Introduction
Hepatocellular carcinoma (HCC) is a common cancer
and the third most frequent cause of cancer-related mortalities
worldwide (1,2). The prognosis of HCC patients remains
poor even following curative treatment, owing to the high incidence
of post-treatment recurrence. Additionally, no effective
chemotherapy regimens have been established for treating HCC.
Furthermore, mechanisms by which HCC recurs are not fully
understood (3). To improve the
prognosis of HCC patients, the mechanism underlying HCC recurrence
requires clarification and new, effective therapies based on that
mechanism require development.
Disseminated tumor cells (DTCs) represent the
beginning of systemic disease arising from localized cancer
(4,5).
Over the past 20 years, evidence has been accumulating that the
presence of DTCs in bone marrow (BM) is a prognostic indicator for
patients with various types of cancer. However, recent studies
indicate that all the patients with DTCs do not necessarily develop
recurrence and/or metastasis (6,7). In a
recent study we demonstrated that DTCs are observed in BM even in
patients with early-stage gastric cancer (8). These findings indicate that DTCs from
different individuals may have varying characteristics that
influence the capacity to develop cancer recurrence and/or
metastasis.
microRNAs (miRNAs) constitute a class of small
non-coding RNAs that function as a novel class of global,
post-transcriptional gene regulators by binding to partially
complementary sequences in the 3′ untranslated regions of
downstream target mRNAs (9–11). Studies have indicated that miRNAs
determine cancer metastasis and recurrence through regulating
multiple target genes in numerous types of cancer, including HCC
(12–15). Furthermore, our recent study showed
that miRNA profiles in DTCs in BM differ significantly between
colorectal cancer patients with and without metastasis and that the
expression level of specific miRNAs correlates significantly with
malignant potential and long-term prognosis (16).
These findings led us to consider that an
investigation of the miRNA expression profiles in DTCs from the BM
of HCC patients may be useful to identify the mechanisms of HCC
recurrence and metastasis based on miRNA-regulated DTC
characteristics, which will in turn lead to the development of
novel, mechanism-dependent effective therapies. To clarify the
mechanism underlying postoperative HCC recurrence, BM samples were
prospectively collected from preoperative HCC patients and their
miRNA expression profiles were analyzed. Using patient clinical
data, an attempt was made to identify the miRNA expression pattern
responsible for postoperative recurrence by comparing miRNA
expression profiles from the BM of patients with and without
postoperative recurrence.
Materials and methods
Cell lines
The human HCC cell lines PLC/PRF/5, HuH7, HLE and
HLF were obtained from the Japan Cancer Research Resources Bank
(Tokyo, Japan). These cells were maintained in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified
incubator with 5% CO2.
HCC patients
The study enrolled nine HCC patients who had
undergone curative hepatic resection for HCC at the Department of
Surgery, Osaka University Hospital (Osaka, Japan) between May 2010
and August 2010. The patient characteristics are shown in Table I. Curative resection was defined as
the complete removal of all the macroscopically evident tumors. All
the patients were postoperatively followed at regular intervals of
3–4 months to check for postoperative recurrences. The
Institutional Review Board of Osaka University Hospital approved
the study protocol (IRB #09142) and a signed consent form was
obtained from each patient.
 | Table I.Characteristics of the HCC patients
enrolled in the study. |
Table I.
Characteristics of the HCC patients
enrolled in the study.
| No. | Gender | Age, years | Risk factor | Child-Pugh
classification | Multiplicity | Maximum tumor size,
cm | Tumor thrombus | Postoperative
recurrencea |
|---|
| 1 | M | 37 | HBV | A | Solitary | 1.1 | – | – |
| 2 | F | 69 | HBV | A | Solitary | 2.0 | – | + |
| 3 | M | 70 | HCV | A | Multiple | 1.7 | + | – |
| 4 | M | 72 | HCV | B | Solitary | 3.0 | – | – |
| 5 | M | 65 | – | A | Multiple | 5.0 | – | + |
| 6 | M | 60 | HCV | A | Solitary | 0.9 | – | – |
| 7 | M | 40 | HBV | A | Multiple | 8.0 | + | + |
| 8 | M | 76 | – | A | Solitary | 1.8 | – | + |
| 9 | M | 47 | HBV, HCV | A | Multiple | 3.5 | – | – |
Drugs
5-Fluorouracil (5-FU) and cisplatin (CDDP) were
kindly supplied by Kyowa Hakko Kogyo Co., Ltd., (Tokyo, Japan) and
Pfizer Japan, Inc. (Tokyo, Japan), respectively.
BM samples
BM was aspirated under general anesthesia
immediately before surgery, as previously described (17). The BM aspirate was obtained from the
ilium of each patient using a BM aspiration needle. The mononuclear
cell fraction was isolated and RNA was extracted from the BM
sample.
Magnetic-activated cell sorting
(MACS)
Cell sorting was performed as previously described
(16,18). Briefly, the mononuclear cell fraction
was isolated using density-gradient centrifugation. Each fraction
was separated by MACS. First, cluster of differentiation 14
(CD14)-allophycocyanin and CD45-fluorescein isothiocyanate
antibodies (Miltenyi Biotec, Auburn, CA, USA) were applied to
detect human monocytes and macrophages, respectively, and the
sorted cells were removed as host-derived cells. Subsequently,
using the epithelial cell adhesion molecule (EpCAM)-phycoerythrin
antibody (Miltenyi Biotec.), EpCAM-positive cells were sorted from
the remaining fraction and used as viable epithelial tumor
cells.
Transfection
The oligonucleotide hsa-miR-615-3p
(pre-miR-615-3p) and the antisense oligonucleotide inhibitor
of hsa-miR-615-3p (anti-miR-615-3p) were purchased
from Ambion Inc., (Austin, TX, USA). Pre/anti-miR-615-3p
were transfected using Lipofectamine® RNAiMAX (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
Each scrambled oligonucleotide was transfected in the same way as a
matched negative control.
RNA extraction
Total RNA was isolated from cell lines and BM
samples with Trizol reagent (Invitrogen) as previously described
(19). RNA quality was assessed with
a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies,
Wilmington, DE, USA) at 260 and 280 nm wavelengths.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for miRNA expression
RT was performed with the TaqMan MicroRNA RT kit
(Applied Biosystems, Foster City, CA, USA) and real-time RT-qPCR
was performed with TaqMan MicroRNA assays (Applied Biosystems)
using the ABI7900HT system (Applied Biosystems). The expression of
the target miRNA was normalized relative to that of the endogenous
control, RNU48. Data were analyzed according to the comparative Ct
method (20).
RT-qPCR for mRNA expression
RT was performed as previously described (21). Real-time RT-qPCR was performed using
designed oligonucleotide primers and the LightCycler 480 Real-Time
PCR system (Roche Diagnostics, Mannheim, Germany). To detect the
amplification products, the LightCycler-DNA master SYBR-green I
(Roche Diagnostics) was used as described previously and the amount
of target gene expression was calculated (22). The expression of the target gene was
normalized relative to the expression of GAPDH, which was
used as an endogenous control. The designed PCR primers were as
follows: E-cadherin forward, 5′-TGCCCAGAAAATGAAAAAGG-3′; and
reverse primer, 5′-GTGTATGTGGCAATGCGTTC-3′; N-cadherin forward,
5′-TGAAACGCCGGATAAAGAACG-3′; and reverse primer,
5′-TGCTGCAGCTGGCTCAAGTCAT-3′; vimentin forward,
5′-TCTGGATTCACTCCCTCTGG-3′; and reverse primer,
5′-TGCACTGAGTGTGTGCAATTT-3′; fibronectin forward,
5′-CAGTGGGAGACCTCGAGAAG-3′; and reverse primer,
5′-TCCCTCGGAACATCAGAAAC-3′; and GAPDH forward,
5′-GTCGGAGTCAACGGATTTGGT-3′; and reverse primer,
5′-GCCATGGGTGGAATCATATTGG-3′.
miRNA microarray analysis
Extracted total RNA was labeled with Hy5 using the
miRCURY LNA Array miR labeling kit (Exiqon, Vedbæk, Denmark). The
labeled RNAs were hybridized onto 3D-Gene Human microRNA Oligo
chips containing 941 antisense probes printed in duplicate spots
(Toray, Kamakura, Japan). The annotation and oligonucleotide
sequences of the probes conformed to the miRBase microRNA database
(http://www.mirbase.org/). Following stringent
washes, fluorescent signals were scanned with the ScanArray Express
Scanner (PerkinElmer, Waltham, MA, USA) and analyzed using GenePix
Pro version 5.0 (Molecular Devices, Sunnyvale, CA, USA). The raw
data for each spot were normalized by substitution with a mean
intensity of the background signal, which was determined by the
signal intensities of all the blank spots with 95% confidence
intervals. Measurements of the duplicate spots with signal
intensities greater than two standard deviation (SD) of the
background signal intensity were considered valid. The relative
expression level of miRNA was calculated by comparing the signal
intensities of the averaged valid spots with their mean value
throughout the microarray experiments following normalization by
their equivalently adjusted median values.
Cell proliferation assay
Cells were uniformly seeded (3×103/well)
in triplicate into 96-well dishes (day 0). Cells were counted using
the Cell Counting kit-8 (Dojindo, Kumamoto, Japan) on days 1–3.
Plate absorbance was measured in a microplate reader at 450 nm.
Growth inhibition assay
The growth inhibition assay was performed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
(Sigma-Aldrich Co., St. Louis, MO, USA) assay, as described
previously (23). Briefly, cells were
incubated for 72 h in several concentrations of 5-FU and CDDP.
After re-incubation for 4 h with MTT solution, an acid-isopropanol
mixture was added to dissolve the resulting formazan crystals.
Plate absorbance was measured in a microplate reader at a
wavelength of 550 nm with a 650 nm reference and the results were
expressed as a percentage of absorbance relative to that of the
untreated controls.
Invasion assay
The invasion assay was performed using transwell
culture chambers (BD Biosciences, Bedford, MA, USA) according to
the manufacturer's instructions. The upper chamber was loaded with
cell suspension and the lower chamber was loaded with 10% FBS.
After a 24-h incubation, cells that had invaded the undersurface of
the membrane were counted under a microscope. Three microscopic
fields were randomly selected for cell counting.
Statistical analysis
Data are presented as mean ± SD. Between-group
differences were assessed using the χ2 test and
continuous variables were compared using Student's t-test.
Statistical analysis was performed using JMP software version 9.0.2
(SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-615-3p expression is higher in the
recurrence group compared to the non-recurrence group
Previously, we reported that postoperative HCC
metastasis is represented by recurrence within a 2-year
postoperative period (24). During 2
years of observation after surgery, postoperative HCC recurrence
was identified in four of nine patients (the recurrence group); the
five patients without recurrence were termed the non-recurrence
group. To investigate the miRNA expression profiles in DTCs and
their regulation of postoperative HCC recurrence, the miRNA from
DTCs isolated preoperatively from the BM of the recurrence group
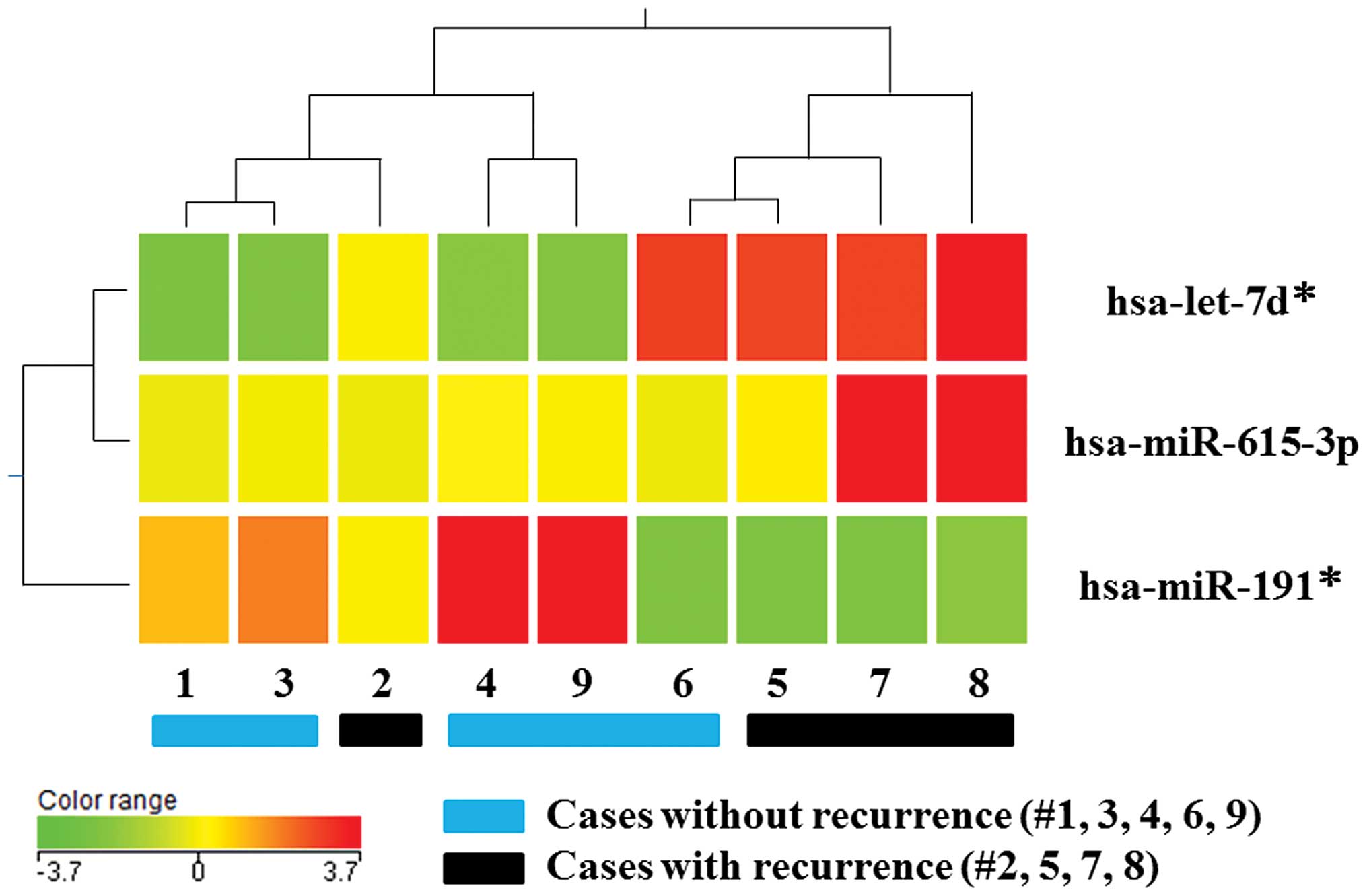
was compared against the non-recurrence group. Cluster analysis
revealed distinct miRNA expression profiles between the two groups;
the expression levels of three out of 941 miRNAs (0.3%) exhibited a
mean change of >1.5-fold in the recurrence group compared to the
non-recurrence group (Fig. 1). When
including adequate expression quantities and excluding miRNA*s,
miR-615-3p was identified as a candidate miRNA in DTCs
regulating HCC recurrence. Subsequently, further in vitro
investigations were performed focusing on the effect of
miR-615-3p on malignant characteristics of HCC cells.
miR-615-3p alters proliferative
activity
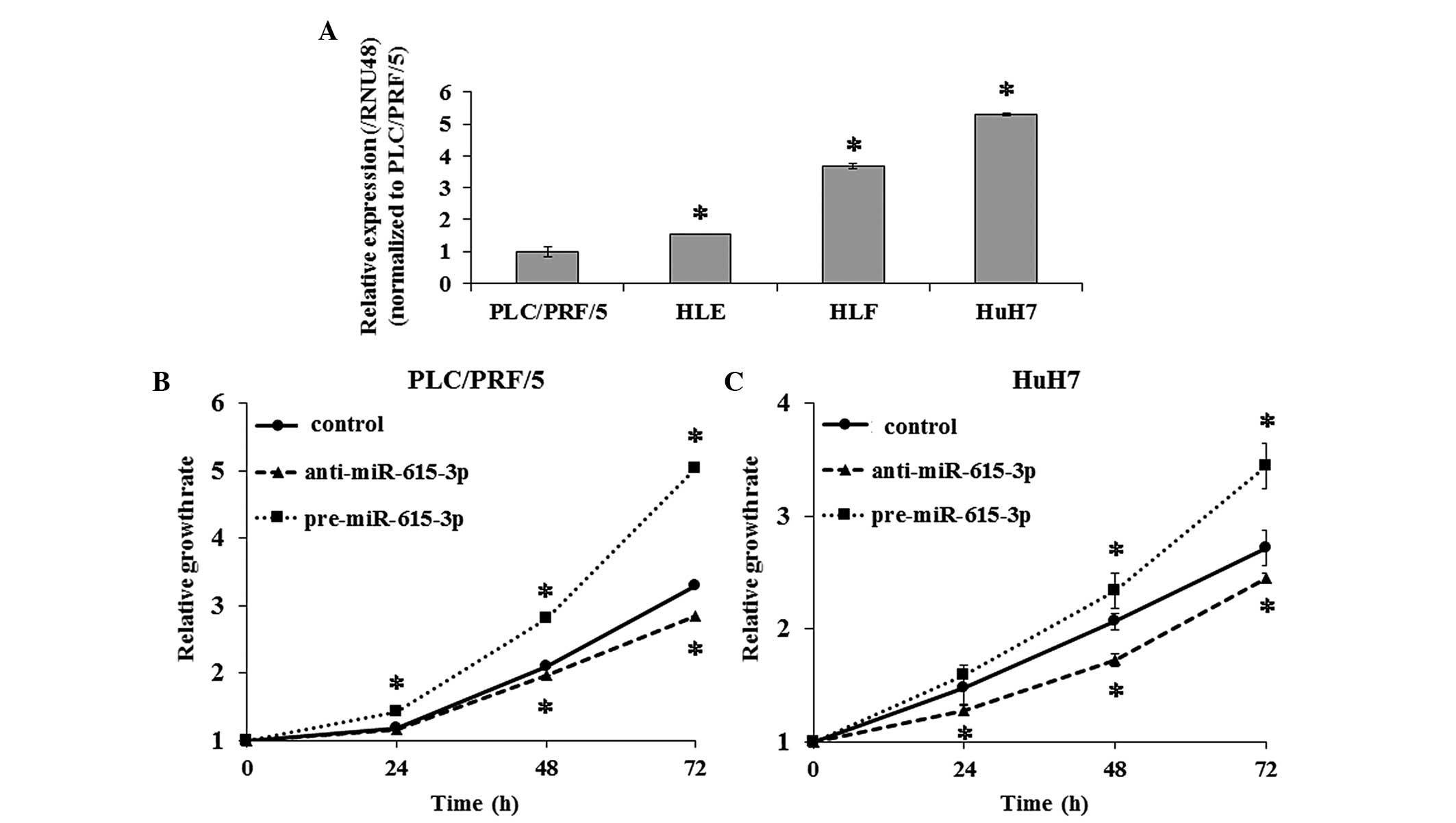
To evaluate the effect of miR-615-3p on cell
proliferation, pre-miR-615-3p and anti-miR-615-3p
were transfected into PLC/PRF/5 and HuH7 cells, which exhibited the
lowest and highest expression of miR-615-3p among the four
HCC cell lines, respectively (Fig.
2A). RT-qPCR confirmed that miR-615-3p expression was
significantly increased and decreased in cells transfected with
pre-miR-615-3p and anti-miR-615-3p, respectively. The
cell proliferation assay demonstrated that PLC/PRF/5 and HuH7 cells
overexpressing miR-615-3p were significantly more
proliferative compared to the controls and that
miR-615-3p-suppressed cells exhibited decreased cell
proliferation compared to the controls (Fig. 2B and C).
miR-615-3p induces resistance to
chemotherapeutic agents
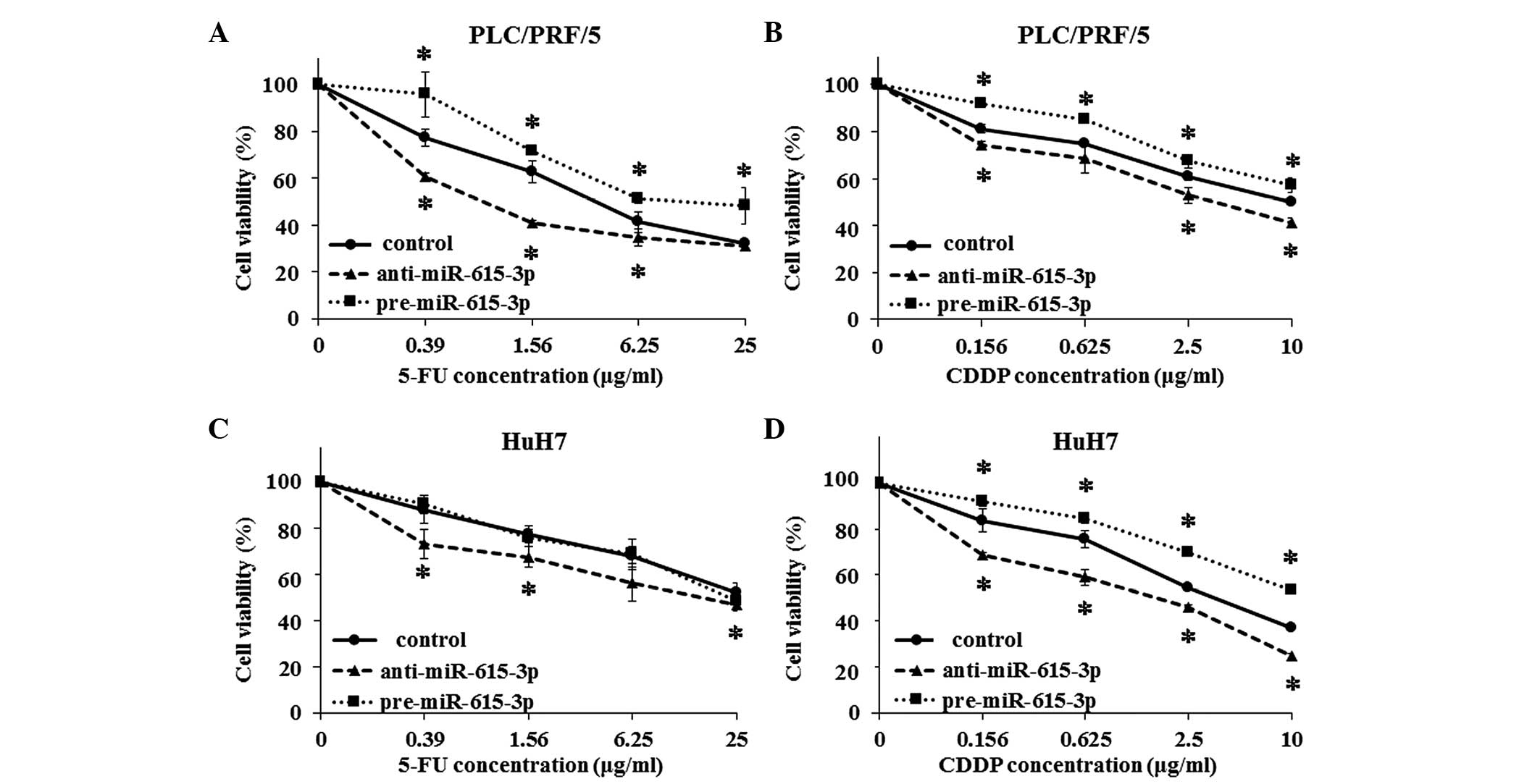
The growth inhibition assay was also used to examine
the resistance to chemotherapeutic agents, such as 5-FU and CDDP.
PLC/PRF/5 cells transfected with pre-miR-615-3p and
anti-miR-615-3p were significantly more and less resistant
to the two drugs, respectively, compared to the control cells
(Fig. 3A and B). HuH7 cells exhibited
a pattern similar to that of PLC/PRF/5 cells, although
miR-615-3p-overexpressing HuH7 cells did not show more
resistance compared to the control cells (Fig. 3C and D).
Expression level of miR-615-3p is
associated with epithelial-mesenchymal transition (EMT)
The EMT, a highly conserved developmental program
activated during mesoderm formation and neural crest development,
is indicated in promoting the dissemination of malignant cells from
primary tumors (25). In this
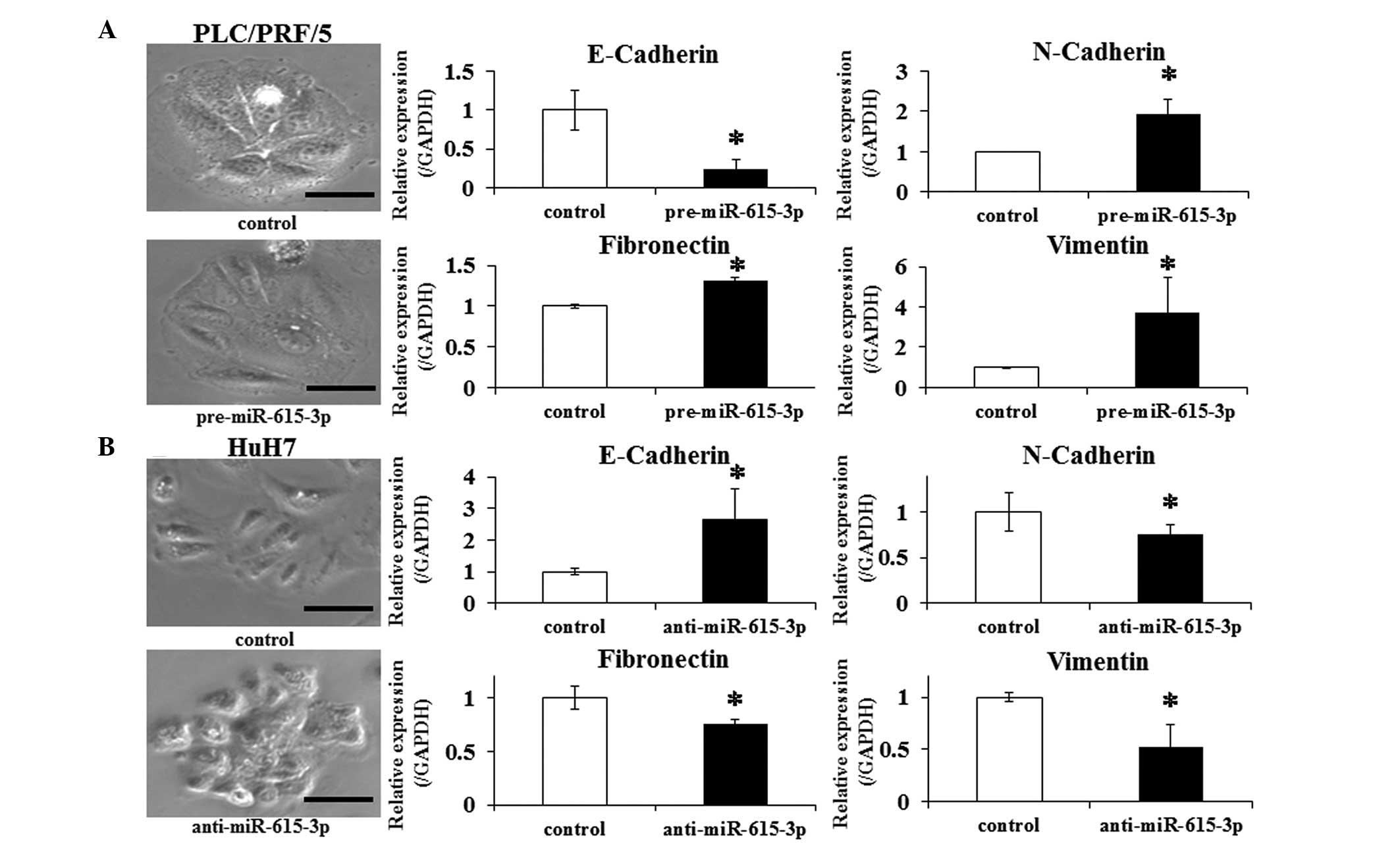
context, whether changing the miR-615-3p expression affects
EMT in HCC cells was investigated. PLC/PRF/5 cells were transfected
with pre-miR-615-3p and HuH7 cells with
anti-miR-615-3p. Control PLC/PRF/5 cells exhibited a
well-defined epithelial polygonal shape, while
pre-miR-615-3p-transfected PLC/PRF/5 cells exhibited a clear
morphological change characterized by a spindle-like fibroblastoid
appearance (Fig. 4A). Consistent with
this finding, a significantly greater expression of N-cadherin,
fibronectin and vimentin, and lower expression of E-cadherin was
observed in the transfected cells compared to the control cells
(Fig. 4B). The changes of the
morphological appearance and expression of the above genes were
also examined in HuH7 cells transfected with
anti-miR-615-3p. The transfected HuH7 cells exhibited a
pattern of the changes that was opposite to the pattern observed in
PLC/PRF/5 cells (Fig. 4B). These
observations indicated that the miR-615-3p-overexpressing
cells promote EMT-like phenotypes in HCC cell lines.
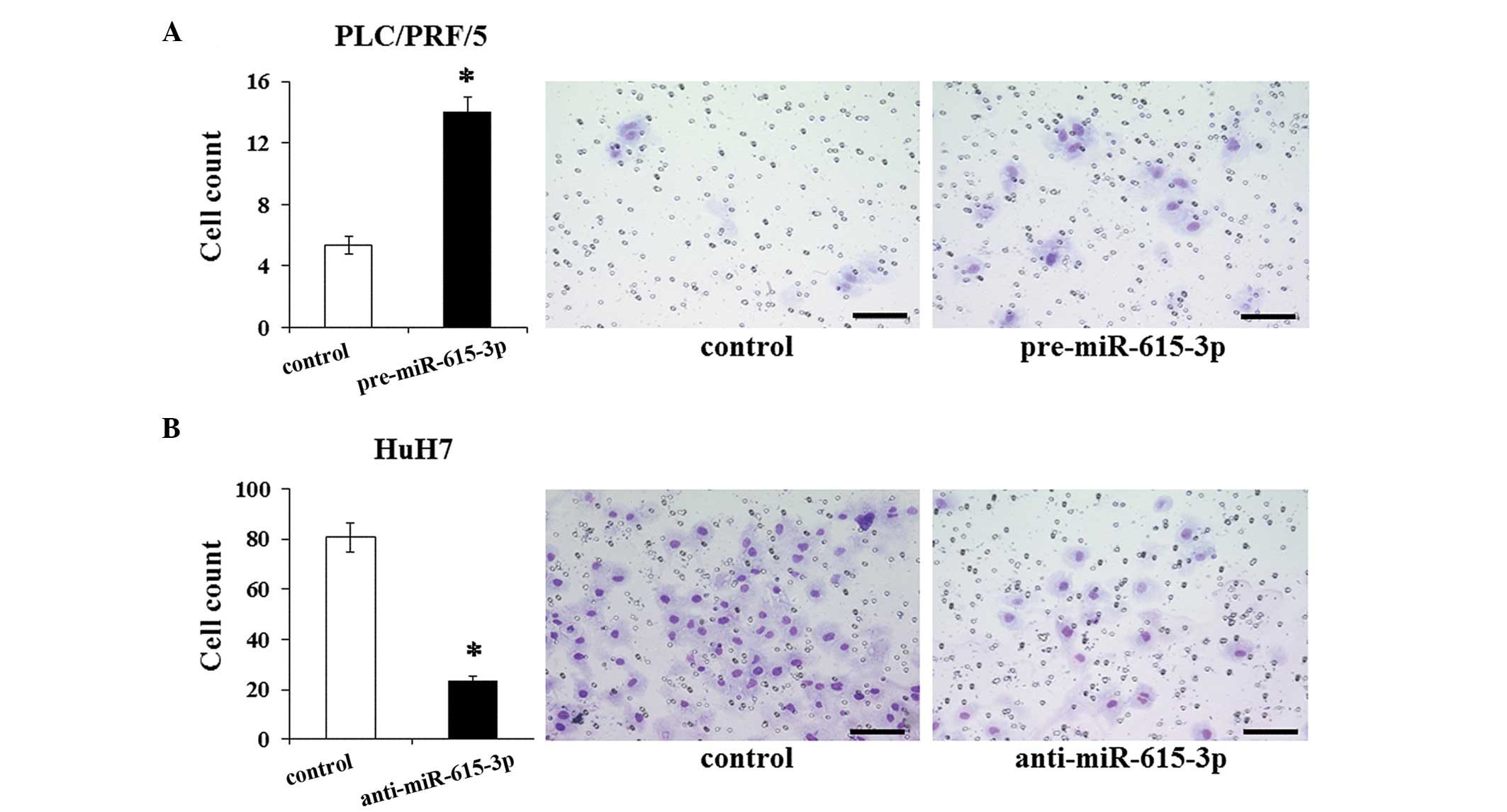
miR-615-3p promotes invasive
capacity
The association of miR-615-3p with EMT led us
to consider the effect of miR-615-3p on the invasive
capacity of cells. The invasive capacity was examined in HCC cells
following the modulation of their miR-615-3p expression
level. The invasion assay demonstrated that the invasive capacity
of PLC/PRF/5 cells transfected with pre-miR-615-3p is
significantly greater than that of the control cells (Fig. 5A). The miR-615-3p-suppressed
HuH7 cells exhibited a significantly less invasive capacity
compared to the control cells (Fig.
5B). These results suggested that miR-615-3p influences
the invasive capacity, which was consistent with the results of the
EMT features induced by miR-615-3p.
Discussion
In the present study, microarray data were used to
compare miRNA expression profiles of DTCs in BM collected
preoperatively from HCC patients with and without postoperative
recurrence. DTCs of the HCC patients with recurrence exhibited
distinctly different miRNA expression profiles compared to the
patients without recurrence. In particular, miR-615-3p
expression was significantly increased in the BM of patients with
recurrence compared to patients without recurrence. Additional
in vitro experiments revealed that the expression level of
miR-615-3p is significantly associated with the malignant
characteristics of HCC cells, such as proliferative capacity,
chemotherapeutic resistance and acquisition of EMT-like features.
These results suggested the possibility that DTCs from different
individuals may have varying characteristics that affect their
capacity to develop recurrence/metastasis, which supported the
concept that characteristics of existing DTCs, rather than the
presence or absence of DTCs, may determine cancer recurrence.
Notably, miR-615-3p induced chemoresistance and acquisition
of EMT, which are representative phenotypes of cancer stem cells
(CSCs) (26,27). This finding indicates that DTCs from
patients with recurrence may have features of CSCs, suggesting
consistency with previous studies that CSC features are frequently
observed in isolated tumor cells in cancer patients with metastatic
lesions (28,29).
Previously, we investigated the prognostic impact of
α-fetoprotein (AFP) mRNA in BM samples from HCC patients and
identified that survival rates did not differ significantly between
patients with AFP mRNA-positive BM verses AFP
mRNA-negative BM (17). However,
during the same period, another study demonstrated that AFP
mRNA expression in the BM of HCC patients is of significant
prognostic value (30). The
controversy appears to be unexpected when considering the
possibility that characteristics of DTCs play a role in
postoperative tumor recurrence. These previous studies subjected
the entire mononuclear cell fraction to analysis; by contrast, the
present study analyzed a sorted fraction of viable epithelial tumor
cells. Considering the range of cell characteristics among
fractions, differences in the fraction analyzed may be a source of
the controversy regarding the prognostic significance of AFP
mRNA in the whole mononuclear cell fraction of BM.
miR-615-3p, a member of the miR-615
family, reportedly promotes the phagocytic capacity of splenic
macrophages through upregulation of peroxisome
proliferator-activated receptor γ (31). Regarding the correlation of
miR-615-3p with malignant diseases, there has been only one
study, which reported that miR-615-3p is associated with
survival in mantle cell lymphoma (32). However, the mechanism by which
miR-615-3p influences the prognosis has not been
investigated. To validate the results of the present study, it is
also necessary to investigate whether modulating the level of
miR-615-3p expression in an in vivo HCC model would
cause a change in metastatic capacity. In this context, the present
study will help clarify the function of miR-615-3p in
malignant disease. In addition, considering that no studies have
focused on the target genes of miR-615-3p, identifying which
of its target genes regulate cancer recurrence is required.
The results of the present study potentially lead to
several clinical applications in the field of HCC. One is the
development of a novel clinical model for predicting the
postoperative prognosis in HCC patients by examining the
miR-615-3p expression level in DTCs in BM. When considering
the sample origin for this potential prediction model, peripheral
blood may also be used, as circulating tumor cells (CTCs) may be an
alternative to DTCs. CTCs are also reportedly associated with
hematogenous dissemination and poor prognosis in cancer patients
(5,33). When the upregulation of
miR-615-3p is confirmed in the DTCs and the CTCs of HCC
patients with recurrence, prognosis may be more easily predicted by
analyzing CTCs instead of DTCs in BM. However, several studies
comparing the detection rate of the tumor cells (and their
prognostic significance) between DTCs and CTCs have reported that
the two cell populations do not always correlate and further
investigation is required before CTCs are analyzed as a prognostic
alternative to DTCs (34,35). Another possible application is a
potential miRNA-targeted therapeutic option against HCC. New
therapeutic options targeting specific miRNAs have recently been
developed (36,37). The results of the present study may
lead to a novel, effective therapeutic option that targets
miR-615-3p against HCC recurrence; inhibition of
miR-615-3p may lead to the prevention of recurrence in HCC
patients by suppressing DTCs/CTCs with high malignant potential.
However, several challenges must be overcome in order to realize
such a new therapy, including the drug delivery system, off-target
effects and possible toxicities (38).
In conclusion, the results of the present study
demonstrated that DTCs in the BM of HCC patients with and without
postoperative recurrence exhibit distinct miRNA expression
profiles. This finding suggests that the characteristics of DTCs
defined by miRNA expression profiles may act in postoperative tumor
recurrence. Furthermore, among the miRNAs with altered expression,
miR-615-3p was significantly upregulated in the DTCs of
patients with recurrence and its expression level correlated
significantly with malignant characteristics of HCC cells in
vitro. These results may indicate that the expression level of
miR-615-3p in DTCs in the BM is one of the factors that
determine postoperative tumor recurrence and suggest that
miR-615-3p is a potential target molecule for regulating
postoperative HCC recurrence.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schutte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma – epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: how hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braun S, Vogl FD, Naume B, et al: A pooled
analysis of bone marrow micrometastasis in breast cancer. N Engl J
Med. 353:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pantel K, Izbicki J, Passlick B, et al:
Frequency and prognostic significance of isolated tumour cells in
bone marrow of patients with non-small-cell lung cancer without
overt metastases. Lancet. 347:649–653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natsugoe S, Nakashima S, Nakajo A, et al:
Bone marrow micrometastasis detected by RT-PCR in esophageal
squamous cell carcinoma. Oncol Rep. 10:1879–1883. 2003.PubMed/NCBI
|
|
7
|
Oki E, Kakeji Y, Baba H, et al: Clinical
significance of cytokeratin positive cells in bone marrow of
gastric cancer patients. J Cancer Res Clin Oncol. 133:995–1000.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mimori K, Fukagawa T, Kosaka Y, et al:
Hematogenous metastasis in gastric cancer requires isolated tumor
cells and expression of vascular endothelial growth factor
receptor-1. Clin Cancer Res. 14:2609–2616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA-cancer
connection: the beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoshino I and Matsubara H: MicroRNAs in
cancer diagnosis and therapy: from bench to bedside. Surg Today.
43:467–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang P, Li QJ, Feng Y, et al:
TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment
promotes venous metastases of HBV-positive hepatocellular
carcinoma. Cancer Cell. 22:291–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomokuni A, Eguchi H, Tomimaru Y, et al:
miR-146a suppresses the sensitivity to interferon-α in
hepatocellular carcinoma cells. Biochem Biophys Res Commun.
414:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomimaru Y, Eguchi H, Nagano H, et al:
Circulating microRNA-21 as a novel biomarker for hepatocellular
carcinoma. J Hepatol. 56:167–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomimaru Y, Eguchi H, Nagano H, et al:
MicroRNA-21 induces resistance to the anti-tumour effect of
interferon-α/5-fluorouracil in hepatocellular carcinoma cells. Br J
Cancer. 103:1617–1626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeyama H, Yamamoto H, Yamashita S, et
al: Decreased miR-340 expression in bone marrow is associated with
liver metastasis of colorectal cancer. Mol Cancer Ther. 13:976–985.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morimoto O, Nagano H, Miyamoto A, et al:
Association between recurrence of hepatocellular carcinoma and
α-fetoprotein messenger RNA levels in peripheral blood. Surg Today.
35:1033–1041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akiyoshi S, Fukagawa T, Ueo H, et al:
Clinical significance of miR-144-ZFX axis in disseminated tumour
cells in bone marrow in gastric cancer cases. Br J Cancer.
107:1345–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD, Jiang J, Liu Q and Yang L:
A high-throughput method to monitor the expression of microRNA
precursors. Nucleic Acids Res. 32:e432004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori M, Mimori K, Inoue H, et al:
Detection of cancer micrometastases in lymph nodes by reverse
transcriptase-polymerase chain reaction. Cancer Res. 55:3417–3420.
1995.PubMed/NCBI
|
|
22
|
Yamamoto T, Nagano H, Sakon M, et al:
Partial contribution of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to
antitumor effects of interferon-α/5-fluorouracil against
Hepatocellular Carcinoma. Clin Cancer Res. 10:7884–7895. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eguchi H, Nagano H, Yamamoto H, et al:
Augmentation of antitumor activity of 5-fluorouracil by interferon
α is associated with up-regulation of p27Kip1 in human
hepatocellular carcinoma cells. Clin Cancer Res. 6:2881–2890.
2000.PubMed/NCBI
|
|
24
|
Sakon M, Umeshita K, Nagano H, et al:
Clinical significance of hepatic resection in hepatocellular
carcinoma: analysis by disease-free survival curves. Arch Surg.
135:1456–1459. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raimondi C, Gradilone A, Naso G, et al:
Epithelial-mesenchymal transition and stemness features in
circulating tumor cells from breast cancer patients. Breast Cancer
Res Treat. 130:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamiyama T, Takahashi M, Nakagawa T, et
al: AFP mRNA detected in bone marrow by real-time quantitative
RT-PCR analysis predicts survival and recurrence after curative
hepatectomy for hepatocellular carcinoma. Ann Surg. 244:451–463.
2006.PubMed/NCBI
|
|
31
|
Jiang A, Zhang S, Li Z, et al: miR-615-3p
promotes the phagocytic capacity of splenic macrophages by
targeting ligand-dependent nuclear receptor corepressor in
cirrhosis-related portal hypertension. Exp Biol Med (Maywood).
236:672–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goswami RS, Atenafu EG, Xuan Y, et al:
MicroRNA signature obtained from the comparison of aggressive with
indolent non-Hodgkin lymphomas: potential prognostic value in
mantle-cell lymphoma. J Clin Oncol. 31:2903–2911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fehm T, Muller V, Alix-Panabieres C and
Pantel K: Micrometastatic spread in breast cancer: detection,
molecular characterization and clinical relevance. Breast Cancer
Res. 10 (Suppl 1):S12008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ignatiadis M, Georgoulias V and Mavroudis
D: Micrometastatic disease in breast cancer: clinical implications.
Eur J Cancer. 44:2726–2736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kota J, Chivukula RR, O'Donnell KA, et al:
Therapeutic microRNA delivery suppresses tumorigenesis in a murine
liver cancer model. Cell. 137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lanford RE, Hildebrandt-Eriksen ES, Petri
A, et al: Therapeutic silencing of microRNA-122 in primates with
chronic hepatitis C virus infection. Science. 327:198–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar : PubMed/NCBI
|