Introduction
Previous studies have demonstrated that inhibitors
of apoptosis proteins (IAPs), such as survivin, livin and X-linked
IAP (XIAP), are expressed in high concentrations in various cancers
and hematological malignancies (1–4). In
addition, survivin acts not only as an anti-apoptotic factor, but
also plays an important role in cell proliferation and escape from
the immune surveillance system via the induction of human
telomerase reverse transcriptase and Fas ligand (5,6). Other
members of IAPs, particularly livin and XIAP, play distinct roles
in various types of cancer (7,8).
Consequently, these facts suggest that detection of IAPs may be
useful as tumor markers. However, tissue specimens or cells are
required for the detection of IAP mRNA or proteins and, therefore,
the clinical utility of this detection method is limited.
Several studies demonstrated the presence of
autoantibodies against various cancer antigens in the peripheral
blood. We also previously reported that autoantibodies against
survivin and livin were detected in patients with gastrointestinal,
lung, breast and hepatic cancers (9–13). Other
studies also reported survivin autoantibody positivity in various
cancers (14–18). However, there have been no
comprehensive studies establishing an ideal detection combination
regarding IAPs and other tumor markers. Furthermore, IAP positivity
in patients with precancerous conditions remains unknown.
Therefore, we targeted colon cancer and adenomas and investigated
the positivity of autoantibodies against survivin, livin and XIAP,
as well as the presence of anti-p53 antibodies (19,20),
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9). In order to determine the ideal assay for the detection
of colon cancer, we compared the positivity rates of several
combinations of these markers.
Materials and methods
Patients
Blood samples were collected from 250 patients with
untreated colon adenoma and 176 patients with untreated colon
cancer at the Sapporo Medical University Hospital and related
hospitals. Histological diagnosis was performed by expert doctors
at a pathology center. As a control, blood samples were collected
from 62 non-hospitalized adults without any malignancy. Informed
consent was obtained from all the blood donors. Following blood
centrifugation, the sera were divided into aliquots and stored at
−80°C. The patients with colon cancer were diagnosed with stage
I–IV tumors on the basis of the Union for International Cancer
Control TNM classification guidelines (21).
Preparation of recombinant
protein
mRNA was extracted from the SW480 colon cancer cell
line (RNeasy Mini kit; Qiagen, Hilden, Germany) and cDNA was
prepared (SuperScript First-Strand Synthesis System for RT-PCR;
Invitrogen, Carlsbad, CA, USA) for gene amplification of
full-length survivin, livin and XIAP. Subsequently, polymerase
chain reaction (PCR) was performed with primers to amplify each
gene. The primers for survivin (NM001168), livin (AF311388) and
XIAP (U45880) were as follows: Survivin: forward,
5′-CACCATGGGTGCCCCGACGTTG-3′ and reverse,
5′-TCAATCCATGGCAGCCAGCTGCT-3′; livin: forward,
5′-CACCATGGGACCTAAAGACAGTG-3′ and reverse,
5′-CTAGGACAGGAAGGTGCGCAC-3′; and XIAP: forward,
5′-CACCATGACTTTTAACAGTTTTGA-3′ and reverse,
5′-TTAAGACATAAAAATTTTTTGCTTGAA-3′. The PCR conditions were as
follows: Taq polymerase activation at 94°C for 5 min; 35 cycles at
94°C for 15 sec, at55°C for 45 sec andat72°C for 45 sec; and final
extension at 72°C for 10 min. Each band of amplified PCR product in
the gel was extracted and then inserted into the protein expression
vector pRT151 (Champion pET Directional TOPO Expression kits;
Invitrogen). The inserted vector was transformed using BL21
competent cells. Each protein was purified using the HisTrap FF
crude kit (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
Commercial antigens and
antibodies
For the pre-absorption test and for drawing standard
curves, the purchased antigens and antibodies were as follows:
Recombinant human survivin (cat. no. 4160-50; BioVision, Inc.,
Milpitas, CA, USA), recombinant human livin (cat. no. 01-2103;
American Research Products Inc, Grandville, MI, USA), recombinant
human XIAP (cat. no. 822-XF-050; R&D Systems, Minneapolis, MN,
USA), rabbit-anti human survivin (R&D), mouse anti-human livin
(Abcam, Cambridge, MA, USA) and mouse anti-human XIAP
(R&D).
ELISA
Purified recombinant survivin (1 mg/l), livin (5
mg/l) and XIAP (1 mg/l) were used as antigens for coating wells in
ELISA, which were placed in the wells of 96-well plates (Corning,
Corning, NY, USA) and incubated overnight at 4°C. After removing
the antigen solutions, the plate was washed with Block Ace™
(Dainippon Sumitomo Pharma, Inc., Osaka, Japan) and the plates were
blocked using Block Ace for 2 h at room temperature. After emptying
the wells and washing 4 times with 0.5 ml/l Tween-20
[T-phosphate-buffered saline (PBS)], 100 µl of diluted serum sample
(1,000-fold) in 4-fold diluted Block Ace by PBS was added to each
well and incubated for 1 h at room temperature. The samples were
then removed and the wells were washed 4 times with T-PBS, after
which of rabbit anti-human IgG (1:6,000 dilution) conjugated with
horseradish peroxidase (Dako, Carpinteria, CA, USA) was added to
each well and incubated for 30 min at room temperature. Following
removal of this antibody solution and washing 4 times with T-PBS,
each well was developed using tetramethylbenzidine (TMB). After a
30-min incubation in darkness, the reaction was stopped by adding
0.5 mol/l H2SO4 and absorbance was measured
at 450/620 nm using EVOLIS™ system (Bio-Rad, Hercules, CA, USA).
The measured absorbance was converted to values using the standard
curve. The measurement of the anti-p53 antibody was performed using
the MESACUP™ anti-p53 test kit (Medical and Biological
Laboratories, Co., Ltd., Nagoya, Japan) according to the
manufacturer's instructions.
Measurement of concentrations of CEA
and CA19-9
The serum concentrations of CEA and CA19-9 were
measured by the reagent ECLusys® CEA (Roche Diagnostics Japan,
Tokyo, Japan) and ECLusys CA19-9 (Roche Diagnostics Japan) on
cobas® 8000e series (Roche Diagnostics Japan).
Epitope analysis for survivin
The constructed survivin expression vector was used
as a template and the expression vectors for exons 1–2, 3 and 4 of
each protein were constructed using the following primers: Survivin
1: forward, 5′-CACCATGGGTGCCCCGACGTTG-3′ and reverse,
5′-TCATATGGGGTCGTCATCTGG-3′; survivin 2: forward,
5′-CACCGAGGAACATAAAAAGCA-3′ and reverse,
5′-TCAAATTTTGTTCTTGGCTCT-3′; and survivin 3: forward,
5′-CACCCTGGACAGAGAAAGAGCC-3′ and reverse,
5′-TCAATCCATGGCAGCCAGCTGCT-3′. The recombinant protein for each
exon was purified using the HisTrap FF crude kit (GE Healthcare
Bio-Sciences AB) following transformation by BL21 competent cells.
ELISA was performed using these proteins and the reactivity of the
sera obtained from the patients was assessed.
Results
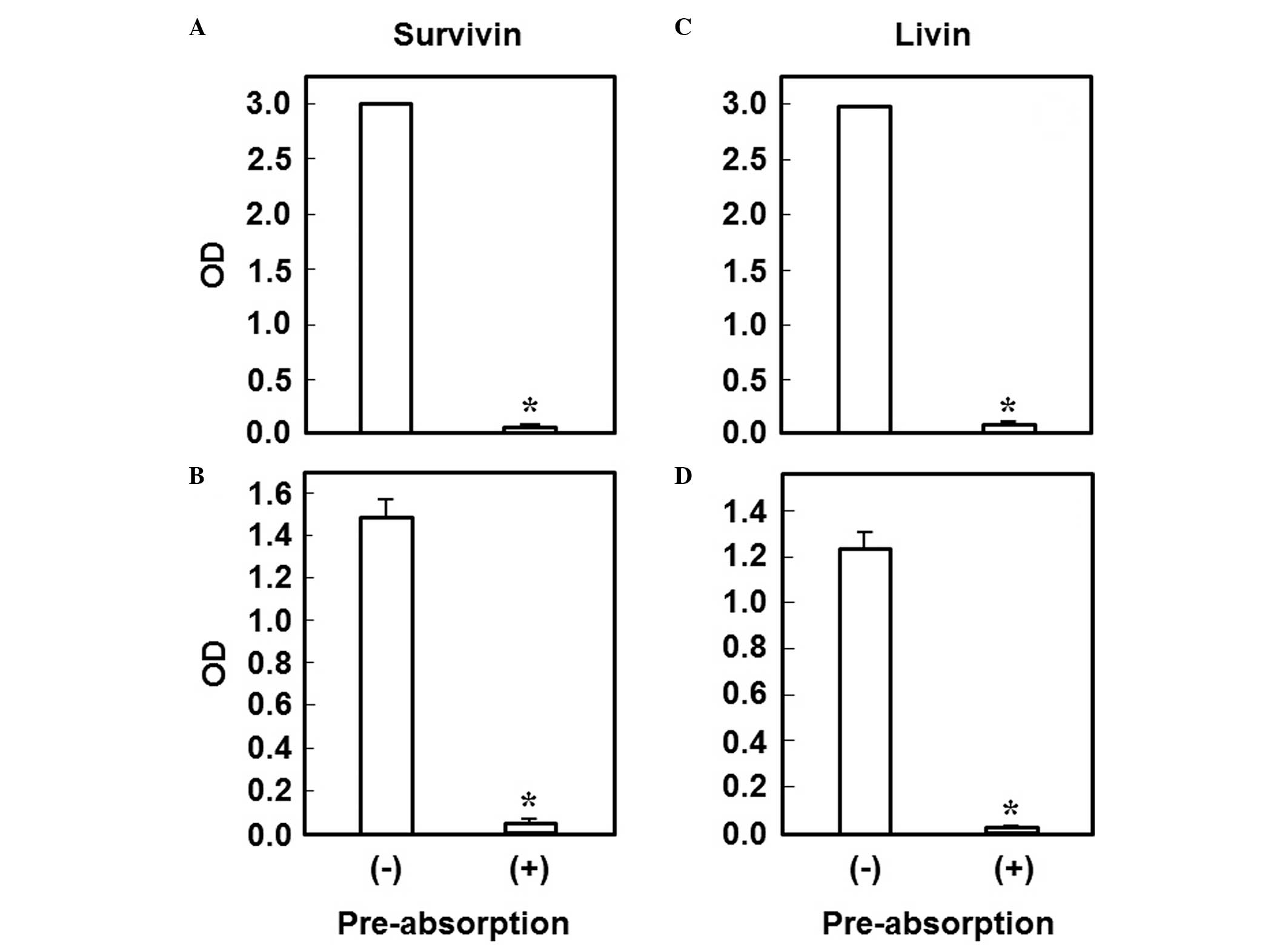
Pre-absorption test
The commercially available antibody was
pre-incubated with the commercially available recombinant antigen
(30 mg/l) for 2 h at 37°C and then subjected to ELISA using the
plate coated with our recombinant antigen, to determinine the
analytical specificity of our own recombinant protein for each
antigen. In addition, we also performed ELISA using the plate
coated with the commercial recombinant antigen. For survivin and
livin, both experiments revealed a significant decrease in the
measured value, indicating absorption of the antibody (Fig. 1). However, for XIAP, the reactivity
between the commercially available antibody and antigen was low
and, therefore, the experiment could not be performed (data not
shown).
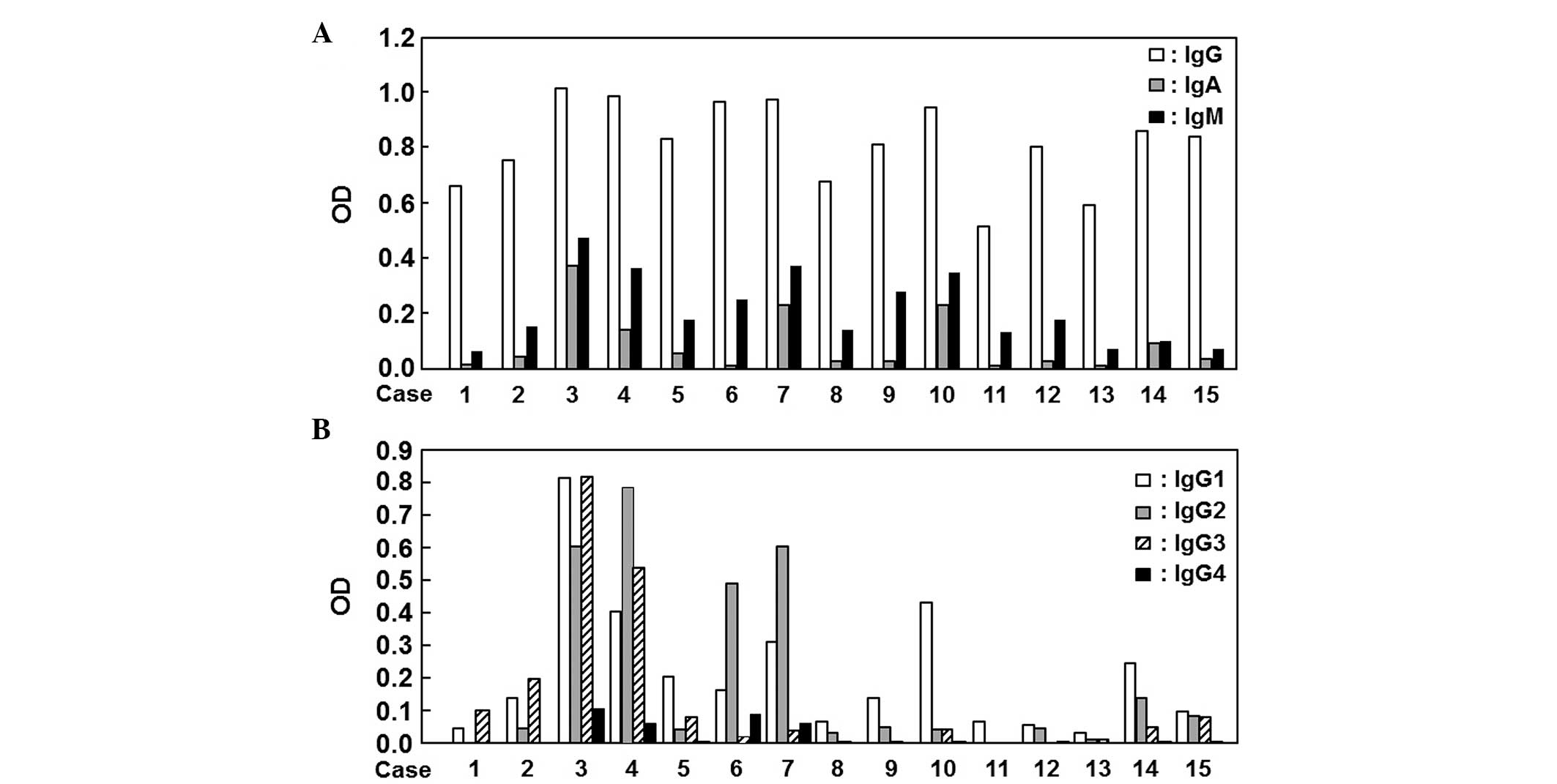
Analysis of autoantibody isotype and
subclass
Using sera from 15 patients with colon cancer, we
analyzed the subclasses of the anti-survivin autoantibody that
reacted to their own recombinant antigens. Among IgG, IgA and IgM,
the difference in reactivity was the least for IgG (Fig. 2A). Moreover, the differences in the
IgG subclasses were examined and IgG1 exhibited the least
significant difference among all patients compared to IgG2, IgG3
and IgG4 (Fig. 2B).
Epitope analysis
Using the recombinant proteins for exons 1–2, 3 and
4 of survivin, the difference in reactivity was examined in 6
patients with colon cancer. As a result, apparent reactivity was
observed in the sets for all exons and all patients, leading to a
higher measured value compared to the negative control samples
without sera (data not shown). Consequently, no apparent
specificity in the reactivity of antibodies in the sera was
observed and it was suggested that our ELISA system for survivin
has universality from the point of potential clinical use.
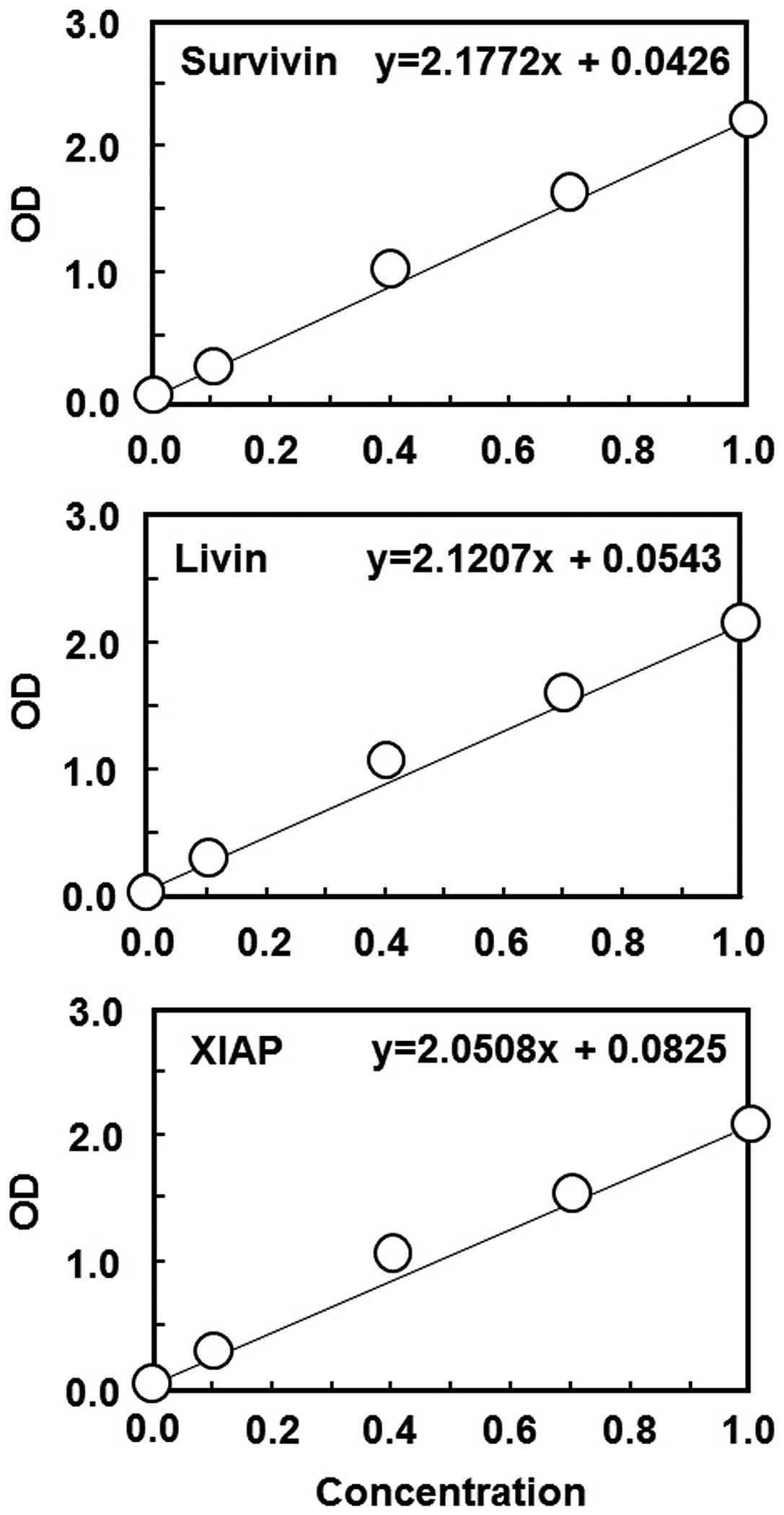
Standard curve
As seen in Fig. 3, our
recombinant antigens and the diluted, commercially available
antibodies for survivin, livin and XIAP were subjected to ELISA and
used to draw a standard curve to obtain the actual values of serum
antibody concentrations. We obtained an almost linear association
for a wide range of concentrations for each set of antigen-antibody
(Fig. 3).
Autoantibody expression profile in
patient sera
The positivity of each antibody was assessed using a
cutoff of the mean + 2 standard deviations (SDs) of the measured
values in healthy individuals to determine the clinical utility of
autoantibodies against IAPs. In all the cancer patients, among all
the IAP autoantibodies, the anti-survivin antibody exhibited the
highest positivity rate using a single assay (Table I). The positivity rate of the survivin
autoantibody was relatively lower compared to that of CEA, but was
similar to that of the anti-p53 antibody. In the analysis by cancer
stage, the positivity rate of the anti-survivin antibody was the
highest in stage 0 (cancer in adenoma) of the tumor, but anti-p53
antibody was not detected in any of the patients. By contrast, the
anti-p53 antibody exhibited the highest positivity rate in patients
with stage I and CEA exhibited the highest positivity rate in
patients with stage IV disease. We then compared the positivity
using combinations of all the markers. In all the patients, the
combination of anti-survivin antibody and CEA exhibited the highest
positivity rate, leading to a diagnosis of 50% of cancers (Table I). Furthermore, in the analysis by
stage, the combination of the anti-survivin antibody and CEA
exhibited a significantly high positivity rate in stage IV tumors,
reflecting the high positivity of only CEA. By contrast, for
early-stage tumors (stages 0-I), there was complete independence
between the anti-survivin and anti-p53 antibodies, resulting in the
highest positivity rate (35.6%) when testing the combination of the
two. Furthermore, in 250 patients with colon adenoma, the
anti-survivin antibody exhibited the highest positivity rate among
all markers (Table II).
 | Table I.Comparison of the positivity (%) in
the single or combination assay at different clinical stages. |
Table I.
Comparison of the positivity (%) in
the single or combination assay at different clinical stages.
| Assays | Total (n=176) | Stage 0 (n=25) | Stage I (n=20) | Stage 0+I (n=45) | Stage II (n=47) | Stage III (n=51) | Stage IV (n=33) |
|---|
| Survivina | 24.4 | 16.0 | 15.0 | 15.6 | 23.4 | 29.4 | 30.3 |
| Livinb | 9.7 | 4.0 | 5.0 | 4.4 | 14.9 | 9.8 | 9.1 |
| XIAPc | 21.6 | 12.0 | 10.0 | 11.1 | 23.4 | 21.6 | 33.3 |
| p53d | 29.0 | 0.0 | 45.0 | 20.0 | 27.7 | 33.3 | 9.1 |
| CEA | 35.2 | 4.0 | 5.0 | 4.4 | 28.8 | 33.3 | 81.8 |
| CA19-9 | 19.9 | 0.0 | 15.0 | 6.7 | 6.7 | 15.7 | 51.5 |
| Survivin |
|
|
|
|
|
|
|
| +CEA | 50.0 | 20.0 | 20.0 | 20.0 | 44.7 | 54.9 | 84.8 |
|
+CA19-9 | 42.6 | 16.0 | 30.0 | 22.2 | 27.7 | 37.3 | 66.7 |
| +p53 | 42.0 | 16.0 | 60.0 | 35.6 | 46.8 | 49.0 | 33.3 |
|
+Livin | 30.1 | 20.0 | 20.0 | 20.0 | 29.8 | 35.3 | 36.4 |
|
+XIAP | 29.0 | 16.0 | 15.0 | 15.6 | 31.9 | 33.3 | 39.4 |
| Livin |
|
|
|
|
|
|
|
| +CEA | 39.8 | 8.0 | 10.0 | 8.9 | 40.4 | 41.2 | 48.5 |
|
+CA19-9 | 26.1 | 4.0 | 20.0 | 11.1 | 21.3 | 25.5 | 57.6 |
| +p53 | 30.7 | 4.0 | 45.0 | 22.2 | 38.3 | 41.2 | 18.2 |
|
+XIAP | 23.9 | 16.0 | 15.0 | 15.6 | 27.7 | 29.4 | 39.4 |
| XIAP |
|
|
|
|
|
|
|
|
+CEA | 44.9 | 16.0 | 15.0 | 15.6 | 46.8 | 41.2 | 84.8 |
|
+CA19-9 | 33.0 | 12.0 | 25.0 | 17.8 | 29.8 | 25.5 | 57.6 |
|
+p53 | 37.5 | 12.0 | 55.0 | 31.1 | 46.8 | 41.2 | 18.2 |
 | Table II.Positivity (%) in patients with colon
adenoma (n=250). |
Table II.
Positivity (%) in patients with colon
adenoma (n=250).
| Assays | Positivity (%) |
|---|
|
Survivina | 18.4 |
| Livinb | 2.4 |
| XIAPc | 3.2 |
| p53d | 3.2 |
| CEA | 2.0 |
| CA19-9 | 1.2 |
As regards the size of the adenoma in the patients
positive for any anti-IAP antibodies, the average size (6.4 mm) was
not significantly different compared to that in all the patients
(7.0 mm). In addition, the average size (6.5 mm) in the patients
positive for anti-survivin antibody did not differ compared to that
in the patients positive for any anti-IAP antibodies (plotting data
not shown). In the analysis of positivity determined by
morphological type, there was no significant difference between the
patients positive for any anti-IAP antibodies and those positive
for anti-survivin antibody (Table
III).
 | Table III.Positivity (%) of anti-IAP antibodies
determined in each morphological type in patients with colon
adenoma. |
Table III.
Positivity (%) of anti-IAP antibodies
determined in each morphological type in patients with colon
adenoma.
| Typea | Total (n=250) | IAPb (n=56) |
Survivinc (n=46) |
|---|
| Ip | 18 (7.2) | 1 (1.8) | 1 (2.2) |
| Isp | 87 (34.8) | 21 (37.5) | 19 (41.3) |
| Is | 91 (36.4) | 26 (46.4) | 20 (43.5) |
| IIa | 48 (19.2) | 7 (12.5) | 5 (10.8) |
| LST | 6 (2.4) | 1 (1.8) | 1 (2.2) |
Discussion
In this study, we demonstrated the diagnostic
relevance of detecting autoantibodies against IAPs, particularly
survivin, as compared to other tumor markers in colon cancer. In
the comparison among IAPs, the positivity in a single assay was the
highest (24.2%) for anti-survivin antibody. As regards the
positivity of anti-survivin antibody, Rohayem et al
(14) reported a relatively lower
positivity rate (8.2%; 4/49) in patients with colorectal cancer
using their ELISA protocol. By contrast, Chen et al
(18) reported high positivity
(sensitivity, 56.9%) in patients with colon cancer. This
significant difference by different investigators is mainly
attributed to the criteria for establishing a cutoff value. Rohayem
et al (14) established a
strict cutoff of the mean + 3 SDs and their data lead to a higher
positivity when the cutoff was set as the mean + 2 SDs. Chen et
al (18) established the cutoff
using different criteria (Yowden's index from receiver operating
characteristic curve analysis: Sensitivity, +; specificity, −1);
therefore, healthy volunteers exhibited a higher positivity rate
(percentage of pseudo-positive patients, 35.9%) compared to the
results of our study (8.1%; 5/62; actual plotting data not
shown).
As regards other IAPs, anti-XIAP antibody also
exhibited a relatively high positivity rate; no significant
increase in positivity was observed with the combination of
anti-XIAP and anti-survivin antibodies (29.0%; 51/176), reflecting
the similar expression profile of these antibodies (actual plotting
data not shown). By contrast, the ability of anti-livin antibody to
detect colon cancer was apparently low throughout all stages, which
was consistent with the relatively lower expression of the livin
protein in colon cancer (22).
As described in previous studies (9,14,15,20), the
anti-p53 antibody may also detect colon cancer and we observed a
similar positivity for anti-p53 and anti-survivin antibodies. As
regards the expression profile of anti-survivin and anti-p53
antibodies, the positivity in the combination assay in each stage
of the tumor has not been elucidated. Our study demonstrated the
complete independence in the reactivity of anti-survivin and
anti-p53 antibodies, resulting in the highest positivity rate when
tested together, particularly in early-stage colon cancer (stages
0-I). An advantage in measuring anti-survivin antibody was also
shown for other stages, particularly stage IV, in which we
confirmed a different positivity rate for the anti-survivin
antibody compared to the anti-p53 antibody. These findings are
considered to be consistent with the low correlation between the
two antibodies that was reported in the study by Rohayem et
al (14). On combining with other
well-established tumor markers, CEA and anti-survivin antibody
exhibited the highest positivity among IAPs, reflecting the
significantly high positivity rate of CEA in advanced-stage
tumors.
In the analysis using the patients with colon
adenoma, anti-survivin antibody detected a higher number of
patients compared to the anti-p53 antibody. Taken together with the
results of patients with carcinoma, we established the significance
of measuring anti-survivin antibody for the diagnosis of
early-stage carcinogenesis. As regards application in the clinical
setting, based on the results from patients with colon adenoma and
cancer, the patients exhibiting positivity for anti-survivin
antibody or for the combination of anti-survivin and anti-p53
antibodies should be investigated and treated by endoscopy.
In this study, we analyzed epitopes for their
reaction with anti-survivin antibodies in patients' sera. Previous
studies have identified several splicing variants resulting in
protein deletion or additional protein insertion (23). When autoantibodies in the serum
recognize only limited and specific epitopes, a limited number of
patients with survivin variants may not be detected. Therefore, we
constructed 3 recombinant proteins for exons 1–2, 3 and 4 of
survivin and investigated the reactivity using patients' sera. In
contrast to our predictions, sera from all 6 patients reacted to
all recombinant proteins, indicating that the autoantibodies in the
serum are able to recognize multiple epitopes, but not specific and
limited epitopes. Consequently, we provided the first evidence on
the non-specific reactivity of autoantibodies against survivin,
indicating the universality of our ELISA system.
Acknowledgements
We would like to thank the medical staff of the
Sapporo Kiyota Hospital and the Steel Memorial Muroran Hospital for
the preparation of the patient's sera.
Glossary
Abbreviations
Abbreviations:
|
IAP
|
inhibitor of apoptosis protein
|
|
CEA
|
carcinoembryonic antigen
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
XIAP
|
X-linked IAP
|
Refernces
|
1
|
Gazzaniga P, Gradilone A, Giuliani L,
Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L and Aglianò
AM: Expression and prognostic significance of livin, survivin and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanabe H, Yagihashi A, Tsuji N, Shijubo Y,
Abe S and Watanabe N: Expression of survivin mRNA and livin mRNA in
non-small-cell lung cancer. Lung Cancer. 46:299–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi H, Kim J, Fujimoto A, Umetani N,
Mori T, Bilchik A, Turner R, Tran A, Kuo C and Hoon DS: X-Linked
inhibitor of apoptosis protein expression level in colorectal
cancer is regulated by hepatocyte growth factor/C-met pathway via
Akt signaling. Clin Cancer Res. 11:7621–7628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Augello C, Caruso L, Maggioni M, et al:
Inhibitors of apoptosis proteins (IAPs) expression and their
prognostic significance in hepatocellular carcinoma. BMC Cancer.
9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asanuma K, Tsuji N, Endoh T, Yagihashi A
and Watanabe N: Survivin enhances Fas ligand expression via
up-regulation of specificity protein 1-mediated gene transcription
in colon cancer cells. J Immunol. 172:3922–3929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Endoh T, Tsuji N, Asanuma K, Yagihashi A
and Watanabe N: Survivin enhances telomerase activity via
up-regulation of specificity protein 1- and c-Myc-mediated human
telomerase reverse transcriptase gene transcription. Exp Cell Res.
305:300–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu T, Brouha B and Grossman D: Rapid
induction of mitochondrial events and caspase-independent apoptosis
in Survivin-targeted melanoma cells. Oncogene. 23:39–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopes RB, Gangeswaran R, McNeish IA, Wang
Y and Lemoine NR: Expression of the IAP protein family is
dysregulated in pancreatic cancer cells and is important for
resistance to chemotherapy. Int J Cancer. 120:2344–2352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yagihashi A, Asanuma K, Nakamura M, Araya
J, Mano Y, Torigoe T, Kobayashi D and Watanabe N: Detection of
anti-survivin antibody in gastrointestinal cancer patients. Clin
Chem. 47:1729–1731. 2001.PubMed/NCBI
|
|
10
|
Yagihashi A, Asanuma K, Tsuji N, Torigoe
T, Sato N, Hirata K and Watanabe N: Detection of anti-livin
antibody in gastrointestinal cancer patients. Clin Chem.
49:1206–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yagihashi A, Asanuma K, Kobayashi D, Tsuji
N, Shijubo Y, Abe S, Hirohashi Y, Torigoe T, Sato N and Watanabe N:
Detection of autoantibodies to livin and survivin in Sera from lung
cancer patients. Lung Cancer. 48:217–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yagihashi A, Ohmura T, Asanuma K,
Kobayashi D, Tsuji N, Torigoe T, Sato N, Hirata K and Watanabe N:
Detection of autoantibodies to survivin and livin in sera from
patients with breast cancer. Clin Chim Acta. 362:125–130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagihashi A, Asanuma K, Kobayashi D, Tsuji
N, Torigoe T, Sato N and Watanabe N: Autoantibodies to survivin in
patients with chronic hepatitis and hepatocellular carcinoma.
Autoimmunity. 38:445–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rohayem J, Diestelkoetter P, Weigle B,
Oehmichen A, Schmitz M, Mehlhorn J, Conrad K and Rieber EP:
Antibody response to the tumor-associated inhibitor of apoptosis
protein survivin in cancer patients. Cancer Res. 60:1815–1817.
2000.PubMed/NCBI
|
|
15
|
Koziol JA, Zhang JY, Casiano CA, Peng XX,
Shi FD, Feng AC, Chan EK and Tan EM: Recursive partitioning as an
approach to selection of immune markers for tumor diagnosis. Clin
Cancer Res. 9:5120–5126. 2003.PubMed/NCBI
|
|
16
|
Karanikas V, Khalil S, Kerenidi T,
Gourgoulianis KI and Germenis AE: Anti-survivin antibody responses
in lung cancer. Cancer Lett. 282:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uemura N, Kodama S, Nomi N, Okamoto T and
Suzuki M: Correlation between anti-survivin antibody and survivin
mRNA expression in head and neck cancer patients. Acta Otolaryngol.
130:959–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JS, Chen KT, Fan WC, Yu JS, Chang YS
and Chan EC: Combined analysis of survivin autoantibody and
carcinoembryonic antigen biomarkers for improved detection of
colorectal cancer. Clin Chem Lab Med. 48:719–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lubin R, Schlichtholz B, Teillaud JL,
Garay E, Bussel A and Wild CP: p53 antibodies in patients with
various types of cancer: assay, identification, and
characterization. Clin Cancer Res. 1:1463–1469. 1995.PubMed/NCBI
|
|
20
|
Hammel P, Boissier B, Chaumette MT,
Piedbois P, Rotman N, Kouyoumdjian JC, Lubin R, Delchier JC and
Soussi T: Detection and monitoring of serum p53 antibodies in
patients with colorectal cancer. Gut. 40:356–361. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
pp. 100–109. 2009
|
|
22
|
Ding ZY, Zhang H, Adell G, Olsson B and
Sun XF: Livin expression is an independent factor in rectal cancer
patients with or without preoperative radiotherapy. Radiat Oncol.
8:2812013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taubert H, Kappler M, Bache M, Bartel F,
Kohler T, Lautenschlager C, Blumke K, Wurl P, Schmidt H, Meye A and
Hauptmann S: Elevated expression of survivin-splice variants
predicts a poor outcome for soft-tissue sarcomas patients.
Oncogene. 24:5258–5261. 2005. View Article : Google Scholar : PubMed/NCBI
|