Introduction
Gastric cancer is one of the most common malignant
tumors in the world with a poor prognosis. Although its incidence
and mortality has been decreasing in recent years, it is still the
second leading cause of cancer-related mortality (1,2). In order
to administer and treat gastric cancer optimally, studies on the
prediction for the prognosis and the treatment efficacy of gastric
cancer were focused for a long time. The prognosis of gastric
cancer was affected by numerous aspects, such as gastric cancer
staging, location, histological type, biological behavior and
therapeutic measures. However, a previous study showed that
mutation or gene polymorphism in certain important molecules, such
as epidermal growth factor receptor, human epidermal growth factor
receptor 2 and c-Met, are also involved in the prognostic
prediction (3). However, there are no
commonly accepted prognostic biomarkers that are used clinically.
Therefore, further investigation and analysis is required.
Microsatellite DNA are widespread, short and
repetitive DNA sequences that are randomly distributed in the human
genome (4). When mismatch repair
genes, including hMLH1 and hMSH2, are inactivated,
replication errors, such as insertions or deletions of bases within
microsatellite regions, cannot be repaired. These phenomena are
known as microsatellite instability (MSI). Alternatively, MSI can
also be caused via epigenetic promoter methylation (5,6). For
characterizing and classifying MSI, a panel of five markers for the
analysis of MSI was validated and recommended by the National
Cancer Institute (NCI) in 1997, including two mononucleotide
repeats (BAT-25 and BAT-26) and two dinucleotide repeats (D5S346,
D2S123 and D17S250) (7). If ≥2 of the
five markers show instability, these genotypes are grouped into
high-frequency (MSI-H). When only one marker shows instability,
these genotypes are grouped into low-frequency (MSI-L), and when no
marker shows instability, these are grouped into microsatellite
stable (MSS). As more markers have been found, >5 markers were
used in the detection of MSI, such as DP1, NM23, p53, NR-21 or DCC
microsatellite loci, and the principle has been optimized. MSI-H is
defined as having 30–40% instability markers, while MSI-L is
defined when instability markers are <30–40% (7,8). This is
not the only principle, as Bethesda Guidelines have revised that
MSI-H can be defined when having instability at a mononucleotide
loci and MSI-L is having limited instability at only a dinucleotide
loci, and mononucleotide repeats were shown to be more sensitive
compared to dinucleotide loci in detecting MSI (9–11).
MSI, since being first described in hereditary
nonpolyposis colorectal cancer (CRC) (12), has been found in numerous familial and
sporadic human neoplasms (5). MSI is
proved to be a mostly molecular mechanism involved in
carcinogenesis and development. MSI has been used in the prognosis
of numerous types of cancer. For example, MSI-H is associated with
a good prognosis in CRC, but no significant association was found
between MSI-H and prognosis in early endometrial cancer. MSI-H
forecasted a bad prognosis in breast cancer (13–15). In
gastric cancers, the incidence of MSI-H varies from 8.2–37%, which
was decided by the number of cases investigated. Patients with
gastric cancer and MSI-H tend to be older, female, distal located,
with a well-differentiated adenocarcinoma type and in lower tumor
stages (6,16–22). The
association between MSI-H and gastric cancer prognosis remains
ambiguous. Certain studies support that MSI-H is associated with a
good prognosis (6,18–21,23–25)
while others are conflicting (16,26–28).
Therefore, the present study is a meta-analysis to
identify whether MSI is associated with the prognosis in gastric
cancer by assembling the current opinions and the pooled result.
The pooled analysis showed that patients with MSI-H have a reduced
risk of lymph node (LN) metastasis, tumor invasion and mortality
compared to those with MSI-L/MSS. Furthermore, MSI-H gastric
cancers have an improved prognosis. The present analysis will
provide a whole evaluation for the effect of MSI on the prognosis
of gastric cancer.
Materials and methods
Search strategy
The PubMed electronic database was searched until
January 2014 using MeSH terms and key words. The strategy included
‘microsatellite instability’ or ‘MSI’ or ‘replication error
phenotype’ combined with ‘stomach neoplasm’ or ‘stomach cancer’ or
‘stomach carcinoma’ or ‘gastric neoplasm’ or ‘gastric cancer’ or
‘gastric carcinoma’ combined with ‘prognosis’ or ‘outcome’ or
‘survival’ or ‘DFS’ or ‘OS’. In addition, the references and review
studies were manually searched to identify other relevant studies
to ensure integrity. Primary authors were not contacted.
Eligibility criteria
The search was restricted to human studies that were
published in peer-reviewed journals in English. Studies assessing
the association between MSI and prognosis in gastric cancer with
notable outcomes [overall survival (OS) in gastric cancer with MSI]
were included. Reviews, single case reports, unrelated and
duplicated studies were excluded. Studies that did not allow the
extraction of the hazard ratio (HR) and 95% confidence interval
(CI) were included in the systematic review but excluded from
meta-analysis. Two investigators read the full texts of the
relevant studies and applied the eligibility criteria
independently. Any disagreement was assessed by a third
investigator.
Quality assessment
Currently, a standard assessment for observational
studies is not available. The Newcastle-Ottawa Quality Assessment
Scale (NOS) for case-control studies was used for reference
(29). Quality assessment was
performed by the two independent investigators and any disagreement
was solved by a third investigator.
Data extraction
Two investigators extracted the information from all
the studies independently, including: First author, year of
publication, distribution of clinical pathological factors, such as
tumor stage, Lauren's classification and treatment interventions,
and MSI definition.
Data analysis
Meta-analysis was performed between patients with
MSI+ (MSI-H) and with MSI- (MSI-L/MSS) to explore the association
between MSI and OS. Only the studies that reported HR with 95% CI
were included in the meta-analysis. The HR and 95% CI were
calculated for pooling if not available from the study, using the
total number of events (fatalities and relapse) and numbers at risk
in each group or the Kaplan-Meier survival curves provided
(30). In order to explore the
association between the status of MSI and OS comprehensively, the
association between the MSI status and clinicopathological features
in the eight studies were analyzed. Data were analyzed using Stata
12.0 software (StataCorp, College Station, TX, USA) with a random
effects model. Inter-study heterogeneity was estimated using
I2 statistics. Sensitivity was analyzed by removing
certain studies with low quality. All the statistics were two-sided
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Eligible studies
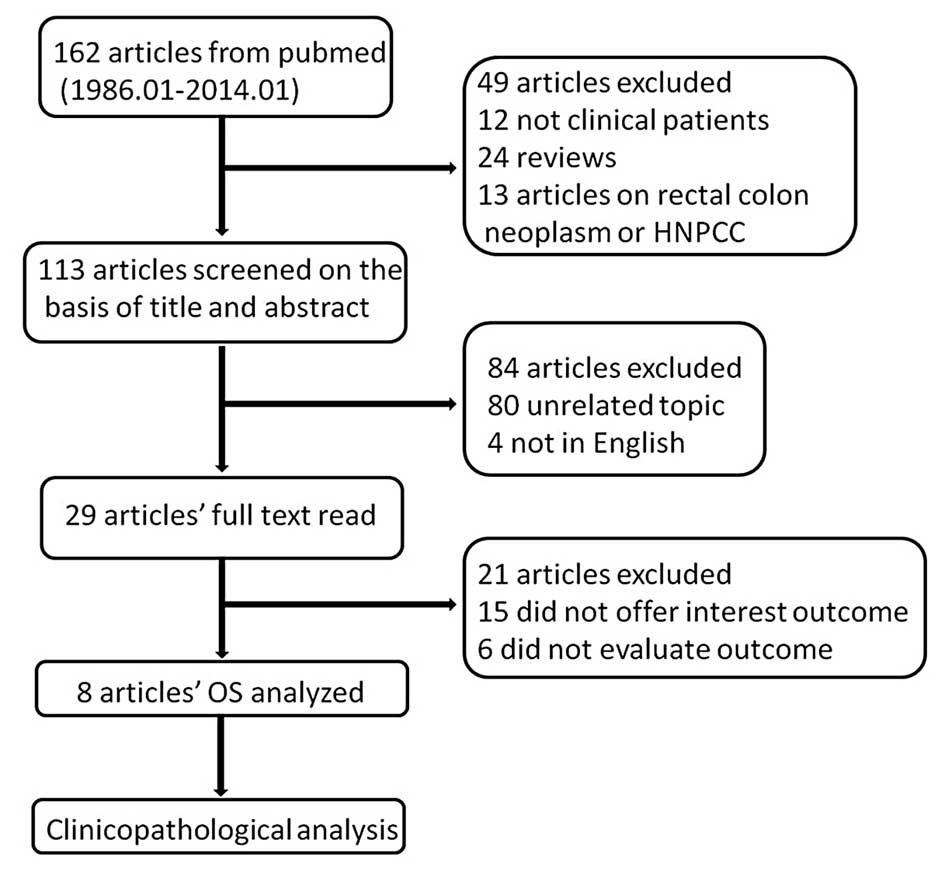
In total, 162 studies were searched using MeSH terms
and key words from the Pubmed database until January 2014. A total
of 24 review studies were removed and 12 were removed for not
assessing clinical patients, while 13 did not analyze gastric
cancer. The title and abstract of the remaining 113 studies were
assessed; however, 80 studies were not regarding the topic between
MSI and gastric cancer prognosis and four were not in English.
Therefore, 29 full texts of potentially eligible studies were read
independently by two investigators, and 15 studies were excluded
for not providing the association between OS and MSI status (MSI-H
and MSI-L/MSS), two were excluded for not reporting HR but reported
the relative risk (RR) of MSI-H compared to MSI-L/MSS, while one
study did not report HR but the odds ratio (OR). Two studies were
excluded for offering the survival curves of MSI-H, MSI-L and MSS
separately so that the HR of MSI-H could not be calculated compared
to MSI-L/MSS and one study offered a rough Kaplan-Meier survival
curve that a HR with an existing error was calculated from, so it
was therefore removed. Finally, there were eight studies used in
the meta-analysis; three of these were manually calculated
(30). There was a good agreement
between the two investigators (Fig.
1).
Characteristics of the studies
The characteristics of the studies are summarized in
the Table I. In total, 1,976 patients
were analyzed, of which there were 431 MSI-H patients, range
11.68–33.82%. Two studies collected the data prospectively. The
follow-up period ranged 1–260.9 months. The majority of the
patients had adenocarcinoma and advanced gastric cancer. More than
half the patients had intestinal type according to the Lauren
classification. Almost all the patients received surgery (Table I).
 | Table I.Characteristics of each study in the
meta-analysis. |
Table I.
Characteristics of each study in the
meta-analysis.
| Study (year) | No. of patients | No. of MSI-H, % | Period of follow-up,
month | Stage distribution,
n | No. of intestinal
type | Treatment
strategy | No. of MSI
marker | MSI-H defination | (Refs.) |
|---|
| Fang et al
(2012) | 214 | 11.7 | 1–243 | Early, 44 Advanced,
170 | 134 | Surgery II/III;got
adjuvant CT | 5 | ≥2 | (6) |
| Corso et al
(2009) | 250 | 25.2 | 36–260.9 | Early, 183 Advanced,
67 | 163 | Surgery; no
neoadjuvant CT | 5 | ≥2 | (18) |
| Chiaravalli et
al (2001) | 185 | 19.5 | NA | All advanced | 108 | Surgery; no
neoadjuvant CT | 3, all
mono-neoadjuvant | ≥1 | (31) |
| Beghelli et al
(2006) | 510 | 16.3 | Until July 2004 | Early, 64 Advanced,
446 | 286 | Surgery; no
neoadjuvant CT | 2, all
mono-nucleotide | ≥1 | (21) |
| Kim et al
(2011) | 476 | 33.8 | 1–57 | Early, 166
Advanced, 310 | 289 | Surgery | 5 | ≥2 | (20) |
| Falchetti et
al (2008) | 159 | 17.0 | 8.8–20.4 | Early, 15 Advanced,
144 | 77 | Surgery | 8 | ≥1
mono-nucleotide | (19) |
| Hayden et al
(1997) | 101 | 20.8 | NA | Early, 15 Advanced,
66 | 75 | Surgery | 11 | ≥1 | (24) |
| An et al
(2005) | 81 | 18.5 | 47–57.7 | Early, 14 Advanced,
67 | 53 | Surgery | 5 | ≥2 | (28) |
MSI analysis
A variety of MSI markers were used in the assessment
of the MSI status within the meta-analysis. Four studies analyzed
the MSI-H status using ≥2 loci of five markers showing instability
(6,9,18,20). Two studies used only mononucleotide
markers (21,31); in which ≥1 loci showed instability,
and was defined as MSI-H. The other two studies used 8 and 11
markers, respectively (19,24). Tumors with MSI-L and MSS showed
similar clinicopathological characteristics, so therefore, these
two types were classified together to compare to the tumors with
MSI-H.
Survival analysis
Eight studies provided the OS data for pooling. The
HR and 95% CI for each study and the summarized HR are shown in
Fig. 2. The estimate pooled was HR,
0.63 (95% CI, 0.52–0.77), with no evidence of heterogeneity
(I2=0.0%). The funnel plot is shown in Fig. 3, and a little asymmetry can be
observed. This result was verified by the quantified evaluation
using Begg's test (P=0.063), therefore no evident publication bias
was discovered in these studies. The studies by Hayden et al
(24) and Beghelli et al
(21) had clear differences in the
definition of MSI-H, so therefore these were removed when
evaluating sensitivity; the outcome changed respectively into HR,
0.62 (95% CI, 0.50–0.75) and HR, 0.66 (95% CI, 0.51–0.85), with no
evidence of heterogeneity (I2=0.0 and 8.6%,
respectively) and the funnel plot showed a little more symmetry
than previously.
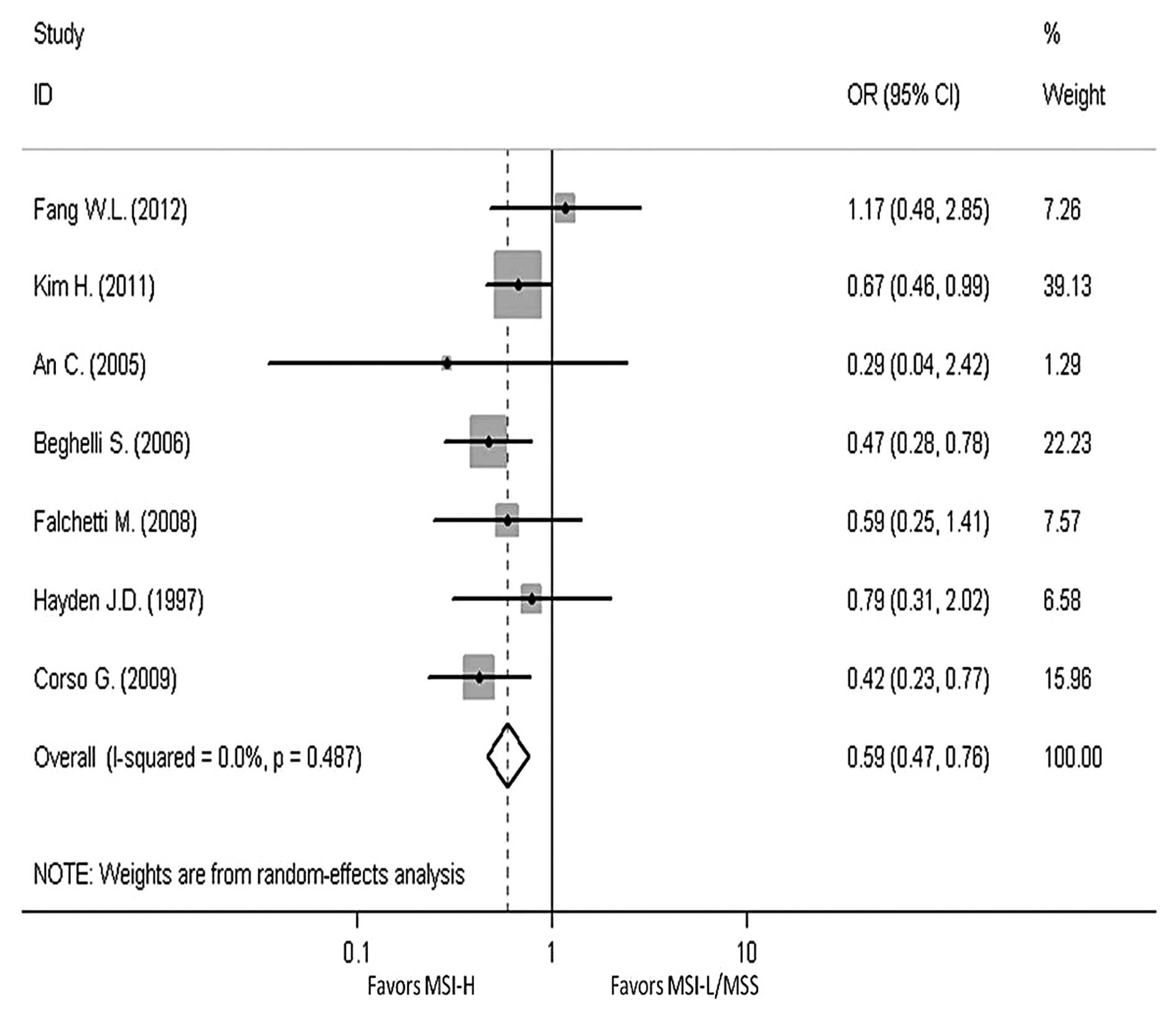
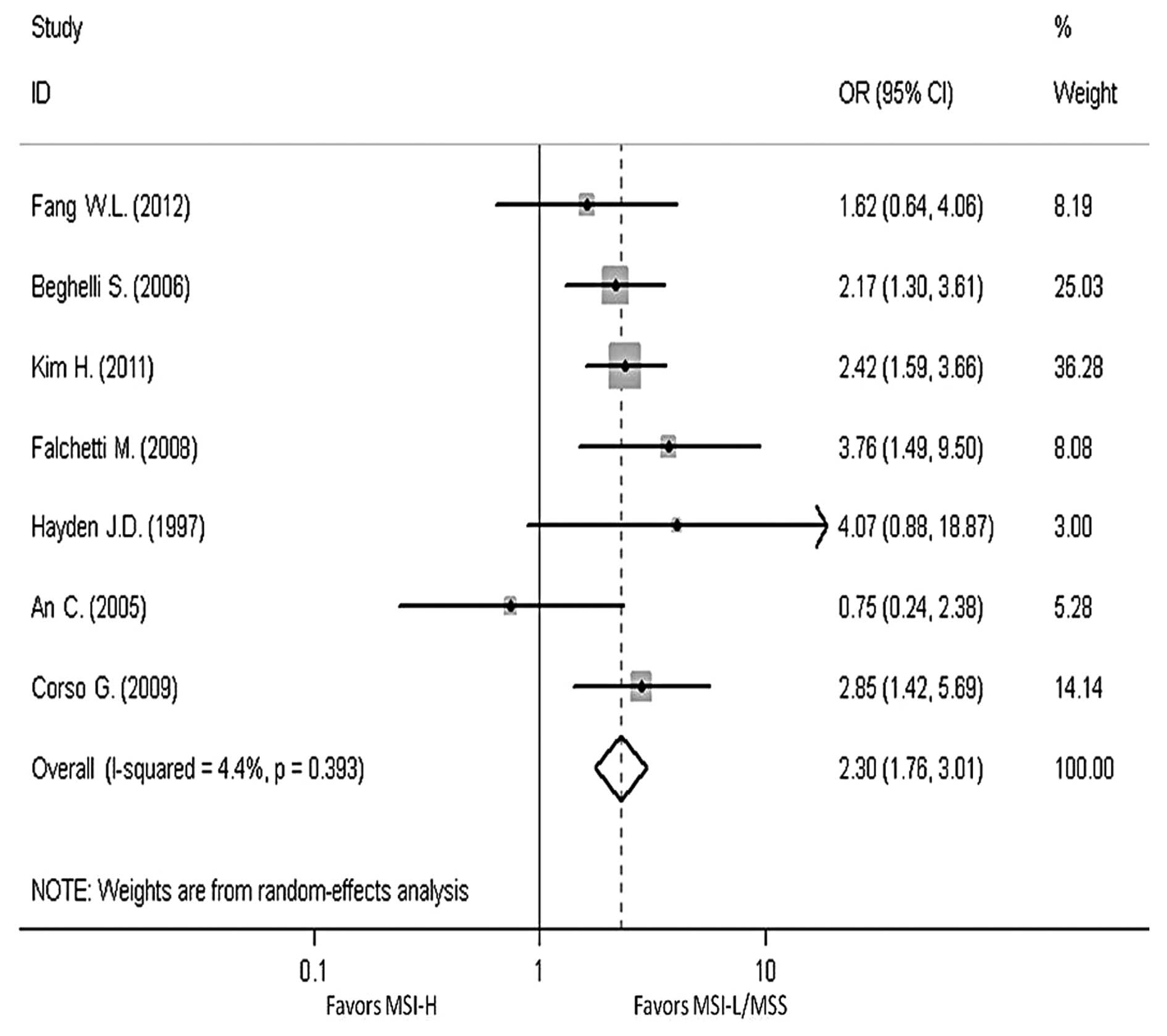
In order to reveal the association, the
clinicopathological characters, such as LN metastasis, tumor
invasion, tumor-node-metastasis (TNM) stage and Lauren
classification, have been analyzed. The pooled OR in LN metastasis
that was positive for MSI-H compared to MSI-L/MSS is 0.59 (95% CI,
0.47–0.76), the pooled OR in T3-T4 invasion is 0.5 (95% CI,
0.39–0.64) and the pooled OR in II–IV stage in TNM phase is 0.7
(95% CI, 0.39–1.24). The pooled OR in intestinal type is 2.3 (95%
CI, 1.76–3.01). All the results have no evident heterogeneity
(Figs. 4–7).
Discussion
Mismatch repair deficiency is an important molecular
mechanism in carcinogenesis and has a clear effect on prognosis. In
CRC patients, cancers with MSI-H have an improved prognosis and a
worse sensitiveness to 5-fluorouracil (5-FU)-based chemotherapy
compared to MSI-L/MSS cancer (32).
While in gastric cancer, the clinical significance remains
controversial. All the studies regarding the effect of MSI status
on prognosis in PubMed were collected and the information was
obtained from 1,976 patients enrolled. The result showed that
patients with MSI-H had a reduced 37% mortality risk compared to
those with MSI-L/MSS, indicating that MSI-H patients have an
improved OS compared to MSI-L/MSS patients, in accordance with CRC.
In addition, the RR of another two studies excluded in the present
meta-analysis were 0.47 (1.23–0.18) and 1.94 (MSI-L vs. MSI-H)
(18,33), while one OR was 0.50 (0.27–0.93)
(34); these studies also supported
an improved OS outcome for MSI-H cancer.
Compared to the previous research, MSI-H patients
have a long survival in gastric cancer. For investigating the
association between MSI-H and a superior prognosis, the information
was repeatedly analyzed and identified that one study (24) used only the English population; this
study was carried out in 1997, which is much earlier than the other
studies. Furthermore, the markers in this study are extremely
different to the other studies (11 markers in which ≥1 was showing
instability and can be classified as MSI+), therefore the study was
excluded. When the study was excluded, the conclusion did not
change and the funnel plot became a little more symmetrical than
previously. The reduced publication bias was also confirmed by
Begg's test (P=0.23). The sample in this study is small (101
patients) and the weight is 2.03, which leads to the meta-analysis
having little sensitivity to the study. Subsequently, another study
was removed that had a different MSI-H definition and the weight
was a little heavier (21); the
conclusion and the publication bias did not change. Therefore,
MSI-H patients have an improved prognosis in gastric cancer. This
conclusion can possibly indicate that MSI-H patients may have an
improved prognosis compared to MSI-L. With this conclusion, the
concrete reflection of the different prognoses is required.
Subsequently, the clinicopathological features of the different
patients were analyzed and it was identified that those with MSI-H
appeared to have a smaller risk in LN metastasis and T invasion.
These patients were also inclined to have the intestinal type. Less
LN metastasis, shallower T invasion and intestinal type are known
to predict a good prognosis, so this is possibly the reason why
patients of MSI-H have a long OS. As a result, increasing attention
should be paid to the MSI-L gastric cancer patient by administering
a stronger treatment.
In contrast to the patients with MSI-L/MSS cancer,
MSI-H patients in CRC had an improved prognosis, as well as a worse
sensitivity to 5-FU-based chemotherapy. This phenomenon indicates
that the MSI-H patients with gastric cancer may also have the same
characteristic. Therefore, the research regarding the medicinal
sensitiveness between MSI-H and MSI-L is extremely necessary, which
may guide the physician to improve the choice of the chemotherapy
treatment. However, there is little research regarding the
medicinal sensitiveness of MSI-H patients in gastric cancer.
The present meta-analysis has certain limitations.
First, the majority of studies are retrospective and cannot be
controlled manually leading to certain selective bias. Therefore,
the random effects model was used. Second, there is not a unified
standard for the evaluation of observational study, so NOS was used
for reference. In addition, the patients analyzed have a variety of
different parameters, including pathology, stage, location,
treatment strategy and MSI-H definition. Four studies used the NCI
panel and three studies used ≥1 mononucleotide loci, while the
remaining study used ≥1 loci that did not limit the type of loci;
this study was removed to conduct a sensitivity analysis and the
result did not show a significant difference. Eight studies were
pooled, only two of which provided the association between the MSI
status and OS information in the tumors of different stage.
Therefore, no pooled conclusion regarding the MSI status and OS in
stages I–IV was achieved.
The pooling of studies is not sufficient, so a large
sample clinical trial focusing on prognosis and prediction is
required, in which the independent outcome in different stages or
different treatment strategies should be stated. A convenient and
economical MSI detection method and evaluation standard of
observational study should be unified.
Acknowledgements
This study was supported by National Science and
Technology Major Project (grant no. 2013ZX09303002) and the Science
and Technology Plan Project of Liaoning Province (grant nos.
2011404013-1 and 2012225001).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chau I, Norman AR, Cunningham D, Waters
JS, Oates J and Ross PJ: Multivariate prognostic factor analysis in
locally advanced and metastatic esophago-gastric cancer - pooled
analysis from three multicenter, randomized, controlled trials
using individual patient data. J Clin Oncol. 22:2395–2403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida K, Yasui W, Yokozaki H, et al: New
molecular prognostic markers in gastric carcinoma. Gan To Kagaku
Ryoho. 25:2021–2027. 1998.(In Japanese). PubMed/NCBI
|
|
4
|
Leung WK, Kim JJ, Kim JG, Graham DY and
Sepulveda AR: Microsatellite instability in gastric intestinal
metaplasia in patients with and without gastric cancer. Am J
Pathol. 156:537–543. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arzimanoglou II, Gilbert F and Barber HR:
Microsatellite instability in human solid tumors. Cancer.
82:1808–1820. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang WL, Chang SC, Lan YT, et al:
Microsatellite instability is associated with a better prognosis
for gastric cancer patients after curative surgery. World J Surg.
36:2131–2138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boland CR, Thibodeau SN, Hamilton SR, et
al: A National Cancer Institute Workshop on Microsatellite
Instability for cancer detection and familial predisposition:
development of international criteria for the determination of
microsatellite instability in colorectal cancer. Cancer Res.
58:5248–5257. 1998.PubMed/NCBI
|
|
8
|
Shemirani AI, Haghighi MM, Zadeh SM, et
al: Simplified MSI marker panel for diagnosis of colorectal cancer.
Asian Pac J Cancer Prev. 12:2101–2104. 2011.PubMed/NCBI
|
|
9
|
Brennetot C, Buhard O, Jourdan F, Flejou
JF, Duval A and Hamelin R: Mononucleotide repeats BAT-26 and BAT-25
accurately detect MSI-H tumors and predict tumor content:
implications for population screening. Int J Cancer. 113:446–450.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umar A, Boland CR, Terdiman JP, et al:
Revised Bethesda Guidelines for hereditary nonpolyposis colorectal
cancer (Lynch syndrome) and microsatellite instability. J Natl
Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baudhuin LM, Burgart LJ, Leontovich O and
Thibodeau SN: Use of microsatellite instability and
immunohistochemistry testing for the identification of individuals
at risk for Lynch syndrome. Fam Cancer. 4:255–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aaltonen LA, Peltomaki P, Leach FS, et al:
Clues to the pathogenesis of familial colorectal cancer. Science.
260:812–816. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guastadisegni C, Colafranceschi M, Ottini
L and Dogliotti E: Microsatellite instability as a marker of
prognosis and response to therapy: a meta-analysis of colorectal
cancer survival data. Eur J Cancer. 46:2788–2798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paulson TG, Wright FA, Parker BA, Russack
V and Wahl GM: Microsatellite instability correlates with reduced
survival and poor disease prognosis in breast cancer. Cancer Res.
56:4021–4026. 1996.PubMed/NCBI
|
|
15
|
Diaz-Padilla I, Romero N, Amir E, et al:
Mismatch repair status and clinical outcome in endometrial cancer:
a systematic review and meta-analysis. Crit Rev Oncol Hematol.
88:154–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oki E, Kakeji Y, Zhao Y, et al:
Chemosensitivity and survival in gastric cancer patients with
microsatellite instability. Ann Surg Oncol. 16:2510–2515. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo HM, Chang YS, Joo SH, et al:
Clinicopathologic characteristics and outcomes of gastric cancers
with the MSI-H phenotype. J Surg Oncol. 99:143–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corso G, Pedrazzani C, Marrelli D, Pascale
V, Pinto E and Roviello F: Correlation of microsatellite
instability at multiple loci with long-term survival in advanced
gastric carcinoma. Arch Surg. 144:722–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falchetti M, Saieva C, Lupi R, et al:
Gastric cancer with high-level microsatellite instability: target
gene mutations, clinicopathologic features, and long-term survival.
Hum Pathol. 39:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H, An JY, Noh SH, Shin SK, Lee YC and
Kim H: High microsatellite instability predicts good prognosis in
intestinal-type gastric cancers. J Gastroenterol Hepatol.
26:585–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beghelli S, de Manzoni G, Barbi S, et al:
Microsatellite instability in gastric cancer is associated with
better prognosis in only stage II cancers. Surgery. 139:347–356.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
An JY, Kim H, Cheong JH, Hyung WJ, Kim H
and Noh SH: Microsatellite instability in sporadic gastric cancer:
its prognostic role and guidance for 5-FU based chemotherapy after
R0 resection. Int J Cancer. 131:505–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
dos Santos NR, Seruca R, Constancia M,
Seixas M and Sobrinho-Simoes M: Microsatellite instability at
multiple loci in gastric carcinoma: clinicopathologic implications
and prognosis. Gastroenterology. 110:38–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden JD, Cawkwell L, Quirke P, et al:
Prognostic significance of microsatellite instability in patients
with gastric carcinoma. Eur J Cancer. 33:2342–2346. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiaravalli AM, Feltri M, Bertolini V, et
al: Intratumour T cells, their activation status and survival in
gastric carcinomas characterised for microsatellite instability and
Epstein-Barr virus infection. Virchows Arch. 448:344–353. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perez RO, Jacob CE, D'Ottaviano FL, et al:
Microsatellite instability in solitary and sporadic gastric cancer.
Rev Hosp Clin Fac Med Sao Paulo. 59:279–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wirtz HC, Muller W, Noguchi T, et al:
Prognostic value and clinicopathological profile of microsatellite
instability in gastric cancer. Clin Cancer Res. 4:1749–1754.
1998.PubMed/NCBI
|
|
28
|
An C, Choi IS, Yao JC, et al: Prognostic
significance of CpG island methylator phenotype and microsatellite
instability in gastric carcinoma. Clin Cancer Res. 11:656–663.
2005.PubMed/NCBI
|
|
29
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiaravalli AM, Cornaggia M, Furlan D, et
al: The role of histological investigation in prognostic evaluation
of advanced gastric cancer. Analysis of histological structure and
molecular changes compared with invasive pattern and stage.
Virchows Arch. 439:158–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carethers JM, Smith EJ, Behling CA, et al:
Use of 5-fluorouracil and survival in patients with
microsatellite-unstable colorectal cancer. Gastroenterology.
126:394–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grogg KL, Lohse CM, Pankratz VS, Halling
KC and Smyrk TC: Lymphocyte-rich gastric cancer: associations with
Epstein-Barr virus, microsatellite instability, histology, and
survival. Mod Pathol. 16:641–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang WL, Chang SC, Lan YT, et al:
Molecular and survival differences between familial and sporadic
gastric cancers. Biomed Res Int. 2013:3962722013. View Article : Google Scholar : PubMed/NCBI
|