Introduction
Acute lymphoblastic leukemia (ALL) is a heterogenous
hematological disease characterized by the proliferation of
immature lymphoid cells in the bone marrow, peripheral blood, and
other organs (1). Statistically, the
incidence rate of ALL in the USA is 1.6/100,000 individuals per
year (2). There are 6,000 estimated
new cases (male:female prevalence of ~1.3:1) of ALL diagnosed
yearly in the USA. Patients are mainly children; ~60% of cases
occur in people aged <20 years (3). ALL represents 75–80% of paediatrics
acute leukemia, and by contrast, it only represents 20% of all
adult leukemia (4). Over the past
several decades, the cure rate and survival outcome of ALL have
been significantly improved, particularly among children with ALL.
In the current treatment therapy, the complete remission (CR) among
children with ALL is ~80% (5–7); but in adults with ALL, the long-term
prognosis with CR is ~30–40% (8–12). The
difference between children and adults in long-term outcomes can be
explained partly by the difference in cytogenetic subtypes of ALL
among age groups (13–15).
Colony-stimulating factors (CSF) are a family of
cytokines, which can regulate the proliferation and differentiation
of hematopoietic cells. Currently, there are >20 molecules
regarded as CSFs (16). Among them,
granulocyte CSF (G-CSF) and granulocyte macrophage CSF (GM-CSF)
have been used clinically. G-CSF regulates the production of
neutrophil lineage. Administration of G-CSF results in a
dose-dependent increase in circulating neutrophils (16,17).
GM-CSF is a growth factor that stimulates the granulocytes,
macrophages and eosinophil colonies. Administration of GM-CSF
results in a dose-dependent increase in blood neutrophils,
eosinophils, macrophages and occasionally lymphocytes (16,17).
Administration of CSF can be used in the following
ways: i) Priming of chemotherapy or bone marrow transplantation.
The purpose of the former is to recruit leukemic cells into the
cell cycle and enhance the effect of chemotherapy, the aim of the
latter is to mobilize the hematopoietic cells to the periphery and
collect stem cells; ii) during or following chemotherapy so as to
accelerate the hematopoietic cells proliferation and decrease the
incidence of febrile neutropenia infection; and iii) during febrile
neutropenia in order to reinforce the recovery of infection. The
first way was excluded in the present study, as the aim was to
evaluate the prophylactic effect of CSF (18).
Thus far there is no conclusive data to ensure the
effectiveness of CSF to prevent myelosuppressive therapy-related
infectious complications. In addition, no evidence shows that the
addition of CSF decreases the mortality in adult ALL patients and
improves the outcomes. Therefore, the present systematic review was
conducted to evaluate the safety and effectiveness of the addition
of G-CSF or GM-CSF to chemotherapy in adult ALL patients.
Materials and methods
Criteria for considering studies
Types of studies
Randomized controlled trials (RCT) with a parallel
design that compared the addition of CSFs during or subsequent to
myelosuppressive therapy to the no treatment or placebo regime in
adult ALL patients were included. Only studies in English were
accepted.
Types of participants
Adults (>19 years old) with ALL in all stages of
treatment following the administration of the chemotherapy
(induction, consolidation and salvage treatment) were included.
Trials with participants <15 years old were included when
separate statistics could not be obtained.
Types of interventions
The interventions were CSFs, including G-CSF or
GM-CSF, administered either intravenously or subcutaneously, and
concomitantly or following chemotherapy and continued for >24 h.
CSFs were administered in a dose of >5 mcg/kg body weight per
day until absolute neutrophil counts reached
>0.5×109/l. The studies using CSF in the priming of
chemotherapy or the bone marrow transplantation were excluded.
Types of outcome measures
Primary outcomes
The primary outcome was mortality at the end of
follow-up.
Secondary outcomes
Secondary outcomes were mortality at day 30 (usually
parallels mortality associated with ALL induction treatment),
number of patients achieving CR, number of patients with infection
or severe infection and duration of neutropenia (median days).
Search methods for identification of
studies
A comprehensive search was conducted between 1966
and 2014 using databases, including the Cochrane Central Register
of Controlled Trials, PubMed, Web of Science and SinoMed. In
PubMed, the search was conducted using combinations of Medical
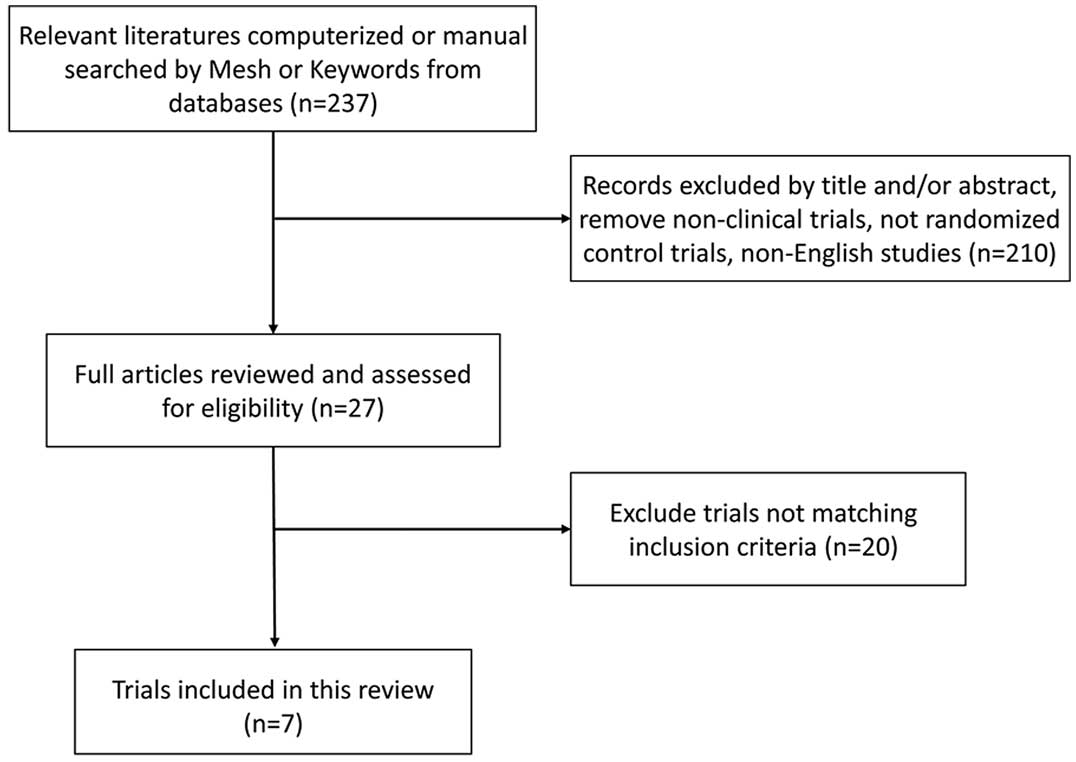
Subject Heading (MeSH) search terms and keywords (Fig. 1). Search details included: (̔Adult̓
[MeSH]) AND (̔Precursor Cell Lymphoblastic Leukemia-Lymphoma̓
[MeSH] or ̔acute lymphoblastic leukemia̓ [All fields] or ̔acute
lymphoblastic leukemia̓ [All fields]) AND (̔Colony-Stimulating
Factors̓ [MeSH] or ̔Hematopoietic Cell Growth Factors̓ [All
fields]). Other databases were queried using identical terms for
keyword searching. Only studies in English were accepted.
Data collection and analysis
Two review authors independently extracted the data
from the included trials. In case of any disagreement, a third
review author extracted the data. A standardized form was used to
extract the relevant data on the characteristics of trials and
patients, intervention protocol and outcomes.
Data synthesis
The Mantel-Haenszel method (Review Manager 5.2)
(19) was used to estimate the risk
ratios (RR) and 95% confidence intervals (CI) for dichotomous data.
A fixed-effect model was used and a sensitivity analysis was
performed by repeating the above analysis using a random-effects
model.
Results
Description of studies
In total, 7 RCTs with 753 patients were included
[Geissler et al (20),
Hallbook et al (21),
Holowiecki et al (22), Ifrah
et al (23), Larson et
al (24), Ottmann et al
(25) and Thomas et al
(26)]. There were only two
double-blind RCTs and the remaining studies were open-label. The
duration of follow-up ranged from 22 months to 8 years. The age of
patients ranged from 15–79 year old. (Table I).
 | Table I.Characteristics of the included
trials. |
Table I.
Characteristics of the included
trials.
|
| Trials | Patients | Intervention |
|---|
|
|
|
|
|
|---|
| No. | First author year
(Ref.) | Recruitment
timea | RCT design | Median follow-up | Group | No. | Males, % | Median age, years
(range) | Chemotherapy | Type | Dose | Method | Total duration |
|---|
| 1 | Geissler | 1993.4–1996.1 | Open-label | 28 months | C | 26 | 65 | 42 (16–79) | Induction (GMALL
protocol 1984) | Filgrastim | 5 µg/kg | s.c. | Day 2 until ANC
>2000/µl |
|
| 1997 (20) |
|
|
| G | 25 | 40 | 36 (17–75) |
| Phase I |
|
|
|
| 2 | Hallbook | 1990.2–1992.5 | Open-label | 8 years | C | 32 | 44 | 51 (17–78) | Induction (L-10
protocol) | Lenograstim | 5 µg/kg | s.c. | Days 3–14,
17–28 |
|
| 2009 (21) |
|
|
| G | 32 | 63 | 44 (16–79) |
|
|
|
|
| 3 | Holowiecki | 1997–1998 | Open-label | 22 months | C | 31 | 58 | 28 (16–54) | Induction (PALG
4–96 protocol) | Lenograstim | 150
µg/m2 | s.c. | Days 2–6, 9–13,
16–20, 23 |
|
| 2002 (22) |
|
|
| G | 33 | 67 | 26 (16–58) |
|
|
|
|
| 4 | Ifrah | 1990.11–1992.4 | Double-blind | 4 years | C | 29 | 62 | 28 (16–52) | Induction
regimen | rGM-CSF | 5 µg/kg | i.v | Day 1 until ANC
>2000/µl |
|
| 1999 (23) |
|
|
| GM | 35 | 77 | 36 (18–55) | Days 1–5 IDA 8
mg/m2 |
|
|
|
|
|
|
|
|
|
| Days 1–3 ara-C 2
g/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Days 1–6 MP 1
mg/Kg |
|
|
|
|
| 5 | Larson | 1991.6–1993.7 | Double-blind | 4.7 years | C | 96 | 46 | 35 (16–79) | Induction
regimen | Filgrastim | 5 µg/kg | s.c. | Day 4 until ANC
>2000/µl |
|
| 1998 (24) |
|
|
| G | 102 | 60 | 37 (16–75) | Day 1 CTX 1,200
mg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Days 1, 2, 3 DNR 45
mg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Days 1, 8, 15, 22
VCR 2 mg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Days 1–21 PED 60
mg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Days 5, 8, 11, 15,
18, 22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| L-Asp 6000
IU/m2 |
|
|
|
|
| 6 | Ottmann | 1992.1–1993.4 | Open-label | 20 months | C | 39 | 64 | 30 (16–58) | Induction (GMALL
protocol 1984) | Filgrastim | 5 µg/kg | s.c. | Day 7 until ANC>
2000/µl |
|
| 1995 (25) |
|
|
| G | 37 | 70 | 27 (16–65) | Phase II |
|
|
|
|
| 7 | Thomas | 1994.6–1995.6 | Open-label | 3.5 years | C | 74 | 70 | 33 (16–55) | Induction (LALA-94
protocol) | Lenograstim | 263 µg | s.c. | Day 9 until ANC
>2000/µl |
|
| 2004 (26) |
|
|
| G | 95 | 64 | 33 (15–55) |
| rGM-CSF | 5 µg/kg | i.v | Day 16 until
ANC> 2000/µl |
|
|
|
|
|
| GM | 67 | 68 | 35 (16–55) |
|
|
|
|
|
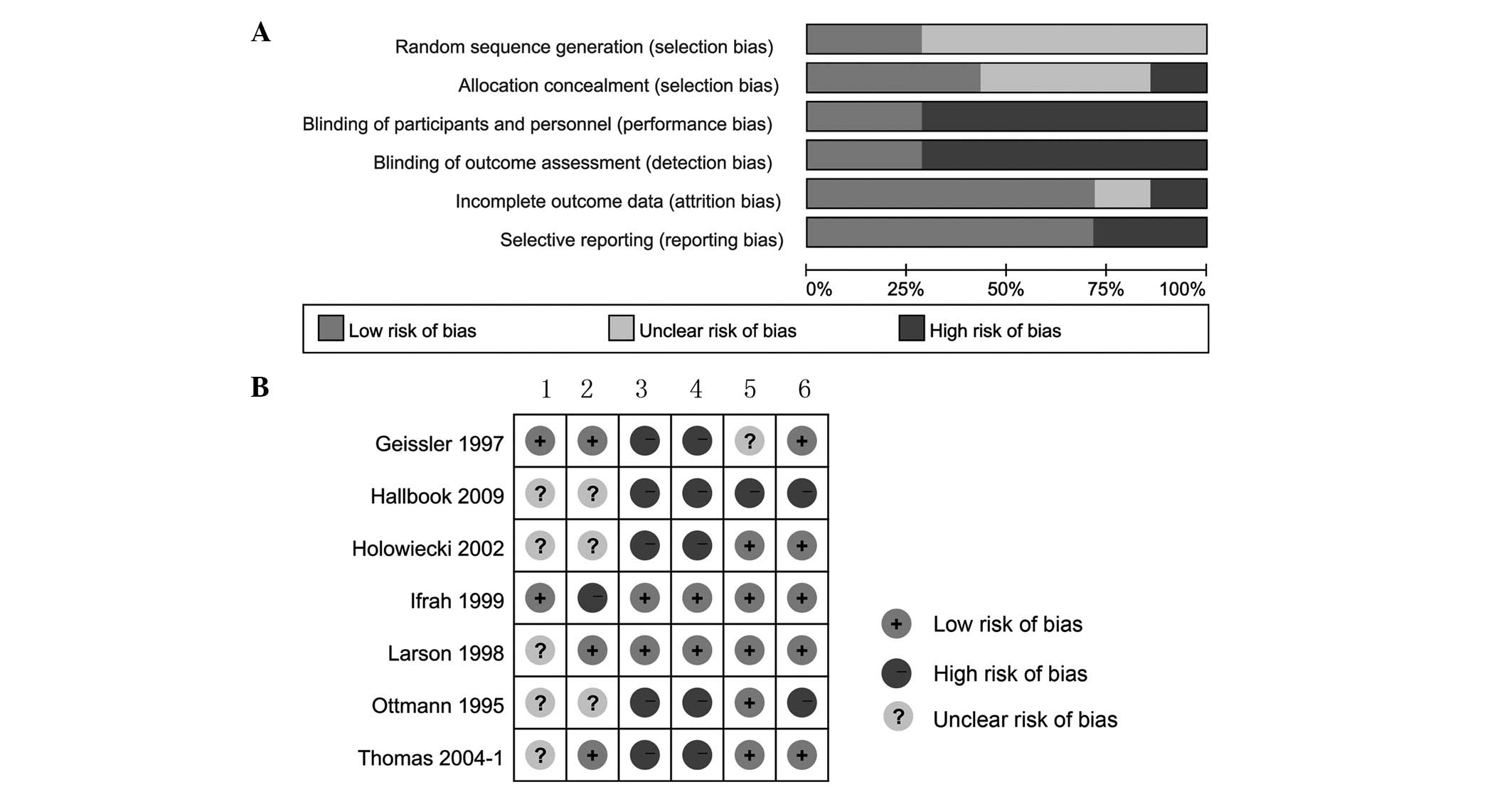
Risk of bias in included studies
Generation of randomization sequence, allocation
concealment, blinding, incomplete outcome data and selective
reporting were assessed for all 7 included trials (Fig. 2).
Effects of interventions
Primary outcomes
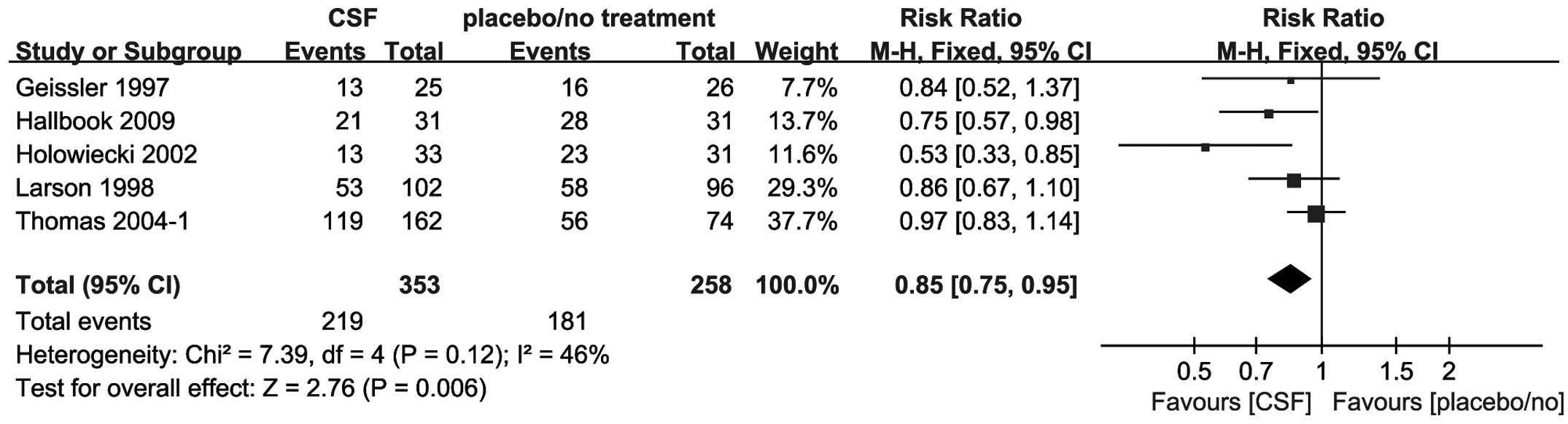
Five trials including 611 patients reported
mortality at the end of follow-up. The end of follow-up ranged from
1 to 5 years. The addition of CSFs to chemotherapy decreased the
mortality between patients treated with chemotherapy and CSFs, and
those treated with chemotherapy alone. Meta-analysis showed a
significant difference to the CSF group with an RR of 0.85 (95% CI,
0.75–0.95) (Fig. 3).
Secondary outcomes
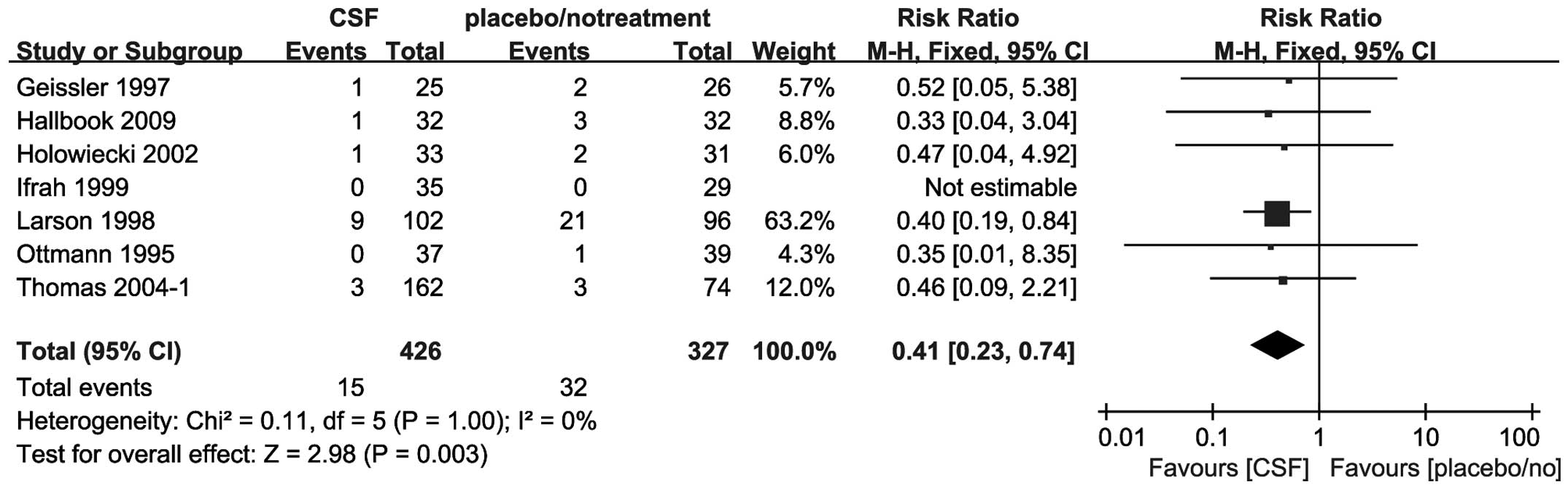
All 7 trials, including 753 patients, reported the
all-cause mortality at day 30. The addition of CSFs to chemotherapy
decreased the mortality at day 30 in the adult ALL patients (RR,
0.41; 95% CI, 0.23–0.74) (Fig.
4).
Number of patients achieving CR
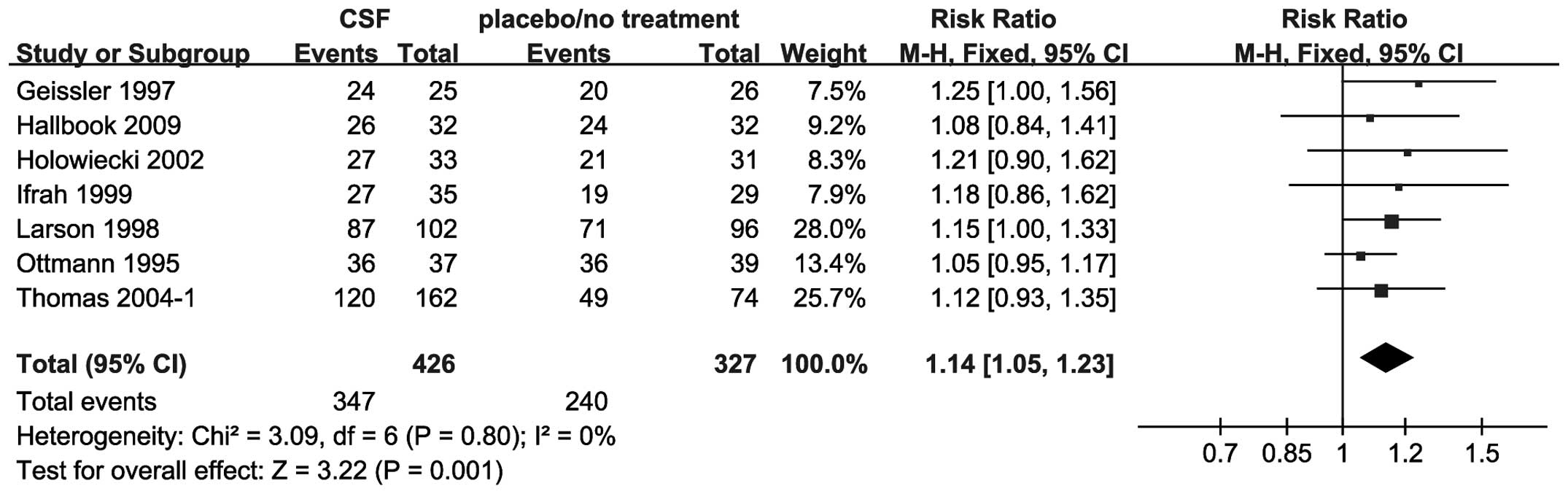
All 7 trials, including 753 patients, reported the
CR rate. The addition of CSF to chemotherapy compared to placebo or
no intervention increased the rate of CR, with an RR of 1.14 (95%
CI, 1.05–1.23) (Fig. 5).
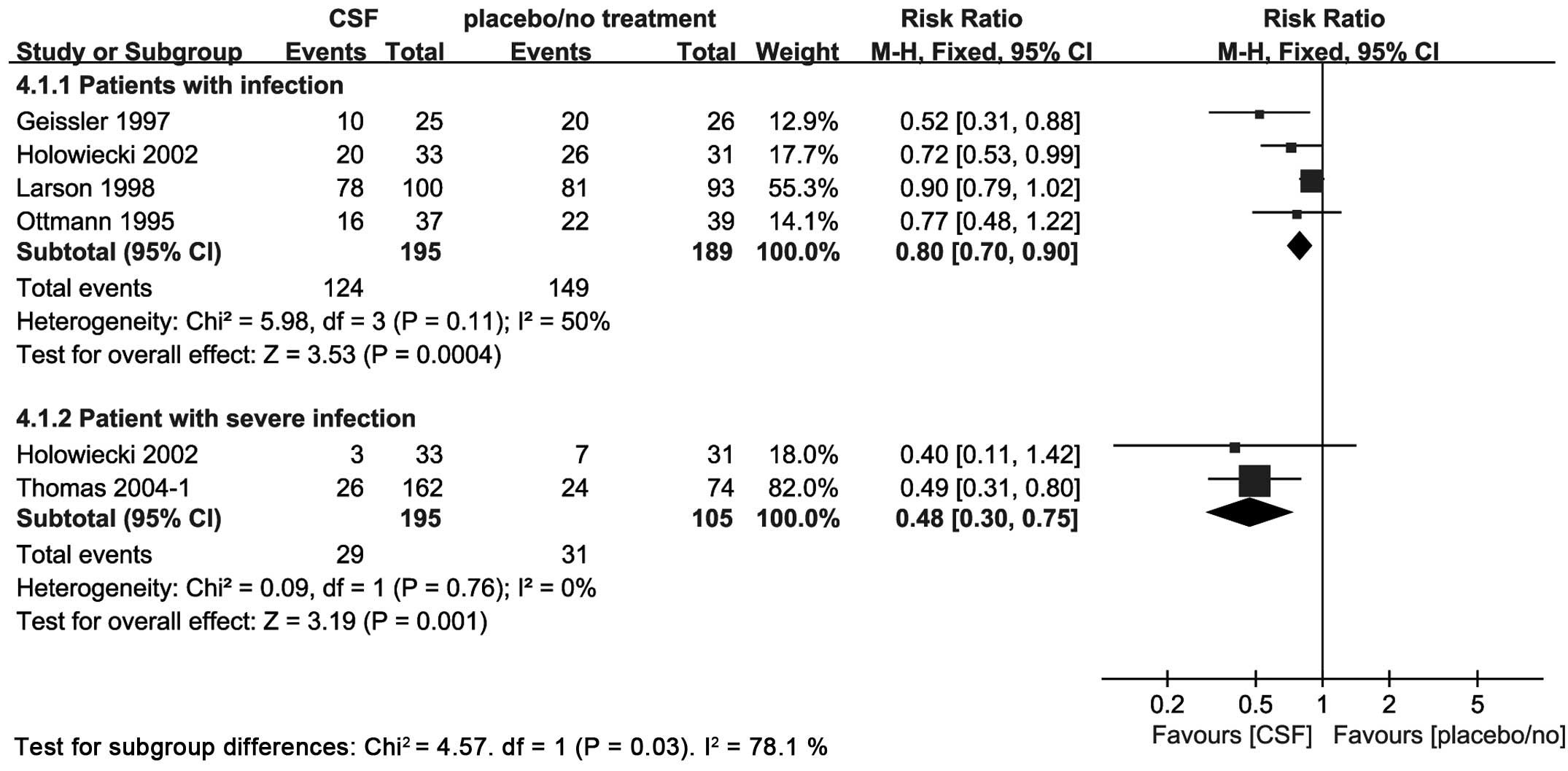
Number of patients with infection and
severe infection
A total of 4 trials reported the number of patients
with infection and 2 trials reported the number of patients with
severe infection (World Health Organization grade ≥III). The
addition of CSF to chemotherapy compared to placebo or no
intervention reduced the occurrence of infection (RR, 0.8; 95% CI,
0.7–0.9) and severe infection (RR, 0.48; 95% CI, 0.3–0.75)
(Fig. 6).
Duration of neutropenia from
randomization
In total, 6 studies reported on neutropenia duration
(20–22,24–26). In
these studies, neutropenia was defined as <0.5×109/l
neutrophils. The median duration of neutropenia ranged between 8
and 17 days in the CSFs arm and between 12.5 and 24 days in the
control arm. A meta-analysis could not be conducted on this outcome
as it is a non-normally dispersed variable and outcomes were
reported as medians in the majority of trials.
Discussion
CSFs are often used to prevent chemotherapy-related
infection and febrile neutropenia in cancer patients. Currently,
there are certain systematic reviews that investigate the effect of
CSF in cancer treatment (27–29), particularly in ALL (30).
In the present study, we conclude that CSF is
effective in the prevention of the myelosuppressive therapy-related
infectious complications in adult ALL. CSFs were also able to
improve the overall mortality. A recent meta-analysis that focused
on the prophylactic use of G-CSF in ALL patients was published by
Giebel et al (31). The study
conducted a joint analysis and obtained a conclusion that is
agreeable with the present study, which is that the prophylactic
use of G-CSF during induction of ALL is effective and associated
with improved long-term outcome (31).
An important issue is that based on the current
available evidence and physician experience, the optimum
application of CSF in clinical practice is unknown.
Five of the included studies used G-CSF and two used
GM-CSF (20–26). The commonly used dosage of G-CSF and
GM-CSF is 5 µg/kg. A subgroup meta-analysis to compare the
difference was conducted, however, the number of studies included
was small.
Another issue is the optimal injection scheme of
CSF. The initial time varies from 2 to 9 days after the
chemotherapy. The end time is until the absolute neutrophil count
recovery (ANCR), however the criteria are different. Certain
studies identified ANCR as ANC 1500/µl, and others identified it as
>2000/µl. Until now, there is no consensus of CSF in the
clinical use.
In all the included studies, the administration of
CSF was only used in the induction phase of chemotherapy. However,
in the treatment of adult ALL, the protocol is often composed of
the induction, intensification, re-intensification and maintenance
phase. The effect and safety of CSF following a long-term treatment
is unknown, which is used in every course of chemotherapy. In
current studies, the identification of receptors to G-CSF, GM-CSF
and interleukin-3 (32) on the
membrane of leukemic lymphoblasts was described. Certain initial
molecular studies identified growth of ALL blast cell colonies
following exposure to G-CSF (33),
GM-CSF and other cytokines (such as interleukin) (34) in in vitro assay. It is possible
that the frequent use of CSF during each myelosuppressive therapy
can influence the prognosis of ALL regarding CR, relapse and
overall mortality rates.
Additionally, the number of included trials is small
and there are no large sample trials. The majority of the studies
are open-label studies, which are known as subject to potential
risk of performance and detection bias. In another aspect, there
are only 7 published studies included in the present study, and the
unpublished studies could not be identified. Therefore, the
publication bias is significant. Furthermore, there are certain
RCTs that were supported by pharmaceutical company funding, which
has an impact on the quality of studies. Pharmaceutical company
funding is empirically linked to potential bias. There is a need to
avoid pharmaceutical funding as much as possible to minimize
results and publication bias (35).
Future RCTs should focus on determination of the
optimal CSF dosage and the timing point, as well as the use of CSFs
during all cycles of the most intensive phases of treatment. As
CSFs are a relatively expensive drug, cost-effectiveness analysis
of the implication of routine use in ALL therapy should be
performed with consideration of different economic scenarios.
In conclusion, it is recommended that CSFs can be
administered to ALL patients during myelosuppressive chemotherapy,
particularly in the induction phase. In statistics, the
administration of CSF reduces the mortality at the end of follow-up
and at day 30, decreases the occurrence of infections and shortens
the duration of neutropenia. By contrast, the administration of CSF
increases the CR rate following the induction course.
Acknowledgements
The present study was supported by a grant from the
National Nature Science Foundation of China (grant no.
81270615).
References
|
1
|
Jabbour EJ, Faderl S and Kantarjian HM:
Adult acute lymphoblastic leukemia. Mayo Clinic Proc. 80:1517–1527.
2005. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hiroto Inaba MG and Charles G Mullighan:
Acute lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esparza SD and Sakamoto KM: Topics in
pediatric leukemia-acute lymphoblastic leukemia. Med Gen Med.
7:232005.
|
|
5
|
Pui CH and Evans WE: Acute lymphoblastic
leukemia. N Engl J Med. 339:605–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pui CH, Pei D, Sandlund JT, et al:
Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B
and 14 for childhood acute lymphoblastic leukemia. Leukemia.
24:371–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaynon PS, Angiolillo AL, Carroll WL, et
al: Long-term results of the children's cancer group studies for
childhood acute lymphoblastic leukemia 1983–2002: a Children's
Oncology Group Report. Leukemia. 24:285–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linker C, Damon L, Ries C and Navarro W:
Intensified and shortened cyclical chemotherapy for adult acute
lymphoblastic leukemia. J Clin Oncol. 20:2464–2471. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kantarjian H, Thomas D, O'Brien S, et al:
Long-term follow-up results of hyperfractionated cyclophosphamide,
vincristine, doxorubicin and dexamethasone (Hyper-CVAD), a
dose-intensive regimen, in adult acute lymphocytic leukemia.
Cancer. 101:2788–2801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowe JM, Buck G, Burnett AK, et al:
Induction therapy for adults with acute lymphoblastic leukemia:
results of more than 1500 patients from the international ALL
trial: MRC UKALL XII/ECOG E2993. Blood. 106:3760–3767. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bassan R and Hoelzer D: Modern therapy of
acute lymphoblastic leukemia. J Clin Oncol. 29:532–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Annino L, Vegna ML, Camera A, et al:
Treatment of adult acute lymphoblastic leukemia (ALL): long-term
follow-up of the GIMEMA ALL 0288 randomized study. Blood.
99:863–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larson RA, Dodge RK, Burns CP, et al: A
five-drug remission induction regimen with intensive consolidation
for adults with acute lymphoblastic leukemia: cancer and leukemia
group B study 8811. Blood. 85:2025–2037. 1995.PubMed/NCBI
|
|
14
|
Takeuchi J, Kyo T, Naito K, et al:
Induction therapy by frequent administration of doxorubicin with
four other drugs, followed by intensive consolidation and
maintenance therapy for adult acute lymphoblastic leukemia: the
JALSG-ALL93 study. Leukemia. 16:1259–1266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas X, Boiron JM, Huguet F, et al:
Outcome of treatment in adults with acute lymphoblastic leukemia:
analysis of the LALA-94 trial. J Clin Oncol. 22:4075–4086. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffin JD: Hematopoietic growth
factorsCancer: Principles and Practice of Oncology. DeVita VT Jr,
Hellman S and Rosenburg SA: 1. 6th. Lippincott Williams &
Wilkins; Philadelphia, PA: pp. 2798–2809. 2001
|
|
17
|
Petros WP: Colony-stimulating
factorsCancer Chemotherapy and Biotherapy: Principles and Practice.
Chabner BA and Longo DL: 5th. Lippincott Williams & Wilkins;
Philadelphia, PA: pp. 357–392. 2001
|
|
18
|
Ozer H, Armitage JO, Bennett CL, et al:
2000 update of recommendations for the use of hematopoietic
colony-stimulating factors: evidence-based, clinical practice
guidelines. American Society of Clinical Oncology Growth Factors
Expert Panel. J Clin Oncol. 18:3558–3585. 2000.PubMed/NCBI
|
|
19
|
The Cochrane Collaboration: Review Manager
(RevMan) 5.2. http://tech.cochrane.org/revman/downloadAccessed.
December 252013.
|
|
20
|
Geissler K, Koller E, Hubmann E, et al:
Granulocyte colony-stimulating factor as an adjunct to induction
chemotherapy for adult acute lymphoblastic leukemia-a randomized
phase-III study. Blood. 90:590–596. 1997.PubMed/NCBI
|
|
21
|
Hallbook H, Bjorkholm M, Hagglund H and
Smedmyr B: Does granulocyte colony-stimulating factor improve
long-term outcome in adult acute lymphoblastic leukemia? Leuk
Lymphoma. 50:1872–1874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holowiecki J, Giebel S, Krzemien S, et al:
G-CSF administered in time-sequenced setting during remission
induction and consolidation therapy of adult acute lymphoblastic
leukemia has beneficial influence on early recovery and possibly
improves long-term outcome: a randomized multicenter study. Leuk
Lymphoma. 43:315–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ifrah N, Witz F, Jouet JP, et al:
Intensive short term therapy with granulocyte-macrophage-colony
stimulating factor support, similar to therapy for acute
myeloblastic leukemia, does not improve overall results for adults
with acute lymphoblastic leukemia. GOELAMS Group. Cancer.
86:1496–1505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Larson RA, Dodge RK, Linker CA, et al: A
randomized controlled trial of filgrastim during remission
induction and consolidation chemotherapy for adults with acute
lymphoblastic leukemia: CALGB study 9111. Blood. 92:1556–1564.
1998.PubMed/NCBI
|
|
25
|
Ottmann OG, Hoelzer D, Gracien E, et al:
Concomitant granulocyte colony-stimulating factor and induction
chemoradiotherapy in adult acute lymphoblastic leukemia: a
randomized phase III trial. Blood. 86:444–450. 1995.PubMed/NCBI
|
|
26
|
Thomas X, Boiron JM, Huguet F, et al:
Efficacy of granulocyte and granulocyte-macrophage
colony-stimulating factors in the induction treatment of adult
acute lymphoblastic leukemia: a multicenter randomized study.
Hematol J. 5:384–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bohlius J, Herbst C, Reiser M, Schwarzer G
and Engert A: Granulopoiesis-stimulating factors to prevent adverse
effects in the treatment of malignant lymphoma. Cochrane Database
Syst Rev: CD003189. 2008. View Article : Google Scholar
|
|
28
|
Clark OA, Lyman G, Castro AA, Clark LG and
Djulbegovic B: Colony stimulating factors for chemotherapy induced
febrile neutropenia. Cochrane Database Syst Rev: CD003039.
2003.
|
|
29
|
Renner P, Milazzo S, Liu JP, Zwahlen M,
Birkmann J and Horneber M: Primary prophylactic colony-stimulating
factors for the prevention of chemotherapy-induced febrile
neutropenia in breast cancer patients. Cochrane Database Syst Rev:
CD007913. 2012. View Article : Google Scholar
|
|
30
|
Gurion R, Belnik-Plitman Y, Gafter-Gvili
A, et al: Colony-stimulating factors for prevention and treatment
of infectious complications in patients with acute myelogenous
leukemia. Cochrane Database Syst Rev: CD008238. 2011. View Article : Google Scholar
|
|
31
|
Giebel S, Thomas X, Hallbook H, et al: The
prophylactic use of granulocyte-colony stimulating factor during
remission induction is associated with increased leukaemia-free
survival of adults with acute lymphoblastic leukaemia: a joint
analysis of five randomised trials on behalf of the EWALL. Eur J
Cancer. 48:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inukai T, Sugita K, Iijima K, et al:
Leukemic cells with 11q23 translocations express granulocyte
colony-stimulating factor (G-CSF) receptor and their proliferation
is stimulated with G-CSF. Leukemia. 12:382–389. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benko I, Kovacs P, Szegedi I, et al:
Effect of myelopoietic and pleiotropic cytokines on colony
formation by blast cells of children with acute lymphoblastic
leukemia. Naunyn-Schmiedeberg's Arch Pharmacol. 363:499–508. 2001.
View Article : Google Scholar
|
|
34
|
Gattei V, Aldinucci D, Attadia V, et al:
Human granulocyte-macrophage colony-stimulating factor supports the
clonogenic growth of B-lineage acute lymphoblastic leukemias
expressing myeloid antigens. Cytokines Cell Mol Ther. 3:141–151.
1997.PubMed/NCBI
|
|
35
|
Lexchin J, Bero LA, Djulbegovic B and
Clark O: Pharmaceutical industry sponsorship and research outcome
and quality: systematic review. BMJ. 326:1167–1170. 2003.
View Article : Google Scholar : PubMed/NCBI
|