Introduction
Acute promyelocytic leukemia (APL) is a subtype of
acute myeloid leukemia (AML) characterized by the presence of the
PML/RARA fusion gene, which is formed by the reciprocal
chromosomal translocation t(15;17)(q22;q21) (1). The prognosis of APL was previously
the worst among all the types of AML, mostly due to the almost
inevitable occurrence of disseminated intravascular coagulation at
diagnosis or during chemotherapy. However, all-trans
retinoic acid (ATRA) has significantly improved the prognosis of
APL, which has now become a highly curable disease with ATRA and
anthracycline-based chemotherapy (1). This combination results in complete
remission (CR) rates of ≤95%, with >80% of the patients
surviving without relapse. Thus, the combination of ATRA and
chemotherapy is currently considered to be the standard treatment
for newly diagnosed APL (1).
One of the major concerns regarding the treatment of
low-to-intermediate-risk APL are currently treatment-related
adverse events, which led to reduction of the anthracycline dose
during consolidation therapy in the PETHEMA LPA2005 protocol
compared to the LPA96/99, without any compromise in disease-free
survival (DFS) or overall survival (OS) (2). Adverse events are also expected to
occur during maintenance therapy, which is included in a number of
clinical trials and continues for ∼2 years. Of note, DFS was
similar between the maintenance and non-maintenance groups in
patients who achieved molecular CR (MCR) following consolidation
therapy in the AIDA0493 trial (3).
Very recently, a new combination therapy consisting of ATRA and
arsenic trioxide (ATO) was associated with fewer treatment-related
toxicities, with possibly better therapeutic effects compared to
ATRA plus chemotherapy (4).
Furthermore, for low-to-intermediate-risk patients, ATRA plus ATO
without maintenance therapy was found to be at least comparable to
ATRA plus chemotherapy with maintenance therapy (4). Therefore, maintenance therapy may not
be essential for low-to-intermediate-risk patients who have
achieved MCR following efficient induction and consolidation
therapy.
To evaluate the clinical efficacy of maintenance
therapy for such patients, we retrospectively analyzed the clinical
outcomes of 11 consecutive low-to-intermediate-risk APL patients in
MCR, with and without subsequent maintenance therapy.
Patients and methods
Patients
We retrospectively analyzed 11 consecutive cases of
newly diagnosed low-to-intermediate-risk APL patients who received
induction and consolidation therapy according to the PETHEMA LPA
protocols (5, 6) between January, 2001 and March, 2013
at Tokyo Medical and Dental University Hospital, Tokyo, Japan. All
the patients were morphologically diagnosed with APL and confirmed
by reverse transcription-polymerase chain reaction (RT-PCR) for the
PML/RARA rearrangement. According to the guideline published
by the Ministry of Health, Labour and Welfare of Japan, this
retrospective study was approved by the Institutional Review Board
of the Tokyo Medical and Dental University.
Treatment protocol and risk
classification
The details of the PETHEMA LPA protocol (LPA96,
LPA99 and LPA2005) regimens have been previously published
(2, 5). In brief, induction therapy consisted
of ATRA with idarubicin (IDA). Patients who achieved CR received 3
monthly consolidation courses with ATRA and anthracycline-based
chemotherapy. Maintenance therapy, consisting of mercaptopurine,
methotrexate and ATRA, was continued for 2 years. According to the
relapse risk categories used by the joint PETHEMA-GIMEMA study
(6), low-to-intermediate risk was
defined as a white blood cell count at diagnosis of ≤
10,000/µl.
Statistical analysis
MCR was defined as negativity for
PML/RARA by RT-PCR. Relapse was defined as the
re-appearance of morphologically abnormal leukemic cells following
initial clearance of the marrow or extramedullary sites. OS and
event-free survival (EFS) were calculated from the date of
induction therapy initiation, while DFS was calculate from the date
of CR achievement. The event was defined as relapse, death, or
secondary primary malignancy (SPM) for EFS and relapse or death for
DFS. Time-to-event analyses were performed using the Kaplan-Meier
estimate and comparisons were performed using log-rank tests. All
the statistical analyses were performed with EZR (Saitama Medical
Center, Jichi Medical University, Saitama, Japan), which is a
graphical user interface for R (The R Foundation for Statistical
Computing) (7). The patients who
had received maintenance therapy for <3 months were classified
into the maintenance therapy-free group.
Results
Patient characteristics and
responses
A total of 12 patients were diagnosed with
low-to-intermediate-risk APL between January, 2001 and March, 2013
in our hospital. One patient received only induction therapy due to
severe infection and was excluded from the following analyses. The
patient characteristics are summarized in Tables I and II. According to the LPA
risk-classification (6), 3
patients were classified into the low-risk and 8 patients into the
intermediate-risk group. There were 3 internal tandem duplications
of FLT3-positive patients and no D835Y-positive patient. All the
patients achieved CR following induction therapy (Table II). The PML/RARA transcript
levels became undetectable by quantitative RT-PCR in all the
patients following consolidation-2 (Table III). Furthermore, the nested
RT-PCR method failed to detect the PML/RARA transcript in
all 10 cases examined by this method (Table III).
 | Table I.Patient characteristics (n=11). |
Table I.
Patient characteristics (n=11).
| Maintenance
therapy |
|---|
|
|
|---|
| Variables | Yes | No |
|---|
| Gender | | |
| Male | 2 | 3 |
|
Female | 2 | 4 |
| Age, years | | |
|
Range | 26-71 | 19-81 |
|
Median | 66 | 46 |
| WBC count/µl | | |
|
Range | 700-6,800 | 600-4,200 |
|
Median | 1,200 | 1,500 |
| PLT count
×104/µl | | |
|
Range | 1.1-6.7 | 0.4-11.7 |
|
Median | 2.3 | 2.5 |
| Risk | | |
| Low | 1 | 2 |
|
Intermediate | 3 | 5 |
| Days to CR,
median | 40 | 36 |
| Days to MCR,
median | 136 | 110 |
 | Table II.Clinical characteristics of 11
patients. |
Table II.
Clinical characteristics of 11
patients.
| Patient profile | Leukemia profile | Induction
therapy |
|---|
|
|
|
|
|---|
| Case no. | Gender/age (yrs) | Past history | /µ1 |
×104µ1 | Risk | ACA | CD34 | CD56 | FLT3/ITD | Protocol | DIC | DS | CR(days) | Adverse events |
|---|
| MT |
| 1 | M/63 | | 700 | 1.1 | Int | + | − | − | − | LPA96 | + | + | 50 | FN |
| 2 | F/26 |
| 6,800 | 1.2 | Int | NA | + | − | + | LPA96 | + | + | 39 | Sepsis |
| 3 | F/69 | Colon Ca | 1,300 | 3.4 | Int | − | NA | NA | − | LPA99 | + | − | 41 | Genitalulcer |
| 4 | M/71 |
| 1,100 | 6.7 | Low | + | NA | NA | NA | LPA99 | − | − | 36 | FN |
| No MT |
| 5 | M/19 |
| 600 | 4.2 | Low | NA | NA | NA | − | LPA96 | + | − | 58 | HPS |
| 6 | M/54 |
| 1,500 | 0.4 | Int | NA | NA | NA | NA | LPA96 | + | + | 34 | Infection |
| 7 | F/32 |
| 4,200 | 2.5 | Int | + | + | − | − | LPA99 | + | + | 38 |
| 8 | F/81 | RA, sarcoidosis | 1,300 | 11.7 | Low | + | + | − | NA | LPA99 | + | + | 36 | FN |
| 9 | M/62 | Af | 1,800 | 2.5 | Int | + | − | − | + | LPA2005 | + | − | 34 | Liver
dysfunction |
| 10 | F/28 | Eating disorder | 3,800 | 3.7 | Int | + | + | − | + | LPA2005 | + | − | 31 | Pericoronitis of
wisdom tooth |
| 11 | F/46 | Myoma | 1,100 | 0.4 | Int | − | − | − | − | LPA2005 | + | + | 45 | FN |
 | Table III.Clinical course of 11 patients. |
Table III.
Clinical course of 11 patients.
| PML/RARA
transcript level | Long-term
follow-up |
|---|
|
|
|
|---|
| Case no. | RT-q PCR
negative | Molecular CR | Molecular
relapse | Relapse | SPM (months) | Follow-up
(months) |
|---|
| MT | | | | | | |
| 1 | Ind | Ind | | | Colon Ca (69) | 137 |
| 2 | Ind | C-2 | | | | 126 |
| 3 | C-1 | C-2 | | | Lung Ca (38) | 97 |
| 4 | C-2 | C-2 | | | | 70 |
| No MT | | | | | | |
|
5a | Ind | Ind | | | | 102 |
|
6b | C-1 | C-2 | | | | 85 |
|
7c | Ind | C-2 | Yes (day 269) | Yes (CNS, day
388) | | 95 |
| 8 | C-1 | NA | | | | 27 |
| 9 | Ind | C-1 | | | | 30 |
| 10 | C-1 | C-3 | | | | 28 |
| 11 | Ind | C-1 | | | | 18 |
Maintenance therapy
Although 6 patients were initiated on maintenance
therapy, 2 patients discontinued the therapy within 2 months due to
adverse events, myelosuppression and infection (Table III). These 2 patients were
classified into the group not receiving maintenance therapy.
Relapse and survival
With a median follow-up of 85 months, all the
patients remain alive and APL relapsed in only 1 patient who did
not received maintenance therapy (Table III). In this patient, meningeal
involvement by APL was suspected on brain magnetic resonance
imaging (MRI) during consolidation-3 therapy. The bone marrow
revealed molecular relapse within 2 months after completing
consolidation-3 therapy (day 269), followed by central nervous
system relapse confirmed by cerebrospinal fluid examination on day
388. Following salvage chemotherapy, the patient received umbilical
cord blood transplantation and remains alive without relapse. As
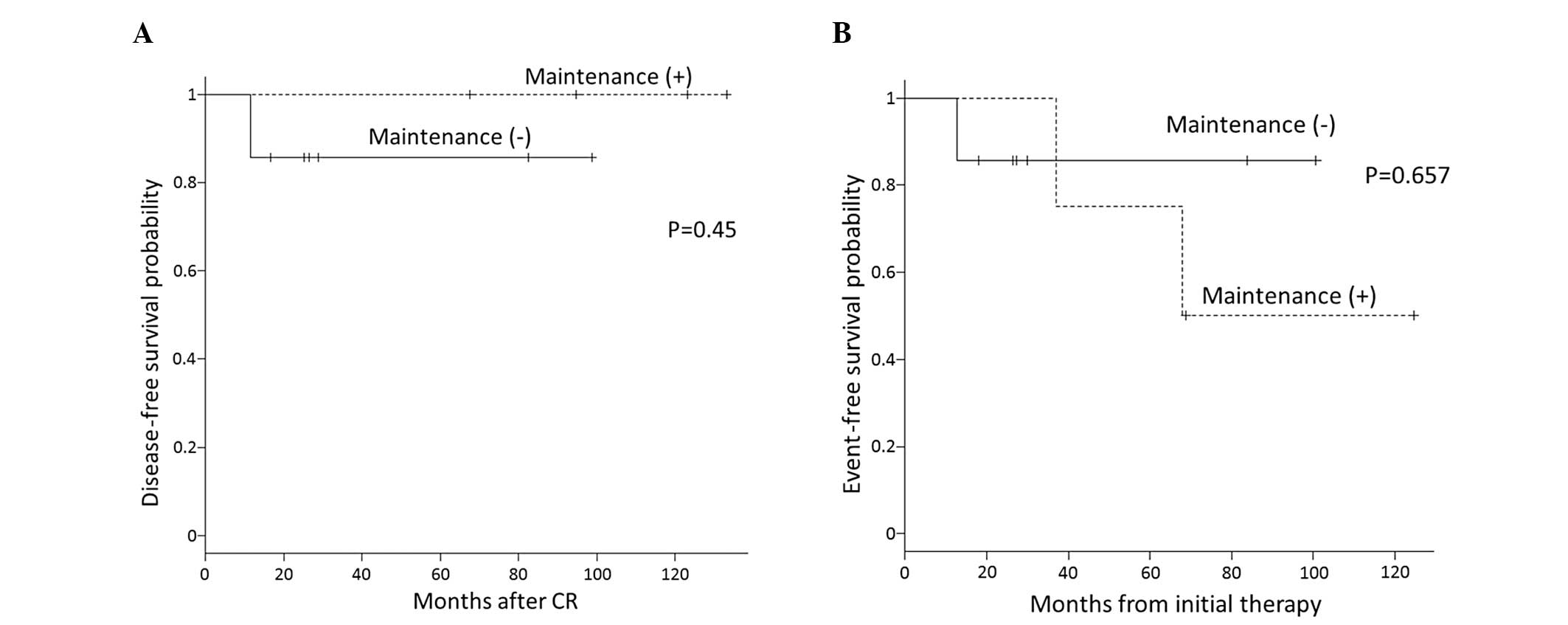
shown in Fig. 1A, no significant
difference in the 5-year estimate of DFS was observed between the
maintenance and maintenance-free group (100 vs. 87.5%, P=0.45).
SPM
SPM was diagnosed in 2 patients in the maintenance
group (Table III). One patient
with colon cancer was treated by curative colon resection and
remains alive without relapse. In the other case, lung cancer was
treated by lung resection but relapsed in the brain. Thus, the
5-year estimate of EFS for the maintenance and maintenance-free
groups was 75 and 85.7%, respectively; however, the difference was
not statistically significant, as shown in Fig. 1B (P=0.657).
Discussion
In the present study, we retrospectively evaluated
the efficacy of maintenance therapy in low-to-intermediate-risk APL
patients consecutively treated in our hospital according to the LPA
protocols, who achieved molecular remission following consolidation
therapy. The 5-year OS was 100% in both the maintenance and
observation groups. Although 1 patient without maintenance therapy
relapsed, the 5-year PFS for patients with and without maintenance
therapy was 100 and 85.7%, respectively, without a statistically
significant difference (P=0.45). It should also be noted that,
although the relapsed case exhibited MCR in the bone marrow sample
following consolidation therapy, the MRI examination during the
last course of consolidation had actually suggested meningeal
relapse. APL relapsed in the bone marrow within 2 months after
completing the consolidation therapy, followed by meningeal relapse
confirmed by cerebrospinal fluid examination. Thus, albeit in MCR,
this patient apparently had a significant amount of residual APL
cells in sanctuary sites at the completition of consolidation
therapy, suggesting that relapse could not have been prevented by
low-dose maintenance therapy. Taken together, these clinical data
strongly suggest that maintenance therapy may not be required to
prevent relapse for low-to-intermediate-risk APL patients who were
treated efficiently by ATRA and anthracycline-based therapy and
achieved MCR.
In contrast to our data, 2 randomized studies
previously reported the significant survival benefit of maintenance
therapy with ATRA and/or low-dose chemotherapy (8, 9),
which was later confirmed in the long-term outcome analyses
(10, 11). However, in those studies, the
patients were not treated concurrently with ATRA and chemotherapy
as induction therapy, or did not receive ATRA during consolidation
therapy. Moreover, the patients were not examined for MCR or
analyzed according to the current risk classification. Thus, it is
hypothesized that maintenance therapy may not confer a survival
benefit selectively in low-to-intermediate -risk APL patients who
were treated with concurrent ATRA and anthracycline-based
chemotherapy for induction and consolidation and achieved MCR. In
accordance with this, a recent study reported that no advantage in
terms of DFS was obtained by maintenance therapy in APL patients
achieving MCR by the AIDA 0493 protocol, which includes the
concurrent administration of ATRA and IDA as induction therapy,
similar to the LPA protocols we employed (3). Furthermore, a recent controlled study
demonstrated that none of the low-to-intermediate-risk APL patients
treated with a consolidation regimen including ATO and achieving
MCR relapsed, with or without maintenance therapy (12). Taken together, these results
strongly suggest that maintenance therapy may not provide any
benefit for low-to-intermediate-risk APL patients treated with
efficient induction and consolidation regimens to achieve MCR.
Maintenance therapy did not provide any significant
survival benefit; however, it was associated with adverse events in
the present study. Maintenance therapy was initiated but abandoned
within 2 months in 2 patients due to myelosuppression and infection
(Table III). In this regard,
fatal infections were observed in 2.5% of the patients randomized
to the maintenance therapy group in a previous study investigating
the efficacy of maintenance therapy (11). Another previous study on the AIDA
0493 protocol also reported sepsis and death due to infection or
hemorrhage during maintenance therapy (3). Thus, the 2 patients were followed
without further attempt at maintenance therapy and observed without
relapse. It is noteworthy that, of the 4 patients receiving
maintenance therapy, 2 patients later developed colon or lung
cancer, while SPM was not reported in the observation group. Of
note, in the Japan Adult Leukemia Study Group APL97 trial,
intensified maintenance chemotherapy was shown to significantly
compromise the OS of APL patients achieving MCR following
consolidation therapy (13). In
that trial, 2 patients in the maintenance group later developed
therapy-related leukemia and succumbed to the disease. Furthermore,
it was suggested that intensified maintenance therapy may have
compromised the sensitivity of APL cells and the tolerance of
patients to subsequent chemotherapy, thereby shortening survival
following relapse (13). These
results suggest that maintenance therapy may actually worsen the
prognosis of low-to-intermediate -risk APL patients in MCR due to
its toxicity.
In conclusion, the present study, conducted on a
well-defined cohort of patients treated at a single institution,
albeit being a retrospective analysis of a limited number of
patients, strongly supports the emerging idea suggested by previous
studies (11–13) that maintenance therapy may not be
required or should be discouraged for low-to-intermediate-risk APL
patients treated efficiently to achieve MCR, as it may be
associated with adverse events, without exerting a significant
preventive effect on leukemia relapse.
References
|
1
|
Elbahesh E, Patel N and Tabbara IA:
Treatment of acute promyelocytic leukemia. Anticancer Res.
34:1507–1517. 2014.PubMed/NCBI
|
|
2
|
Sanz MA, Montesinos P, Rayon C, et al
PETHEMA and HOVON Groups: Risk-adapted treatment of acute
promyelocytic leukemia based on all-trans retinoic acid and
anthracycline with addition of cytarabine in consolidation therapy
for high-risk patients: further improvements in treatment outcome.
Blood. 115:5137–5146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avvisati G, Lo-Coco F, Paoloni FP, et al
GIMEMA AIEOP EORTC Cooperative Groups: AIDA 0493 protocol for newly
diagnosed acute promyelocytic leukemia: very long-term results and
role of maintenance. Blood. 117:4716–4725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo-Coco F, Avvisati G, Vignetti M, et al
Gruppo Italiano Malattie Ematologiche dell'Adulto; German-Austrian
Acute Myeloid Leukemia Study Group; Study Alliance Leukemia:
Retinoic acid and arsenic trioxide for acute promyelocytic
leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanz MA, Martin G, Rayon C, et al: A
modified AIDA protocol with anthracycline-based consolidation
results in high antileukemic efficacy and reduced toxicity in newly
diagnosed PML/RARalpha-positive acute promyelocytic leukemia.
PETHEMA group. Blood. 94:3015–3021. 1999.PubMed/NCBI
|
|
6
|
Sanz MA, Lo Coco F, Martin G, et al:
Definition of relapse risk and role of nonanthracycline drugs for
consolidation in patients with acute promyelocytic leukemia: a
joint study of the PETHEMA and GIMEMA cooperative groups. Blood.
96:1247–1253. 2000.PubMed/NCBI
|
|
7
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tallman MS, Andersen JW, Schiffer CA, et
al: All-trans-retinoic acid in acute promyelocytic leukemia. N Engl
J Med. 337:1021–1028. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fenaux P, Chastang C, Chevret S, et al: A
randomized comparison of all transretinoic acid (ATRA) followed by
chemotherapy and ATRA plus chemotherapy and the role of maintenance
therapy in newly diagnosed acute promyelocytic leukemia. The
European APL Group. Blood. 94:1192–1200. 1999.PubMed/NCBI
|
|
10
|
Tallman MS, Andersen JW, Schiffer CA, et
al: All-trans retinoic acid in acute promyelocytic leukemia:
long-term outcome and prognostic factor analysis from the North
American Intergroup protocol. Blood. 100:4298–4302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ades L, Guerci A, Raffoux E, et al
European APL Group: Very long-term outcome of acute promyelocytic
leukemia after treatment with all-trans retinoic acid and
chemotherapy: the European APL Group experience. Blood.
115:1690–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coutre SE, Othus M, Powell B, et al:
Arsenic trioxide during consolidation for patients with previously
untreated low/intermediate risk acute promyelocytic leukaemia may
eliminate the need for maintenance therapy. Br J Haematol.
165:497–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asou N, Kishimoto Y, Kiyoi H, et al the
Japan Adult Leukemia Study Group: A randomized study with or
without intensified maintenance chemotherapy in patients with acute
promyelocytic leukemia who have become negative for PML-RARalpha
transcript after consolidation therapy: the Japan Adult Leukemia
Study Group (JALSG) AP197 study. Blood. 110:59–66. 2007. View Article : Google Scholar : PubMed/NCBI
|