumors arising in the extremities. Limb-preserving surgery
with a wide margin has achieved positive outcomes; however, loss of
limb function remains an unsolved problem. Marriage is an important
goal for patients, from the physical as well as the psychological
perspective (8). However,
chemotherapeutic agents may cause gonadal toxicity and long-term
impairment of fertility, with infertility being one of the major
long-term adverse effects of chemotherapy. A number of studies have
addressed gonadal toxicity following chemotherapy for hematological
malignancies and testicular and breast cancers (9–11);
however, only a limited number of studies have addressed marriage
and fertility in long-term survivors of high-grade bone and soft
tissue sarcomas (12–15). In this study, we aimed to
investigate specific issues, such as the effect of the treatment of
high-grade bone and soft tissue tumor on marriage and fertility of
long-term survivors, whether it is safe for these patients to
father or conceive offspring, the optimal interval between the
completion of the last chemotherapy and conception and whether the
cumulative dose of chemotherapeutic agents are associated with
subsequent fertility.
Materials and methods
Patients
Between September, 1985 and December, 2013, 272
patients aged < 40 years at diagnosis were treated for
high-grade bone and soft tissue tumors at Osaka City University
Hospital (Osaka, Japan). The inclusion criteria for this study were
male or female patients who had been diagnosed with high-grade
malignant bone or soft tissue tumors, received chemotherapy and
survived for >5 years after the initial treatment; a total of 47
patients fulfilled these criteria. The patients comprised 24 men
and 23 women, with a median age at diagnosis ± standard deviation
(SD) of 18.0±8.9 years (range, 4–40 years). The median age ± SD at
the last follow-up was 33.0±9.5 years. The mean duration of the
follow-up was 11.0 years (range, 5.0-28.0 years). The mean interval
from the last chemotherapy to the last follow-up was 10.1 years
(range, 0.1-27.3 years).
The study protocol was approved by the Institutional
Ethics Review Board of Osaka City University Graduate School of
Medicine.
Treatment
In this series, the treatment protocol for patients
with high-grade bone and soft tissue sarcomas consisted of
preoperative chemotherapy, wide resection and postoperative
chemotherapy. Various surgical procedures, including amputation,
rotationplasty and reconstruction with tumor prostheses, were
performed. When the only option was amputation, this was performed
after providing a full explanation and receiving the patient's
informed consent.
The chemotherapeutic protocols for high-grade
osteosarcoma were as follows : Between 1985 and 1996 (16), chemotherapy was administered
preoperatively for an average of 3.5 months and postoperatively for
7.2 months. Adriamycin (DOX) was administered intravenously (i.v.)
at a dose of 40–60 mg/m2 every 2–3 weeks. Methotrexate
(MTX) was administered i.v. at a dose of 200–300 mg/kg every 2
weeks. Cisplatin (CDDP) was administered i.v. at a dose of 100–130
mg/m2 every 3 weeks. The patients were divided into six
groups according to the protocols of preoperative chemotherapy they
had received : Group 1 had received DOX-DOX-MTX-MTX, group 2
DOX-CDDP-DOX, group 3 CDDP-CDDP-MTX-MTX, group 4 CDDP-CDDP-CDDP,
group 5 MTX-MTX-CDDP-CDDP and group 6
MTX-MTX-CDDP+DOX-MTX-MTX-CDDP-DOX. Postoperative chemotherapy was
repeated twice, using the same protocols as for the preoperative
chemotherapy.
Between 1997 and 2004, intensive doses of
chemotherapy were administered according to the OOS-D protocol
(17), which was based on the T10
and T12 protocols with modifications (1, 2).
Two cycles of DOX 90 mg/m2 plus CDDP 120
mg/m2 and ifosfamide (IFM) 15 g/m2 were
administered as neoadjuvant chemotherapy and two cycles of DOX/CDDP
and IFM plus two cycles of high-dose MTX (10–12 g/m2)
postoperatively.
Between 2005 and 2013, caffeine-assisted
chemotherapy was introduced according to the K2 protocol (18).
Patients with Ewing's sarcoma received high-dose
intensive chemotherapy, according to the European Ewing Tumor
Working Initiative of National Groups 1999 protocol, with
modifications (19). The six
cycles of preoperative chemotherapy comprised vincristine (VCR; 1.4
mg/m2/day; day 1), IFM (3.0 g/m2/day; days
1–3), DOX (20 mg/m2/day; days 1–3) and etoposide (ETP;
150 mg/m2/day; days 1–3). Postoperatively, the patients
received one cycle of VCR (1.5 mg/m2; day 1),
cyclophosphamide (1.5 g/m2; day 1) and actinomycin-D
(0.7 mg/m2; days 1–2). Subsequently, following
pretreatment with busulfan (4 mg/kg/day; days 1–4) and
L-phenylalanine mustard (70 mg/m2; days 1–3), the
patients also received adjuvant peripheral blood stem cell
transplantation.
Patients with high-grade soft tissue sarcomas
received DOX and IFM-based chemotherapy. The preoperative
chemotherapy consisted of DOX (30 mg/m2 i.v.; days 1–2)
and IFM (2 g/m2 i.v.; days 1–5) repeated three times at
3-week intervals. Following tumor resection, two courses of the
same regimen administered preoperatively were given at 3-week
intervals (20).
For patients with relapse in pulmonary or other
sites, second-line chemotherapy comprised IFM (1,800
mg/m2/day; days 0–4), carboplatin (400
mg/m2/day; days 0–1) and ETP (100 mg/m2/day;
days 0–4) with some modifications (21).
Classification by marriage
The proportion of married subjects was defined as
number of married/total number of subjects and was investigated to
clarify whether treatment exerted an effect on achieving marriage.
To assess the true effect of treatment, the ‘true marriage
proportion’ was calculated from the difference in the proportion of
patients already married at presentation and at the final
follow-up. The associations between the proportion of married
patients and gender (male vs. female), age [<20 vs. ≥20 years
(in Japan, individuals aged ≥20 years are officially considered to
be adults)], type of tumor (bone vs. soft tissue tumor) and
surgical procedure were also investigated. The surgical procedures
were classified as amputation or limb preservation groups at the
last follow-up. One patient who had undergone rotationplasty was
classified into the amputation group.
Classification by fertility
The fertility of the 47 patients was also
investigated. Childbirth was selected as the end-point of
fertility, as it was considered important to ascertain whether the
offspring, particularly the first-born, of subjects who had
received chemotherapy were normal. The proportion of fertile
subjects was defined as the number of patients fathering or
conceiving offspring/total number of patients. As some patients
already had children prior to treatment, the data on fertility at
the final follow-up did not represent the true proportion of
fertile subjects. To determine the true effect of chemotherapy on
fertility, the ‘true fertility proportion’ was calculated as the
difference between the proportion of patients with offspring at
presentation and with offspring at the final follow-up. The
associations between the proportion of fertile patients and gender
(male vs. female), age (<20 vs. ≥20 years), type of tumor (bone
vs. soft tissue tumor) and surgical procedure (limb preservation
vs. amputation) were investigated.
The interval from the completion of chemotherapy to
the first childbirth was considered a marker of fertility recovery.
Whether the offspring presented with any problems was also
assessed.
As described above, the main drugs used were DOX,
CDDP, MTX and IFM (22). The
association between fertility and the doses of each of these agents
was evaluated.
Statistical analysis
The Fisher's exact probability test was performed
for statistical comparison of two groups. Statistical analysis was
performed using Excel statistics software, version 2012 (Social
Survey Research Information Co., Ltd., Tokyo, Japan) for Windows.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients
The relevant clinical characteristics of the
patients, including gender, type of tumor, histopathology, site of
primary tumor and state of affected limb are summarized in Table I.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
|
Characteristics | Patient no.
(n=47) |
|---|
| Gender | |
|
Male | 24 |
|
Female | 23 |
| Type of tumor | |
|
Bone | 38 |
| Soft
tissue | 9 |
| Histopathology | |
|
Osteosarcoma | 33 |
| Ewing's
sarcoma | 4 |
|
Synovial sarcoma | 3 |
|
MPNST | 1 |
|
Epithelioid sarcoma | 1 |
| Myxoid
liposarcoma | 1 |
|
Leiomyosarcoma | 1 |
|
Chondrosarcoma | 1 |
|
Extraskeletal Ewing's
sarcoma | 1 |
|
Extraskeletal
osteosarcoma | 1 |
| Site of primary
tumor | |
|
Bone | |
|
Femur | 16 |
|
Tibia | 11 |
|
Humerus | 4 |
|
Pelvis | 3 |
|
Ulna | 1 |
|
Fibula | 1 |
|
Radius | 1 |
|
Phalanx | 1 |
| Soft
tissue | |
|
Thigh | 6 |
|
Upper arm | 2 |
|
Retroperitoneum | 1 |
| State of affected
limb | |
| Limb
preservation | 41 |
|
Amputation | 5 |
|
Rotationplasty | 1 |
Effect of treatment on marriage
The overall proportion of patients who were married
prior to treatment was 6/47 (12.8%) and at the final follow-up
17/47 (36.2%); thus, the true marriage proportion was 23.4%
(11/47). There were no significant associations between marital
status and gender (P=0.093), age (P=0.741), type of tumor
(P=0.925), or surgical procedure (P=0.676) (Table II).
 | Table II.Effect of treatment on marriage. |
Table II.
Effect of treatment on marriage.
| Marriage
proportion | | |
|---|
|
| | |
|---|
| Variables | Initial | Final | True marriage
proportion | P-value |
|---|
| Gender |
0.093 |
|
Male | 4/24 | 7/24 | 3/24 | |
|
Female | 2/23 | 10/23 | 8/23 | |
| Age at diagnosis,
years |
|
|
|
0.741 |
|
<20 | 0/24 | 5/24 | 5/24 | |
|
≥20 | 6/23 | 12/23 | 6/23 | |
| Type of tumor |
0.925 |
|
Bone | 5/38 | 14/38 | 9/38 | |
| Soft
tissue | 1/9 | 3/9 | 2/9 | |
| Surgery |
0.676 |
|
Limb-preser | 5/41 | 15/41 | 10/41 | |
|
Amputation | 1/6 | 2/6 | 1/6 | |
Effect of treatment on fertility
The overall initial proportion of fertile subjects
was 4/47 (8.5%); this had increased to 14/47 (29.8%) by the time of
the final follow-up. Thus, the true proportion of fertile subjects
at the final follow-up was 21.3% (10/47). The true proportion of
fertile subjects was significantly associated with gender
(P=0.036), but not with age (P=0.723), type of tumor (P=0.938), or
surgical procedure (P=0.767). The true proportion of fertile
subjects was significantly lower in men compared to that in women
(Table III).
 | Table III.Effect of treatment on fertility. |
Table III.
Effect of treatment on fertility.
| Fertility
proportion | | |
|---|
|
| | |
|---|
| Variables | Initial | Final | True fertility
proportion | P-value |
|---|
| Gender | 0.036 |
|
Male | 2/24 | 4/24 | 2/24 | |
|
Female | 2/23 | 10/23 | 8/23 | |
| Age at diagnosis,
years |
0.723 |
|
<20 | 0/24 | 4/24 | 4/24 | |
|
≥20 | 4/23 | 10/23 | 6/23 | |
| Type of tumor |
0.938 |
|
Bone | 3/38 | 11/38 | 8/38 | |
| Soft
tissue | 1/9 | 3/9 | 2/9 | |
| Surgery |
0.767 |
|
Limb-preserving | 3/41 | 12/41 | 9/41 | |
|
Amputation | 1/6 | 2/6 | 1/6 | |
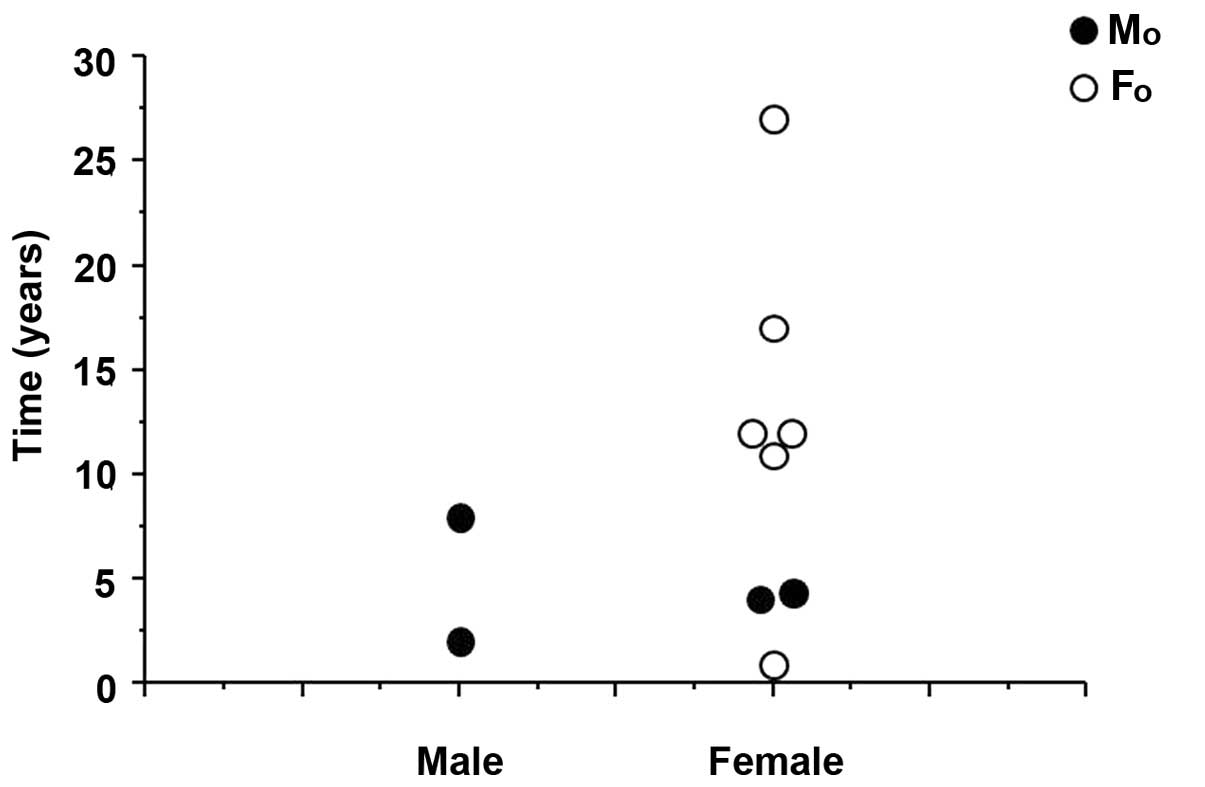
Interval from last chemotherapy to
delivery of first offspring
The overall median interval from the completion of
chemotherapy to the delivery of the first offspring was 9.5 years
(range, 1–27 years). The wife of one of the two fertile men
delivered their first offspring 2 years after the patient's last
chemotherapy and the wife of the other patient 7 years after his
last chemotherapy. The mean interval between the last chemotherapy
and delivery was 11.5 years in the 8 fertile women, with the
shortest interval being 1.0 year (Fig.
1).
Clinical data regarding the first
childbirth following chemotherapy
The clinical data regarding the first childbirth
following treatment of high-grade bone and soft tissue tumors is
summarized in Table IV. At the
last follow-up, 10 patients had a total of 15 offspring after
treatment. Of these 10 subjects, 8 had osteosarcomas and the
remaining 2 synovial sarcomas. Seven of the offspring were male and
8 were female. Of the first offspring, 4 were male and 6 were
female. Six of the first offspring were delivered transvaginally
and 4 by caesarean sections. One female patient (case 3), who had
synovial sarcoma and later conceived, was diagnosed with lung
metastases during her pregnancy and underwent medical termination
of the pregnancy; the fetus had a birth weight of 1,570 g and no
congenital deformities. In case 5, a uterine myoma was detected
during pregnancy and infection of the amniotic fluid necessitated
urgent delivery by caesarean section. In cases 6 and 10, caesarean
sections were required due to prolonged labor caused by abnormal
rotation of the fetus. None of the 15 offspring conceived after
their parents had received chemotherapy had congenital deformities.
Case 10 had received hormone therapy to induce pregnancy and
delivered her first offspring at the age of 40 years, which was 27
years after her cancer treatment.
 | Table IV.Clinical data concerning first
offspring. |
Table IV.
Clinical data concerning first
offspring.
| Case no. | Age at diagnosis,
years | Patient gender | Histopathology | Age at delivery,
years | Gender of first
offspring | First delivery | Congenital
deformities | Other
offsprings |
|---|
| 1 | 30 | M | Osteosarcoma | 32 | Mo | Transvaginal
delivery | None | |
| 2 | 17 | M | Osteosarcoma | 25 | Mo | Transvaginal
delivery | None | Two
Mo |
| 3 | 23 | F | Synovial
sarcoma | 32 | Fo | Caesarean section
medical termination 30 w (BW 1,570 g) | None | |
| 4 | 26 | F | Osteosarcoma | 30 | Mo | Transvaginal
delivery | None | |
| 5 | 14 | F | Osteosarcoma | 26 | Fo | Caesarean section
(infect ed amniotic fluid, myoma uteri) 38 w (BW 2,250 g) | None | |
| 6 | 22 | F | Osteosarcoma | 33 | Fo | Caesarean section
(abnormal rotation of fetus) 42 w (BW 3,240 g) | None | |
| 7 | 17 | F | Osteosarcoma | 34 | Fo | Transvaginal
delivery | None | Fo |
| 8 | 21 | F | Synovial
sarcoma | 25 | Mo | Transvaginal
delivery | None | Fo
(Premature birth 35 w) |
| 9 | 23 | F | Osteosarcoma | 35 | Fo | Transvaginal
delivery | None | Mo |
| 10 | 13 | F | Osteosarcoma | 40 | Fo | Caesarean section
(abnormal rotation of fetus) 39 w (BW 3,345 g) | None |
|
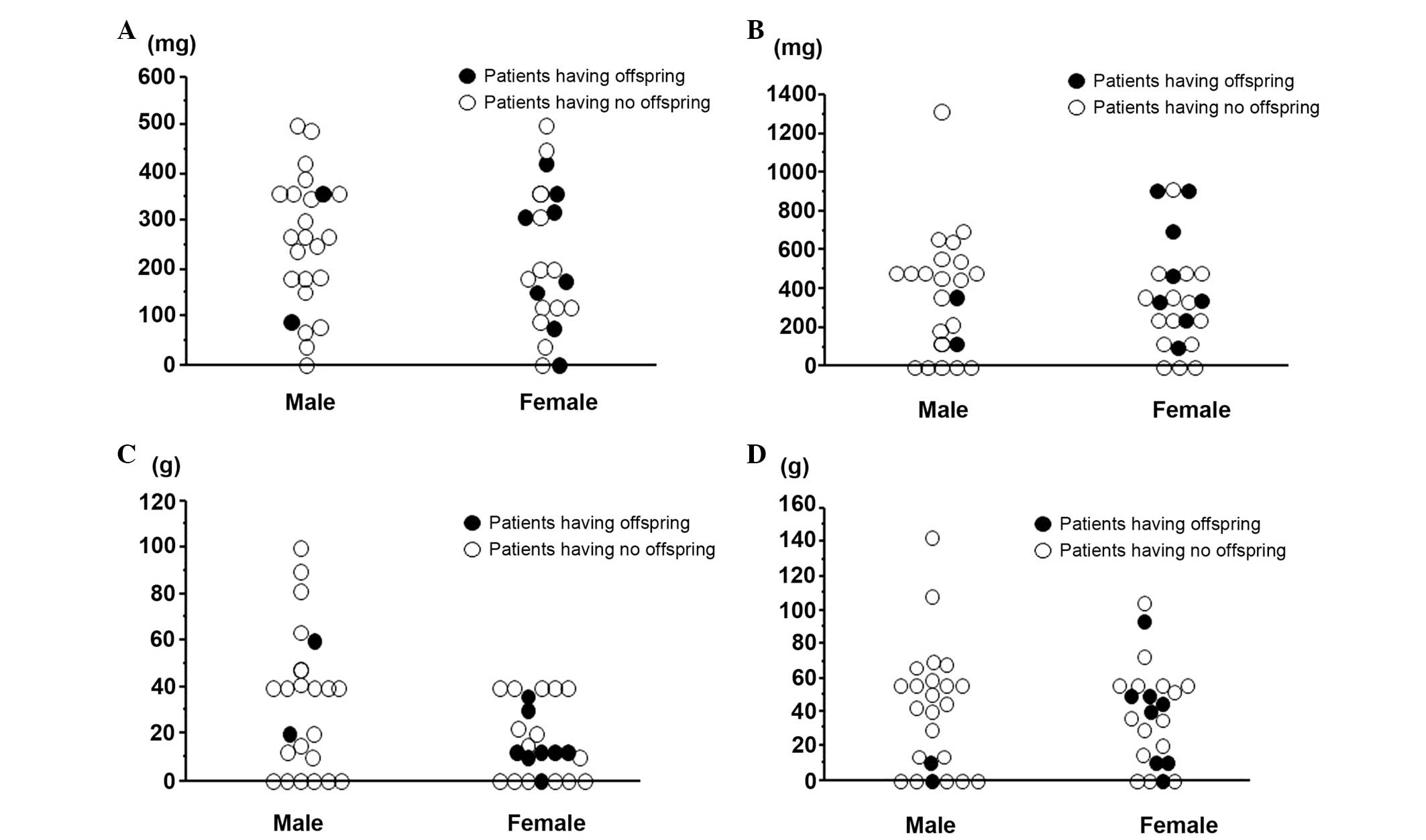
Association between cumulative dose of
each chemotherapeutic agent and childbirth
The associations between the birth of the first
offspring and total cumulative dose of the drugs DOX, CDDP, MTX and
IFM are shown in Fig. 2A-D.
Fertile male subjects had received smaller doses, particularly of
IFM, compared to those who had not fathered any offspring.
Conversely, there was no identified association between childbirth
and cumulative doses of chemotherapeutic agents in the female
subjects.
Discussion
In the present study, the overall proportion of
married subjects of 36.2% (17/47) was lower compared to the 63.0%
reported in equivalent subjects in Japan in 2009 (15). These data may reflect the current
downturn in the marriage rate in Japan (23). In the present study, the proportion
of married men (31.4%; 7/24) tended to be lower compared to that of
married women (43.4%; 10/23); however, this difference was not
statistically significant (P=0.093). The treatment for malignant
bone and soft tissue tumors may exert a negative effect on the
marriage prospects of men. Traditionally, Japanese men are mainly
responsible for the financial support to the family; young men
(mean age, 33 years at the final follow-up) with a certain
impairment of physical function following tumor surgery may
consider it inappropriate to start a family, particularly
considering the recent economical recession affecting Japan.
Regarding fertility, the overall final proportion
and the true proportion of fertile subjects at the last follow-up
were 29.8% (14/47) and 21.3% (10/47), respectively, compared to
previous studies that reported 8.3% in male subjects alone
(12) and 41.6% (15/36) (13) and 27.4% (17/62) in both genders
(15) (Table V). The total fertility rate in
Japan is currently lower compared to that in other developed
countries. According to data on women in Japan (23), the live birth rate (number of
births/total number of women) is ∼75.2% among Japanese women of
similar age. In the present study, 23 women had 13 offspring at the
final follow-up; thus, the live birth rate was 56.6% (13/23).
Therefore, chemotherapy for high-grade bone and soft tissue tumor
apparently exerts a somewhat negative effect on fertility; however,
a statistical analysis of these data is not possible.
 | Table V.Comparison of our findings and those
of previous reports concerning fertility. |
Table V.
Comparison of our findings and those
of previous reports concerning fertility.
| First author
(year) | Histology | Mean
agea, years
(range) | Gender | No. | Patients with
marriage | Patients with
offspr. | Total offspr.
no. | Mean follow-up | Delivery outcome
(no.) | Congenital
deformities Refs. |
|---|
| Longhi (2003) | Osteosarcoma | 17 (10–42) | M | 96 | 26 | 8 | 12 | 9 years | Abortion (1) | None | (12) |
| Hosalkar
(2004) | Osteosarcoma
Ewing's sarcoma | 18 (12–26) | M,F | 36 | NA | 15 | 24 | 5.5 years
(mean) | Medical termination
(1) Cesarean section (Twin)
(1) Miscarriage (1) | None | (13) |
| Yonemoto
(2003) | Osteosarcoma | 16.0 (6–30) | M F | 24 21 | 5 16 | 4 12 | 18 | 176.6 months
(average) | Cesarean section
(5) Abortion (2) | None | (14) |
| Present study | Sarcoma (33
osteosarcoma) | 18 (4–40) | M F | 24 23 | 7 8 | 4 8 | 15 | 11.0 years
(mean) | Cesarean section
(3) Medical termination (1) | None | |
The true male fertility rate of 8.3% (2/24) was
significantly lower compared to the female fertility rate of 34.8%
(8/23). Thus, intensive chemotherapy for malignant bone and soft
tissue tumor exerts a more significant negative effect on male
compared to female fertility. The two men who fathered offspring
had received lower cumulative doses of IFM compared to those who
had not (Fig. 2). In 1948, Spitz
(24) was the first to report the
effects of chemotherapeutic agents on the gonads. Fertility-related
problems following chemotherapy, including male azoospermia and
female amenorrhea, have been well documented. However, several
researchers have reported that chemotherapy for high-grade
malignant bone tumors does not affect the fertility rate (13, 14). Recently, however, more intensive
chemotherapy has been used for high-grade bone tumors, with a
resultant increase in the number of long-term survivors. The
sequelae of this more intensive chemotherapy scheme have been
re-evaluated and found to negatively affect fertility in male
long-term cancer survivors (12,
15). The chemotherapeutic dose
has been associated with damage to the gonads (25). The cumulative dose of
chemotherapeutic agents was the most significant determinant,
largely due to only few patients receiving single-agent
chemotherapy, as multiple-drug chemotherapy is widespread and
highly effective. DOX, CDDP, MTX and IFM are the key
chemotherapeutic drugs used to treat high-grade bone and soft
tissue tumors. Among chemotherapeutic drugs, it has been
demonstrated that alkylating agents are the most toxic to the
gonads (26, 27). Longhi et al (12) reported that 20/26 male patients
exhibited oligospermia or azoospermia following chemotherapy with
high-dose IFM (median, 42 g/m2) for osteosarcoma.
Williams et al (28)
reported that gonadal dysfunction occurs after total IFM doses of
>60 g/m2. Longhi et al (29) reported that patient age and dose of
alkylating agent are the most significant predictors of early
menopause in young female patients with osteosarcoma. Meistrich
et al (30) reported that
high-dose CDDP (≥600 mg/m2) permanently reduces sperm
counts in male patients, whereas according to Wallace et al
(31), total doses of CDDP ≥490
mg/m2 compromise gonadal function. MTX causes transient
testicular dysfunction, but not ovarian dysfunction (32). By contrast, DOX is rarely
associated with gonadal toxicity (33).
In our study, the mean interval between the last
chemotherapy and the first delivery was 9.5 years (1–14 years),
which is similar to previously reported findings (13, 14). The shortest interval to first
delivery was only 1 year in a female patient (case 3; Table IV). Of note, in that patient, the
cumulative doses of chemotherapeutic agents prior to delivery were
as follows: DOX, 420 mg/m2; CDDP, 100 mg/m2;
IFM, 105 g/m2; and MTX, 10 g/m2. Four months
after the completion of chemotherapy, the patient conceived; the
fetus was delivered by caesarean section. This suggests that female
fertility is relatively resistant to the damaging effects of
intensive chemotherapy or that there is rapid recovery following
completion of chemotherapy.
Patients, particularly female patients, of
childbearing age often desire a pregnancy after completing
treatment for high-grade bone and soft tissue sarcoma, as they have
not yet had the planned number of offspring. As regards the
recommendations of health providers and oncologists to such
patients regarding the minimal interval between cancer treatment
and attempting conception, there are no available published studies
for patients with high-grade malignant bone and soft tissue tumors.
The Royal College of Obstetricians and Gynaecologists (34) currently recommends that women with
breast cancer delay pregnancy for ≥2 years after the diagnosis, as
most recurrences occur within this time range. Similarly, the
Society of Obstetricians and Gynaecologists of Canada (35) advises women with breast cancer to
defer pregnancy for 3 years. The guidelines for soft tissue sarcoma
published by the European Society of Medical Oncology (36) recommend that patients with
high-grade tumors who have been treated surgically should be
followed up every 4 months for the first 2–3 years, this being the
period during which relapse usually occurs. Therefore, the minimum
recommended interval between pregnancy and last chemotherapy should
be 2–3 years.
In the present study, only few subjects experienced
complications during delivery (Table
IV). A total of 4 patients (40%) underwent caesarean sections :
One for medical termination as the patient had developed lung
metastases; one for infected amniotic fluid caused by a uterine
myoma; and the remaining 2 patients due to prolonged labor caused
by abnormal rotation of the fetus. Uterine myomas are common benign
gynecological tumors, with an incidence of ∼20%. The tumor size is
affected by estrogen concentrations; this tumor is unrelated to
previous chemotherapy. The incidence of caesarean sections is
similar to that previously reported (Table V). The remaining 6 infants were
delivered uneventfully (normal transvaginal delivery). Thus,
uterine exposure to chemotherapeutic agents for high-grade bone and
soft tissue tumors apparently exerts no negative effect on
childbirth.
Another concern regarding sarcoma treatment is that
any offspring may be at increased risk of congenital deformities
(7). As chemotherapeutic agents
are potential mutagens, there is a theoretical risk of chemotherapy
increasing the incidence of congenital deformities and miscarriage.
A previous review (37) concluded
that the overall incidence of congenital malformations following
cancer treatment is 3–4%, which is similar to the incidence in the
general population. In the present study, no congenital deformities
were detected in any of the offspring of subjects who had received
intensive chemotherapy (Table
IV). Thus, intensive chemotherapy for high-grade bone and soft
tissue tumors exerted no detectable effect on the offspring of our
patients.
This study has certain limitations, namely its
retrospective design, limited patient sample, lack of control cases
and the fact that it was conducted in a single Japanese
institution. Moreover, we collected limited clinical information
regarding the pregnancies and used delivery as the endpoint for
functional assessment of fertility. It would have been preferable
to investigate variables associated with the menstrual cycle in our
female subjects and to perform semen analysis in the men. In
addition, measurements of the serum concentrations of sex-related
hormones, such as follicle-stimulating hormone, luteinizing hormone
and testosterone (38), would have
provided more objective indicators of fertility, as would the
radiological assessment of the size of the testes or ovaries
(39). However, high-grade bone
and soft tissue tumors are rare and data on pregnancies are
extremely difficult to obtain, as there are so few long-term
survivors. Finally, as these patients had been administered various
chemotherapeutic agents under different protocols, we were unable
to accurately identify the effect of any single agent on gonadal
function.
The 2006 American Society of Clinical Oncology
clinical practice guidelines (40), updated in 2012 (41), recommend that health care
providers, including oncologists, discuss the possibility of
infertility and other potential risks with patients who are
scheduled to receive chemotherapy during their reproductive years
prior to the administration of any treatment. All the patients
should understand the possible risk of infertility following
chemotherapy. Our institution has a standard practice of offering
sperm, embryo and oocyte cryopreservation prior to treatment
initiation.
In conclusion, intensive chemotherapy for high-grade
bone and soft tissue tumors may compromise the fertility of young
patients, particularly men. However, there is no evidence for the
offspring of such patients being at increased risk of congenital
deformities. All young patients with high-grade bone and soft
tissue tumors receive counseling regarding the possibility of
infertility prior to the initiation of chemotherapy.
Acknowledgements
We would like to thank Daisuke Tachibana for
obstetric assistance during the deliveries and interpretation of
our data.
References
|
1
|
Meyers PA, Heller G, Healey J, Huvos A,
Lane J, Marcove R, Applewhite A, Vlamis V and Rosen G: Chemotherapy
for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering
experience. J Clin Oncol. 10:5–15. 1992.PubMed/NCBI
|
|
2
|
Meyers PA, Gorlick R, Heller G, Casper E,
Lane J, Huvos AG and Healey JH: Intensification of preoperative
chemotherapy for osteogenic sarcoma: results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol. 16:2452–2458.
1998.PubMed/NCBI
|
|
3
|
Yang JC, Chang AE, Baker AR, Sindelar WF,
Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM,
Merino MJ and Rosenberg SA: Randomized prospective study of the
benefit of adjuvant radiation therapy in the treatment of soft
tissue sarcomas of the extremity. J Clin Oncol. 16:197–203.
1998.PubMed/NCBI
|
|
4
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.PubMed/NCBI
|
|
5
|
Kawaguchi N, Ahmed AR, Matsumoto S, Manabe
J and Matsushita Y: The concept of curative margin in surgery for
bone and soft tissue sarcoma. Clin Orthop Relat Res. 419:165–172.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longhi A, Ferrari S, Tamburini A, Luksch
R, Fagioli F, Bacci G and Ferrari C: Late effects of chemotherapy
and radiotherapy in osteosarcoma and Ewing sarcoma patients: the
Italian Sarcoma Group Experience (1983–2006). Cancer.
118:5050–5059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schover LR, Rybicki LA, Martin BA and
Bringelsen KA: Having children after cancer. A pilot survey of
survivors' attitudes and experiences. Cancer. 86:697–709. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagarajan R, Neglia JP, Clohisy DR, Yasui
Y, Greenberg M, Hudson M, Zevon MA, Tersak JM, Ablin A and Robison
LL: Education, employment, insurance, and marital status among 694
survivors of pediatric lower extremity bone tumors: a report from
the childhood cancer survivor study. Cancer. 97:2554–2564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drasga RE, Einhorn LH, Williams SD, Patel
DN and Stevens EE: Fertility after chemotherapy for testicular
cancer. J Clin Oncol. 1:179–183. 1983.PubMed/NCBI
|
|
10
|
Pryzant RM, Meistrich ML, Wilson G, Brown
B and McLaughlin P: Long-term reduction in sperm count after
chemotherapy with and without radiation therapy for non-Hodgkin's
lymphomas. J Clin Oncol. 11:239–247. 1993.PubMed/NCBI
|
|
11
|
Reichman BS and Green KB: Breast cancer in
young women: effect of chemotherapy on ovarian function, fertility,
and birth defects. J Natl Cancer Inst Monogr. 16:125–129.
1994.PubMed/NCBI
|
|
12
|
Longhi A, Macchiagodena M, Vitali G and
Bacci G: Fertility in male patients treated with neoadjuvant
chemotherapy for osteosarcoma. J Pediatr Hematol Oncol. 25:292–296.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosalkar HS, Henderson KM, Weiss A,
Donthineni R and Lackman RD: Chemotherapy for bone sarcoma does not
affect fertility rates or childbirth. Clin Orthop Relat Res.
428:256–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yonemoto T, Tatezaki S, Ishii T and
Hagiwara Y: Marriage and fertility in long-term survivors of high
grade osteosarcoma. Am J Clin Oncol. 26:513–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yonemoto T, Ishii T, Takeuchi Y, Hagiwara
Y, Iwata S and Tatezaki S: Recently intensified chemotherapy for
high-grade osteosarcoma may affect fertility in long-term male
survivors. Anticancer Res. 29:763–767. 2009.PubMed/NCBI
|
|
16
|
Ishida T and Takami M: Evaluation of
chemotherapy before limb-sparing surgery for osteosarcoma. Int
Orthop. 16:59–61. 1992.PubMed/NCBI
|
|
17
|
Kudawara I, Aoki Y, Ueda T, Araki N, Naka
N, Nakanishi H, Matsumine A, Ieguchi M, Mori S, Myoui A, Kuratsu S,
Hashimoto N and Yoshikawa H: Neoadjuvant and adjuvant chemotherapy
with high-dose ifosfamide, doxorubicin, cisplatin and high-dose
methotrexate in non-metastatic osteosarcoma of the extremities: a
phase II trial in Japan. J Chemother. 25:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuchiya H, Tomita K, Mori Y, Asada N and
Yamamoto N: Marginal excision for osteosarcoma with caffeine
assisted chemotherapy. Clin Orthop Relat Res. 358:27–35. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juergens C, Weston C, Lewis I, Whelan J,
Paulussen M, Oberlin O, Michon J, Zoubek A, Juergens H and Craft A:
Safety assessment of intensive induction with vincristine,
ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of
Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr
Blood Cancer. 47:22–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka K, Kawamoto H, Saito I, Yoshimura
K, Fukuda H and Iwamoto Y: Preoperative and postoperative
chemotherapy with ifosfamide and adriamycin for adult high-grade
soft-tissue sarcomas in the extremities: Japan Clinical Oncology
Group Study JCOG0304. Jpn J Clin Oncol. 39:271–273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Winkle P, Angiolillo A, Krailo M,
Cheung YK, Anderson B, Davenport V, Reaman G and Cairo MS:
Ifosfamide, carboplatin, and etoposide (ICE) reinduction
chemotherapy in a large cohort of children and adolescents with
recurrent/refractory sarcoma: the Children's Cancer Group (CCG)
experience. Pediatr Blood Cancer. 44:338–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byrne J, Mulvihill JJ, Myers MH, et al:
Effects of treatment on fertility in long-term survivors of
childhood or adolescent cancer. N Engl J Med. 317:1315–1321. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
2013 Statistics of the Ministry of Health
Labor and Welfare of Japan, . Marriage, Childbirth Childrearing,
Natality. http://www.mhlw.go.jp/toukei/youran/index-kourou.htmlAccessed.
January 30–2014
|
|
24
|
Spitz S: The histological effects of
nitrogen mustards on human tumors and tissues. Cancer. 1:383–398.
1948. View Article : Google Scholar
|
|
25
|
Bacci G, Ferrari S, Bertoni F, Ruggieri P,
Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M and
Campanacci M: Long-term outcome for patients with nonmetastatic
osteosarcoma of the extremity treated at the Istituto Ortopedico
Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2
protocol: an updated report. J Clin Oncol. 18:4016–4027.
2000.PubMed/NCBI
|
|
26
|
Kenney LB, Laufer MR, Grant FD, Grier H
and Diller L: High risk of infertility and long term gonadal damage
in males treated with high dose cyclophosphamide for sarcoma during
childhood. Cancer. 91:613–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meistrich ML, Wilson G, Brown BW, da Cunha
MF and Lipshultz LI: Impact of cyclophosphamide on long-term
reduction in sperm count in men treated with combination
chemotherapy for Ewing and soft tissue sarcomas. Cancer.
70:2703–2712. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams D, Crofton PM and Levitt G: Does
ifosfamide affect gonadal function? Pediatr Blood Cancer.
50:347–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Longhi A, Pignotti E, Versari M, Asta S
and Bacci G: Effect of oral contraceptive on ovarian function in
young females undergoing neoadjuvant chemotherapy treatment for
osteosarcoma. Oncol Rep. 10:151–155. 2003.PubMed/NCBI
|
|
30
|
Meistrich ML, Chawla SP, Da Cunha MF,
Johnson SL, Plager C, Papadopoulos NE, Lipshultz LI and Benjamin
RS: Recovery of sperm production after chemotherapy for
osteosarcoma. Cancer. 63:2115–2123. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wallace WH, Shalet SM, Crowne EC,
Morris-Jones PH, Gattamaneni HR and Price DA: Gonadal dysfunction
due to cis-platinum. Med Pediatr Oncol. 17:409–413. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shamberger RC, Rosenberg SA, Seipp CA and
Sherins RJ: Effects of high-dose methotrexate and vincristine on
ovarian and testicular functions in patients undergoing
postoperative adjuvant treatment of osteosarcoma. Cancer Treat Rep.
65:739–746. 1981.PubMed/NCBI
|
|
33
|
Waxman J: Chemotherapy and the adult
gonad: a review. J R Soc Med. 76:144–148. 1983.PubMed/NCBI
|
|
34
|
Royal College of Obstetricians and
Gynaecologists, . Pregnancy and B reast Cancer (Green-top Guideline
No. 12). http://www.rcog.org.uk/guidelinesAccessed.
January 27–2014
|
|
35
|
Helewa M, Lévesque P, Provencher D, Lea
RH, Rosolowich V and Shapiro HMBreast Disease Committee and
Executive Committeee and Council, Society of Obstetricians
Gynaecologists of Canada: Breast cancer, pregnancy, and
breastfeeding. J Obstet Gynaecol Can. 24:164–184. 2002.(In English,
French).
|
|
36
|
ESMO/European Sarcoma Network Working
Group, . Soft tissue and visceral sarcomas: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 23
(Suppl 7):vii92–vii99. 2012.PubMed/NCBI
|
|
37
|
Nicholson HS and Byrne J: Fertility and
pregnancy after treatment for cancer during childhood or
adolescence. Cancer. 71 (Suppl 10):3392–3399. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shamberger RC, Sherins RJ and Rosenberg
SA: The effects of postoperative adjuvant chemotherapy and
radiotherapy on testicular function in men undergoing treatment for
soft tissue sarcoma. Cancer. 47:2368–2374. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siimes MA, Elomaa I and Koskimies A:
Testicular function after chemotherapy for osteosarcoma. Eur J
Cancer. 26:973–975. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SJ, Schover LR, Partridge AH, Patrizio
P, Wallace WH, Hagerty K, Beck LN, Brennan LV and Oktay KAmerican
Society of Clinical Oncology: American Society of Clinical Oncology
recommendations on fertility preservation in cancer patients. J
Clin Oncol. 24:2917–2931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Loren AW, Mangu PB, Beck LN, Brennan L,
Magdalinski AJ, Partridge AH, Quinn G, Wallace WH and Oktay
KAmerican Society of Clinical Oncology: Fertility preservation for
patients with cancer: American Society of Clinical Oncology
clinical practice guideline update. J Clin Oncol. 31:2500–2510.
2013. View Article : Google Scholar : PubMed/NCBI
|