Introduction
The insulin-like growth factor (IGF) family is a
complex molecular signaling pathway, which plays an important role
in oncogenesis, tumor progression, metastasis and chemoresistance
(1). One of the key factors in this
family is IGF-1, which is an endocrine and autocrine/paracrine
peptide expressed in the majority of cell types and circulating at
high levels (2). More than 90% of
circulating IGF-1 is bound to IGF binding protein 3 (IGFBP-3),
which stabilizes IGF-1 in the blood and regulates its
bioavailability (3). IGF-1 and
IGFBP-3 are involved in cellular proliferation, differentiation and
apoptosis, which are indicated in cancer etiology (4). Blood concentrations of IGF-1/IGFBP-3
have been associated with the risk of prostate, colorectal and
premenopausal breast cancer (5–9). Certain
epidemiological studies have suggested an association of
circulating IGF-1/IGFBP-3 levels with ovarian cancer risk (10–12). As
the results of previous studies have been inconsistent, the present
review was conducted to evaluate the evidence from existing studies
examining the association of circulating IGF-1/IGFBP-3 with ovarian
cancer risk via meta-analysis.
Materials and methods
Search strategy
A systematic literature search of Pubmed and Embase
databases was conducted to identify all the studies published
before May 1, 2014, which investigated the association of
circulating IGF-1 or IGFBP-3 with the ovarian cancer risk. The
following keywords were used during the search: ‘Insulin-like
growth factor’ and ‘ovarian cancer’. Reference lists of relevant
studies and general reviews were also searched. No restriction of
languages was imposed. The systematic review was performed
following the guideline of the Meta-analysis Of Observational
Studies in Epidemiology (13).
Eligibility criteria
The eligibility of studies was assessed by two
investigators (Q.W. and H.L.P.) independently. Studies were
included into the meta-analysis when they met the following
criteria: i) Cohort or case-control studies published in full
texts; ii) researching the association of circulating IGF-1/IGFBP-3
with ovarian cancer risk; iii) categorizing circulating
IGF-1/IGFBP-3 concentrations into tertile or quartile levels; and
iv) reporting odds ratios (ORs) with 95% confidence intervals
(CIs), with results from crude and adjusted (adjusted for
confounders) models. Study quality was assessed using the nine-star
Newcastle-Ottawa scale (14).
Data extraction
The two investigators mentioned performed data
extraction independently and discussed the results to make a
consensus. The following variables were recorded: First author's
name, publication year, geographic region where the study was
conducted, study period, study design, sample size, IGFs assay,
categorizing and corresponding cut-points of IGF-1/IGFBP-3 levels,
ORs with 95% CIs for each category verses reference (the lowest
category) of IGF-1/IGFBP-3, the numbers of cases and controls in
each exposure category, and confounders considered in the
adjustment models. When necessary, the primary authors were
contacted for additional information.
Statistical analysis
Considering that the IGF assays and categorizing
cut-points differed across studies, a random effects model
(15) was conducted by combining
study-specific maximally adjusted ORs for the highest verses lowest
exposure levels to evaluate the association of IGF-1/IGFBP-3 with
ovarian cancer risk. The pooled ORs and 95% CIs were used to assess
the strength of association. Studies reporting the exact numbers of
cases and controls in each exposure level were included in the
random effects dose-response meta-analysis. The method proposed by
previous studies (5,16) was used to calculate the linear trends
by relating the log of ORs for different exposure levels.
The degree of heterogeneity among studies was
assessed via Q and I2 statistics. Subgroup analyses and
meta-regression models (17) were
conducted to investigate sources of heterogeneity. Sensitivity
analyses were performed to evaluate the stability of the results.
To estimate the publication bias, funnel plots were used. Funnel
plot asymmetry was assessed by Egger's regression test (P<0.05
was considered to indicate a statistically significant publication
bias) (18). All the analyses were
repeated with unadjusted ORs.
All the statistical tests were performed with the
Stata software (version 12.0; StataCorp, College Station, TX, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study characteristics
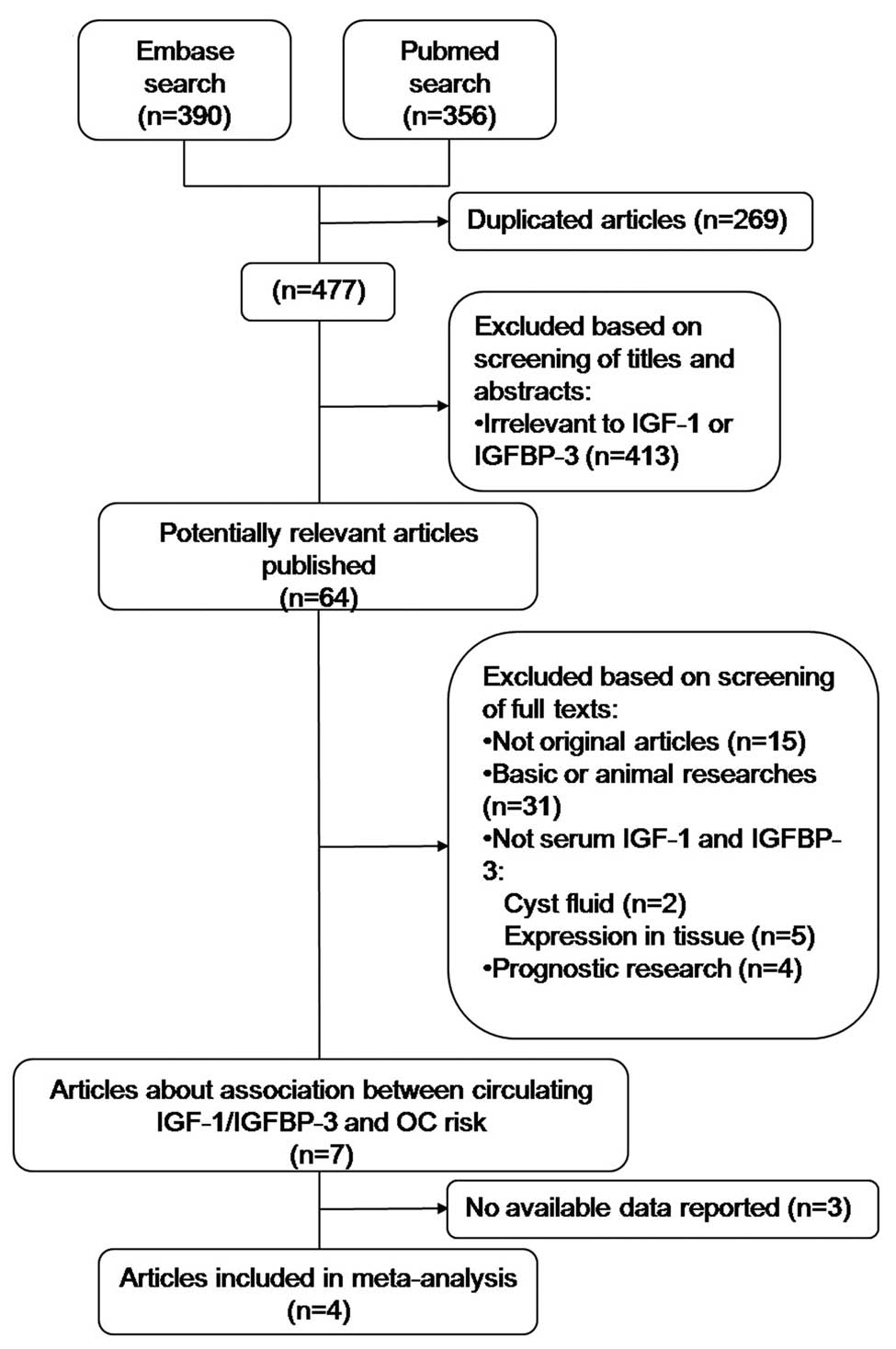
A flow diagram of the literature search is shown in
Fig. 1. Among the 64 potentially
relevant studies, four were finally included in the
meta-analysis.
The remaining four studies (10–13,19) were
published in English between 2002 and 2007 and involved a total of
1,958 patients (627 cases and 1,358 controls). Overall, the
methodology qualities of studies were satisfying, since study
designs, sample sizes, exclusion criteria and diagnosis of cases
were clearly demonstrated, except for the limited information of
follow-up in certain studies. Of these four included case-control
studies, three were nested within large prospective cohorts
(10–13). The study of Peeters et al
(10) was population-based and the
others were hospital-based. All three prospective studies reported
analyses while excluding cases diagnosed within 1 or 2 years of
blood donation. Peeters et al (10) and Lukanova et al (11) reported exclusions of females currently
using exogenous hormones at the time of blood donation, which was
not mentioned in other studies. Categories of IGF-1/IGFBP-3 levels
were calculated on the basis of the distribution of controls
(10,19) or the distribution of cases and
controls (11,12). Intra-assay coefficients of variation
were reported as generally low in all the studies. All the studies
adjusted crude ORs with ≥3 confounders. ORs of three studies were
further adjusted; IGF-1 models for levels of IGFBP-3, and IGFBP-3
models for levels of IGF-1. One study [Dal Maso et al
(19)] reported the results of free
and total circulating IGF-1 levels, where the data of total IGF-1
was used. Study characteristics are demonstrated in Table I.
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| First author
(year) | Region | Study period | Study design | No. of
case/control | Matched | IGFs test | Measure/range of
exposure (ng/ml) | Adjustment for
covariates | Study
qualitya | (Refs.) |
|---|
| Peeters (2007) | Denmark, France,
Greece, Germany, Italy, The Netherlands, Spain, UK | 1999–2003 | Prospective nested
c/c | 214/388 | Yes | Serum, ELISA | <156 (tertfile 1)
≥216 (tertfile 3) | Parity, BMI, ever use
of HRT or OCont and fertility problems | 9 | (10) |
| Lukanova (2002) | USA, Sweden,
Italy | 1985–2000 | Prospective nested
c/c | 132/263 | Yes | Serum/plasma, IRMA,
blind test | Tertile | Parity, smoking
status, BMI and IGFs system | 8 | (11) |
| Tworoger (2007) | USA | 1976–2004 | Prospective nested
c/c | 222/599 | Yes | Plasma, ELISA, blind
test | <139 (quartile 1)
169 to <212 (quartile 3) | Parity, ever use of
HRT or OCont, simple hysterectomy, tubal ligation, physical
activity, age at menarche and menopause, IGFs system | 8 | (12) |
| Dal Maso (2004) | Italy | 1999–2003 | c/c | 59/108 | NR | Plasma, ELISA | <103 (tertfile 1)
≥151 (tertfile 3) | Parity, HRT, OCont,
smoking status and IGFs system | 7 | (19) |
Associations with ovarian cancer
risk
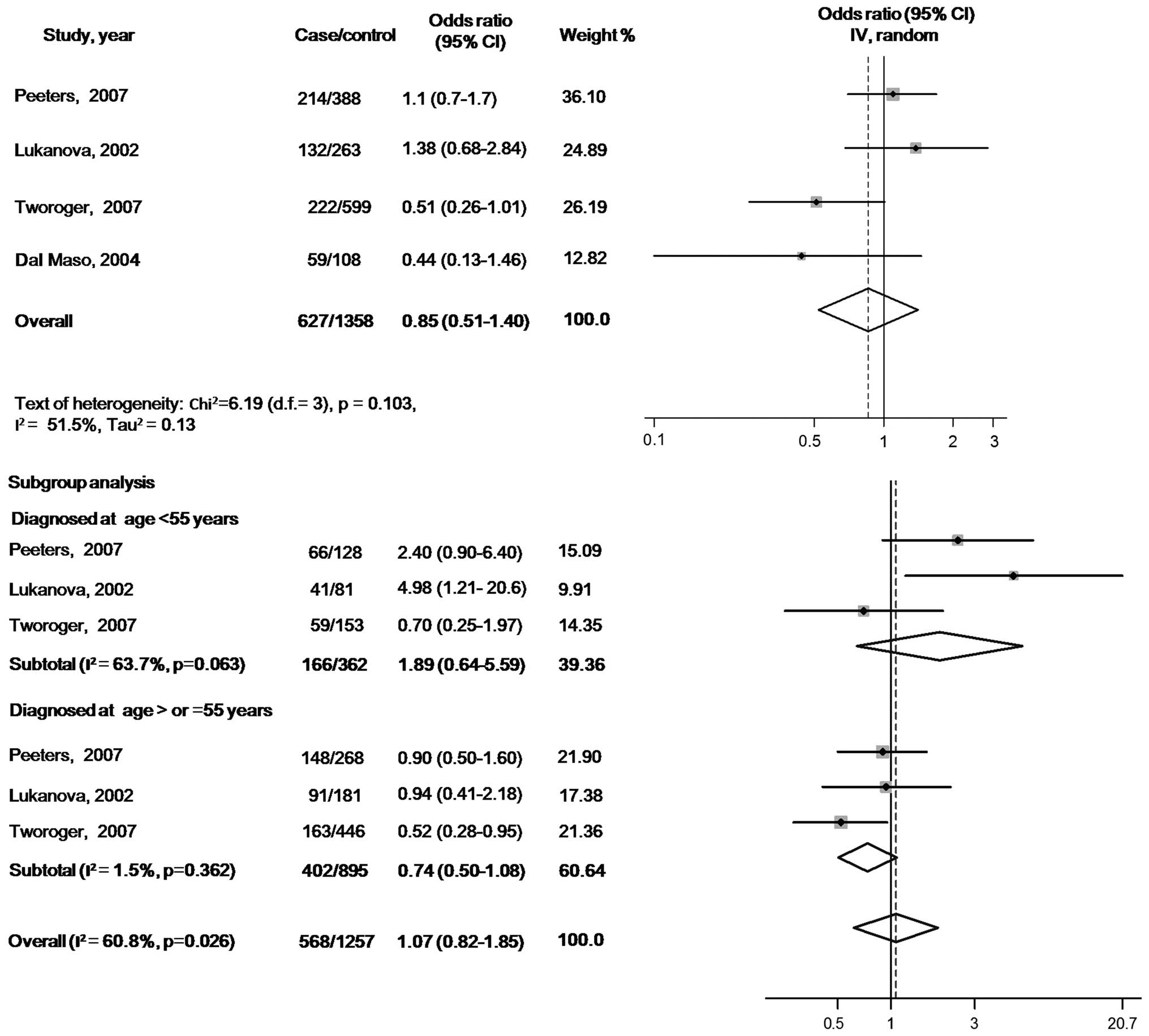
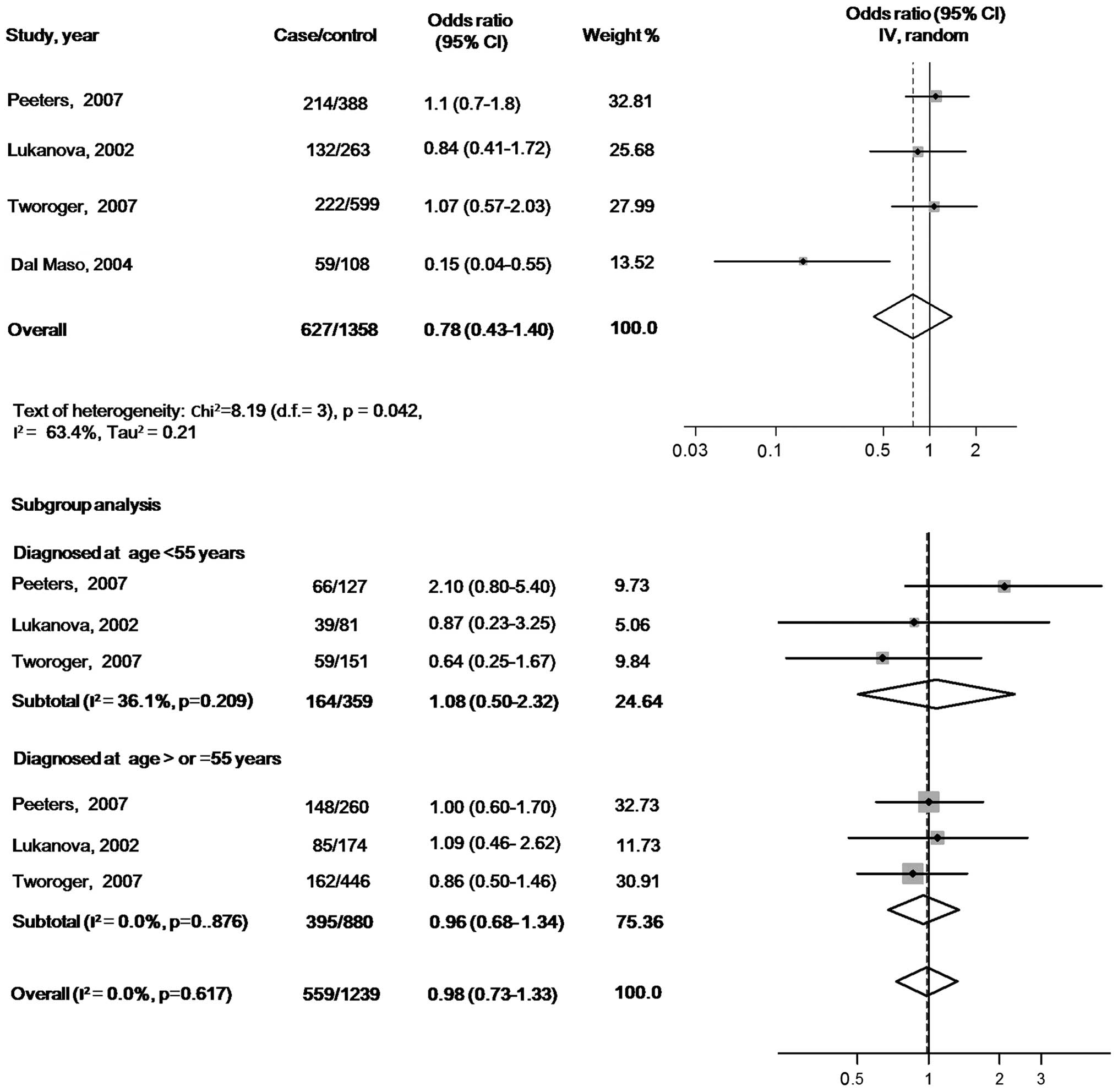
There were no statistically significant associations
of circulating IGF-1 or IGFBP-3 with ovarian cancer risk when the
maximally adjusted ORs for the highest verses lowest exposure
levels in each study were pooled into meta-analysis [OR, 0.85 (95%
CI, 0.51–1.40) for IGF-1 and OR, 0.78 (95% CI, 0.43–1.40) for
IGFBP-3]. When participants in all the studies were analyzed as a
whole, potential heterogeneity among the studies was represented in
the analysis model (P=0.103, I2=51.5% in the IGF-1
model; and P=0.042, I2=63.4% in the IGFBP-3 model)
(Figs. 2 and 3).
In the subgroup analyses according to the age at
diagnosis, the pooled ORs of IGF-1 were 1.89 (95% CI, 0.64–5.59)
for the subgroup with cases diagnosed before 55 years (tests of
heterogeneity: P=0.063, I2=63.7%) and 0.74 (95% CI,
0.50–1.08) for the subgroup with cases diagnosed at ≥55 years
(tests of heterogeneity: P=0.362, I2=0.0%). The pooled
ORs of IGFBP3 were 1.08 (95% CI, 0.50–2.32) for the subgroup cases
diagnosed before 55 years (tests of heterogeneity: P=0.209,
I2=36.1%) and 0.98 (95% CI, 0.73–1.33) for the subgroup
with cases diagnosed at ≥55 years (tests of heterogeneity: P=0.617,
I2=0.0%) (Figs. 2 and
3).
In the meta-regression models, the residual
variation due to heterogeneity was not changed by geographic
regions, year of publication, sample type (serum or plasma), assays
(immunoradiometric assay or ELISA), confounders (smoking status or
ever use of hormones) or range of exposure levels. However, the
Knapp-Hartung meta-regression model (17) reported that the heterogeneity in the
meta-analysis model of IGF-1 was mainly from studies that did not
exclude females using exogenous hormones at the time of blood
donation (residual I2=0.0%). However, no significant
variation in pooled OR and 95% CI with Knapp-Hartung modification
was identified. No evidence of interactions with the above
variables was found in the meta-regression analyses of the IGFBP-3
model. The results of the sensitivity analyses indicated that the
significance of pooled ORs was not influenced by each individual
study. The funnel plot shapes revealed no clear asymmetry. Further
Egger's test (18) suggested no
evidence of publication bias (P=0.404 in IGF-1 model and P=0.062 in
IGFBP-3 model).
Three studies reporting the exact numbers of cases
and controls in each exposure category were included in the random
effects dose-response meta-analysis (15). No linear association was found between
circulating IGF-1/IGFBP-3 levels and ovarian cancer risk [OR, 0.39
(95% CI, 0.13–1.13), P=0.083 for IGF-1 and OR, 0.93 (95% CI,
0.72–1.21), P=0.584 for IGFBP-3]. All the analyses were repeated
with unadjusted ORs and similar findings were identified.
Discussion
No significant association of circulating
IGF-1/IGFBP-3 with ovarian cancer risk was indicated in the present
meta-analysis or in the dose-response analysis. Potential
heterogeneity was represented when regarding participants in all
the studies as a whole. However, heterogeneity in the IGFBP-3 model
was significantly diminished using subgroup analysis based on age
at diagnosis. Furthermore, meta-regression models were conducted
and it was found that inclusion of females that were using
exogenous hormones at the time of blood donation in studies was the
main source of heterogeneity in analysis of IGF-1. This could be
explained by the complicated interactions between sex steroid
hormones and the IGF system. A previous study (20) reported that exogenous hormone use may
affect the peptide levels in the IGF signaling pathway, which
induced the decrease of circulating IGF-1 concentration.
In all the studies reviewed in the present analysis,
two prospective nested case-control studies [Peeters et al
(10) and Lukanova et al
(11)] addressed the hypothesis that
higher circulating levels of IGF-1 increase ovarian cancer risk and
suggested an association of circulating IGF-1 concentrations with
ovarian cancer risk for females diagnosed before 55 years. The
study by Lukanova reported a 5-fold higher risk for the highest
verses lowest exposure levels of IGF-1 in cases diagnosed at <55
years. However, this systemic review did not represent consistent
findings in the subgroup meta-analysis.
Compared to the above two studies, an inverse
association of circulating IGF-1 with ovarian cancer risk was
observed in the case-control study [Dal Maso et al (19)] included, which also reported a
negative association of IGFBP-3 with ovarian cancer. Considering
the lower quality level in the methodology of the retrospective
study, a meta-analyses was conducted while excluding this
case-control study. As a result, heterogeneity in the overall
analysis model of IGFBP-3 was eliminated, though no significant
association with ovarian cancer or similar change in IGF-1 model
was shown [OR, 0.93 (95% CI, 0.55–1.59), P=0.096,
I2=59.4% in the IGF-1 model; and OR, 1.03 (95% CI,
0.74–1.44), P=0.819, I2=0.0% in the IGFBP-3 model]. The
variation of heterogeneity in the IGFBP-3 model may be partly
explained by the blood samples in the retrospective observational
study not being obtained prior to the incidence of disease, which
could affect the circulating IGFBP-3 levels in complicated
ways.
The present meta-analysis was conducted to
comprehensively and precisely evaluate the association of
circulating IGF-1/IGFBP-3 with ovarian cancer, by combining the
inconsistent findings of independent but similar studies. However,
the meta-analyses found no sufficient evidence to confirm that
circulating IGF-1/IGFBP-3 levels are associated with ovarian cancer
risk. This result may be limited by the small number of current
relevant studies and sample size included. In addition, as the
majority of control groups were set in hospitals and health care
centers, controls were not definitely free of other benign diseases
that may affect the circulating levels of IGFs (21). In addition, although considerable
effort was put into assessing the variation from heterogeneity and
finally identifying three potential sources of heterogeneity
(inclusion of females using exogenous hormones at blood donation in
the IGF-1 model, and age at diagnosis and study type in the IGFBP-3
model), solving all the problems without sufficient relevant
studies with available additional data on the confounders was not
possible. Therefore, a more precise analysis of larger samples
remains if future studies with improved quality are available.
Despite the limitations, the present meta-analysis
currently represents the overall view regarding the association of
circulating IGF-1/IGFBP-3 levels and ovarian cancer risk, which has
not been systematically reviewed previously. Additionally, the
review exhibits several important problems that should be
considered in future research. Despite the importance of
prospective study design and stratified analyses of age, which were
already discussed in the previous studies and systematic review in
associating IGF-1/IGFBP-3 with other cancers (5–9), the
influence caused by the status of exogenous hormone use in
participants is indicated. Even though all the studies in the
review adjusted ORs with oral contraceptive use as one of
covariates in logistic models, inclusion of participants currently
using exogenous hormones at blood donation was supposed to
significantly contribute to heterogeneity in analyses, particularly
in the IGF-1 model. Thus, whether exclusion of this group of
participants or subgroup set is necessary should be considered in
future study design.
In conclusion, the present meta-analysis found no
significant association of IGF-1/IGFBP-3 with ovarian cancer risk.
However, a more precise analysis with larger sample sizes should be
conducted if more studies with improved quality are available in
the future.
References
|
1
|
King ER and Wong KK: Insulin-like growth
factor: current concepts and new developments in cancer therapy.
Recent Patents Anticancer Drug Discov. 7:14–30. 2012. View Article : Google Scholar
|
|
2
|
Adams TE, Epa VC, Garrett TP and Ward CW:
Structure and function of the type 1 insulin-like growth factor
receptor. Cell Mol Life Sci. 57:1050–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khandwala HM, McCutcheon IE, Flyvbjerg A
and Friend KE: The effects of insulin-like growth factors on
tumorigenesis and neoplastic growth. Endocr Rev. 21:215–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Renehan AG, Zwahlen M, Minder C, O'Dwyer
ST, Shalet SM and Egger M: Insulin-like growth factor (IGF)-I, IGF
binding protein-3 and cancer risk: systematic review, and
meta-regression analysis. Lancet. 363:1346–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toniolo P, Bruning PF, Akhmedkhanov A, et
al: Serum insulin-like growth factor-I and breast cancer. Int J
Cancer. 88:828–832. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stattin P, Bylund A, Rinaldi S, et al:
Plasma insulin-like growth factor-I, insulin-like growth
factor-binding proteins, and prostate cancer risk: a prospective
study. J Natl Cancer Inst. 92:1910–1917. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan JM, Stampfer MJ, Giovannucci E, et
al: Plasma insulin-like growth factor-I and prostate cancer risk: a
prospective study. Science. 279:563–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palmqvist R, Hallmans G, Rinaldi S, et al:
Plasma insulin-like growth factor 1, insulin-like growth factor
binding protein 3, and risk of colorectal cancer: a prospective
study in northern Sweden. Gut. 50:642–646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peeters PH, Lukanova A, Allen N, et al:
Serum IGF-I, its major binding protein (IGFBP-3) and epithelial
ovarian cancer risk: the European Prospective Investigation into
Cancer and Nutrition (EPIC). Endocr Relat Cancer. 14:81–90. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lukanova A, Lundin E, Toniolo P, et al:
Circulating levels of insulin-like growth factor-I and risk of
ovarian cancer. Int J Cancer. 101:549–554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tworoger SS, Lee IM, Buring JE, Pollak MN
and Hankinson SE: Insulin-like growth factors and ovarian cancer
risk: a nested case-control study in three cohorts. Cancer
Epidemiol Biomarkers Prev. 16:1691–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greenland S and Longnecker MP: Methods for
trend estimation from summarized dose-response data, with
applications to meta-analysis. Am J Epidemiol. 135:1301–1309.
1992.PubMed/NCBI
|
|
17
|
Thompson SG and Sharp SJ: Explaining
heterogeneity in meta-analysis: a comparison of methods. Stat Med.
18:2693–2708. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dal Maso L, Augustin LS, Franceschi S, et
al: Association between components of the insulin-like growth
factor system and epithelial ovarian cancer risk. Oncology.
67:225–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fox EM, Miller TW, Balko JM, et al: A
kinome-wide screen identifies the insulin/IGF-I receptor pathway as
a mechanism of escape from hormone dependence in breast cancer.
Cancer Res. 71:6773–6784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suikkari AM, Tiitinen A, Stenman UH,
Seppala M and Laatikainen T: Oral contraceptives increase
insulin-like growth factor binding protein-1 concentration in women
with polycystic ovarian disease. Fertil Steril. 55:895–899.
1991.PubMed/NCBI
|
|
22
|
Tas F, Karabulut S, Serilmez M, Ciftci R
and Duranyildiz D: Clinical significance of serum insulin-like
growth factor-1 (IGF-1) and insulin-like growth factor binding
protein-3 (IGFBP-3) in patients with epithelial ovarian cancer.
Tumour Biol. 35:3125–3132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dursun P, Gultekin M and Esin S:
Preoperative serum IGF-1 and IGFBP-3 levels in patients with
ovarian carcinoma: preliminary results. Int J Gynecol Cancer.
14:105–106. 2004.
|
|
24
|
Serin IS, Tanriverdi F, Yilmaz MO, Ozcelik
B and Unluhizarci K: Serum insulin-like growth factor (IGF)-I, IGF
binding protein (IGFBP)-3, leptin concentrations and insulin
resistance in benign and malignant epithelial ovarian tumors in
postmenopausal women. Gynecol Endocrinol. 24:117–121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El-Roeiy A, Chen X, Roberts VJ, et al:
Expression of the genes encoding the insulin-like growth factors
(IGF-I and II), the IGF and insulin receptors, and IGF-binding
proteins-1–6 and the localization of their gene products in normal
and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab.
78:1488–1496. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flyvbjerg A, Mogensen O, Mogensen B and
Nielsen OS: Elevated serum insulin-like growth factor-binding
protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian
cancer: correlation with cancer antigen 125 and tumor-associated
trypsin inhibitor. J Clin Endocrinol Metab. 82:2308–2313. 1997.
View Article : Google Scholar : PubMed/NCBI
|