Introduction
Currently, echocardiographic departments are
constantly suffering a reduction in the human resources in parallel
with a significant increase in demands. Thus, the organization of
these departments must be modified in order to provide the patient
with the best management at the precise time. Furthermore, the
patients who require an echocardiogram must move to the echo
department with a consequent cost in terms of money and time
(1).
Oncology is a continually growing medical branch.
New drugs have been combined to delay the progression of the
disease and even, to cure it. However, these may induce the
development of cardiotoxicity (2,3).
Anthracyclines and new targeted therapies have been succeeding in
breast cancer. Since anthracyclines induce an irreversible
cardiotoxicity, the efforts have been directed at improving the
understanding of the cardiotoxicity incidence and optimizing the
measures to address the benefits of new potential cardiotoxic
cancer-targeted therapies as oncological treatment. As a result,
breast cancer oncologists must acknowledge the time between
prescribing anthracyclines and anti-human epidermal growth factor
receptor 2 therapies or the possibility of a non-anthracycline
schedule to be evaluated in selected patients that would otherwise
not be treated (4,5). Optimal cardiac management requires a
good relationship between specialists, in this case with
cardiologists. The aim of this relationship must pursue the
patient's benefit as the oncological prognosis should not be
affected by a cardiac event that may force trastuzumab to be
withdrawn. In fact, a significant improvement in cardiac
examination (such as intervals and tools) and an early detection of
cardiac damage with a precocious and well-directed treatment would
increase the number of patients with treatment achievement
(6). This is the reason why these
patients should be echocardiographically monitored for the early
detection of cardiotoxicity to adapt or even change their
management (7,8). The echocardiographic evaluation of these
patients is targeted and it is principally focused on the left
ventricular (LV) global systolic function. Furthermore, these
patients have peculiar characteristics, such as the impairment in
their mobility, due to the chemotherapy adverse events and
immunodeficiency. Therefore, these patients become a group of
individuals prone to benefit from hand-held echocardiography
performed in the oncology clinic.
During the last decades, hand-held echocardiographic
devices have been developed (9–11).
Currently, they may carry out a complete echo study, without a
significant degradation of image quality. Furthermore, the price
and associated costs have considerably decreased. Thus, the
performance of a focused echocardiographic evaluation (known as
echoscopic heart evaluation) at the patient's location (such as the
outpatient clinic and hospital room) by a non-cardiologist appears
to be feasible for limited diagnostic issues (12).
The aim of the present study was to assess the
accuracy of echoscopic heart evaluation performed by an oncologist
with basic echocardiographic training with a simplified imaging
protocol in the outpatient clinic using a hand-held device. The
results of the echocardiogram performed the same day to the same
patient by a cardiologist expert in cardiovascular imaging using a
‘premium’ device was considered the reference method. The main
target variable was LV ejection fraction (LVEF).
Patients and methods
Patient population
The study cohort comprised consecutive unselected
patients who attended the oncology outpatient clinic at a tertiary
university hospital (Clinical Hospital San Carlos, Health Research
Institute, Madrid, Spain) between October 2013 and March 2014, and
had an indication to undergo a transthoracic echocardiogram. For
statistical purposes, the subjects included in the study were
divided into three groups, depending on the time when they were
enrolled. The first third comprised patients enrolled between
October 2013 and December 2013 (~2 months). The second third
comprised patients enrolled between December 2013 and January 2014
(~2 months) and the remaining third comprised patients between
January 2014 and March 2014 (~2 months). All the patients provided
written informed consent in accordance with a protocol approved by
the Institutional Review Committee.
Oncologist training
Two breast cancer oncologists attended a one-week
training period that included theoretical and practical teaching by
an expert cardiologist. This period focused on the evaluation of LV
diameters and LVEF. Each oncologist performed 20 studies during
that period. After the initial teaching period, the oncologists
were able to consult the cardiologists once each patient was
evaluated; however at that point, no previous measurement was
modified for the study.
Echocardiography
Every subject underwent two echo examinations. The
first examination was performed by one of the two oncologists
working in the study, using a hand-held device (Mindray M7 system
with a P4-2s transducer; Mindray Bio-Medical Electronics Co., Ltd.,
Shenzhen, China). The second was performed by a cardiologist expert
in cardiovascular imaging using a ‘premium’ (top-of-the-line,
full-feature echocardiographic system) device (Philips IE33 system
with an X5-1 transducer; Philips Healthcare, Andover, MA, USA). The
two echocardiograms were performed on the same day. Cardiologists
were blinded to the results obtained by the oncologists.
Standard 2D and M-mode echocardiographic
measurements were determined in accordance with the current
American Society of Echocardiography guidelines (13). All the values were analyzed according
to the same guidelines (13). Each
physician completed a report, including LV end-diastolic diameter
(LVEDD), LV end-systolic diameter (LVESD) and LVEF by means of the
Teichholz method from M-mode acquisition.
Statistical analysis
Categorical variables were described as absolute
number (%). Continuous variables were described as mean ± standard
deviation. Kolmogorov-Smirnov test was used to assess normal
distribution in continuous variables. Inter-observer agreement was
evaluated by means of the intra-class correlation coefficient
(ICC). Bland-Altman's plots were also constructed. A difference in
LVEF >10% between the measurement performed by the oncologist
and the one performed by the cardiologist was considered to be
clinically significant. The same consideration was received by
those patients with an LVEF <50% in one study and >50% in the
other one. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis was performed with
SPSS PASW statistics 15.0 package (SPSS Inc., Chicago, IL,
USA).
Results
Patient characteristics
Out of the 101 enrolled patients, 32 were men
(31.7%) and the mean age was 56.03±16.88 years. Two patients (2%)
had a poor-quality acoustic window. The mean echocardiographic
characteristics based on the measurements performed by oncologists
and cardiologists are detailed in Table
I.
 | Table I.Main characteristics. |
Table I.
Main characteristics.
| Characteristics | Values |
|---|
| Male gender, n
(%) | 32 (31.7) |
| Age, mean years ±
SD | 56.03±16.88 |
| Poor-quality acoustic
window, n (%) | 2 (2) |
| LVEDD-ONCO, mean cm ±
SD | 4.52±0.82 |
| LVESD-ONCO, mean cm ±
SD | 2.75±0.70 |
| LVEF-ONCO, mean % ±
SD | 68.35±9.98 |
| LVEDD-CARDIO, mean cm
± SD | 4.77±0.79 |
| LVESD-CARDIO, mean cm
± SD | 2.82±0.66 |
| LVEF-CARDIO, mean % ±
SD | 64.75±8.76 |
Global agreement analysis
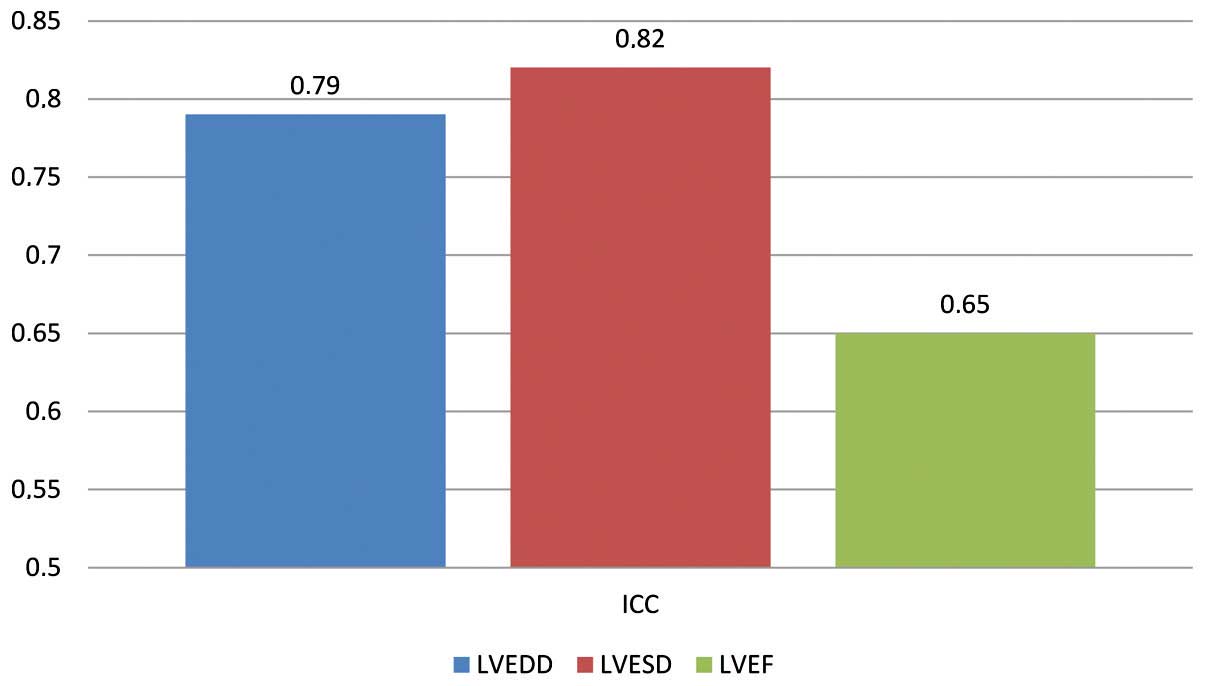
Agreement analysis is depicted in Fig. 1. There was a good global agreement for
the three mean analyzed variables. ICC for LVEDD was 0.79, 0.82 for
LVESD and 0.65 for LVEF. These data were obtained for the whole
population, not taking into account the period of time when each
study was performed.
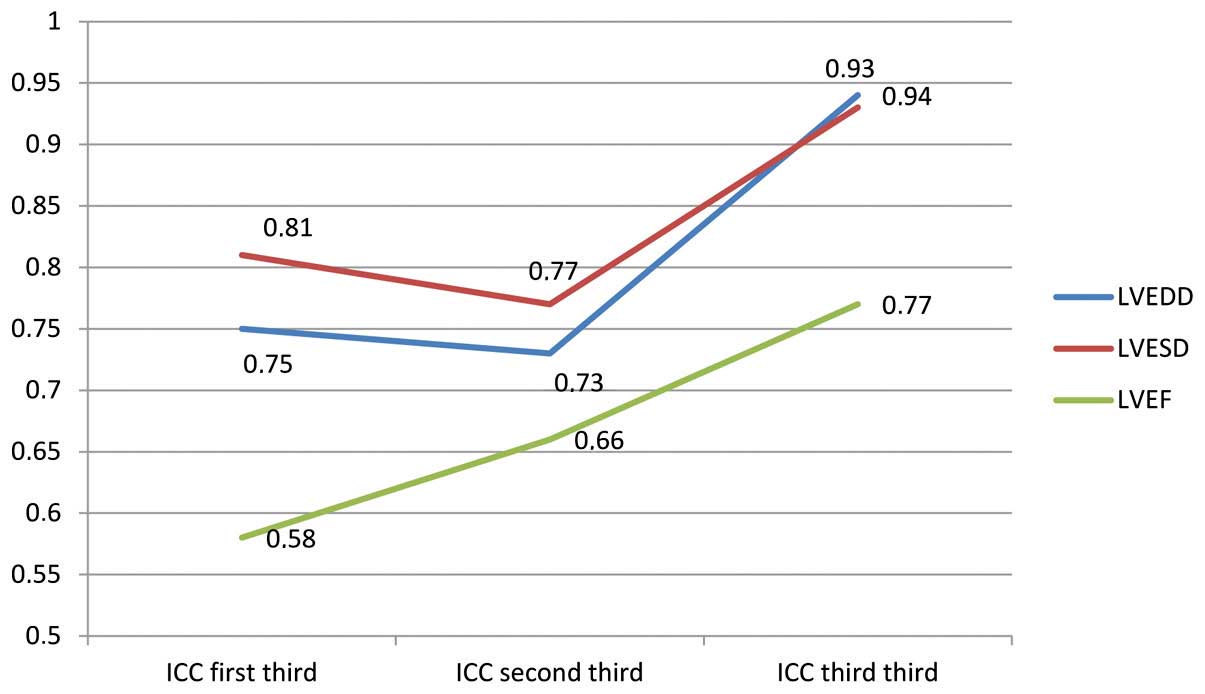
Agreement analysis based on temporal
division
When the results were analysed depending on the
period of time when the echo studies were performed, a clear
learning curve was observed. For LVEDD, ICC increased from 0.75 to
0.73 and to 0.94 along the three periods of time. For LVESD, ICC
progressed from 0.81 to 0.77 and to 0.93. Finally, LVEF started at
ICC=0.58, increased to 0.66 and in the third period of time reached
0.77. These results and its temporal evolution are shown in
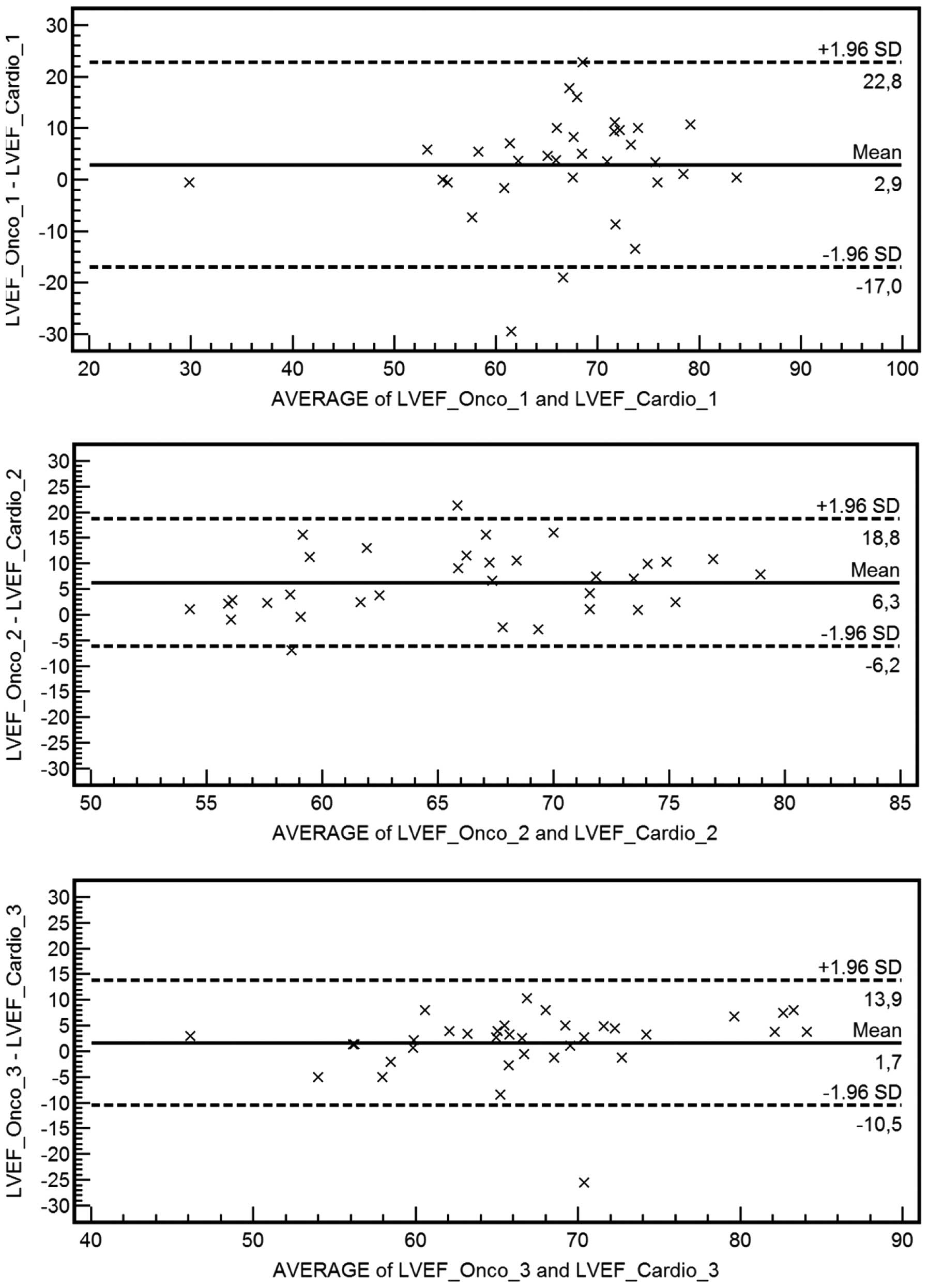
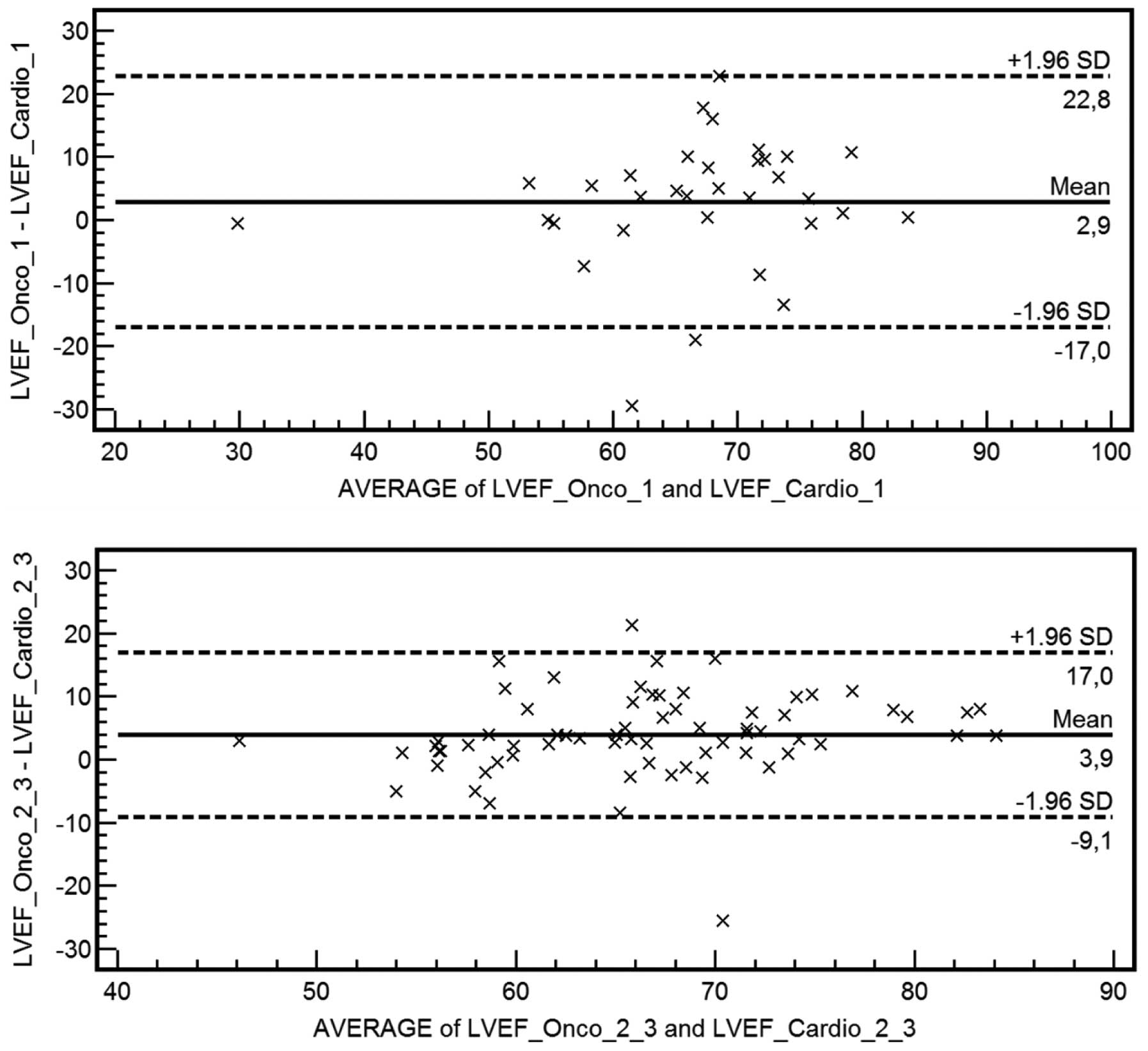
Fig. 2. Bland-Altman's graphs are
also depicted in Figs. 3 and 4.
Clinically significant
discrepancies
As previously defined, a clinically significant
difference was considered to be present if the difference in LVEF
was >10% between the measurement performed by the oncologist and
the one performed by the cardiologist or the LVEF was <50% in
one study (performed by oncologist or cardiologist) and >50% in
the other one. Results are shown in Table II. In these results, a clear learning
curve is also evident, particularly in the results for a difference
in LVEF >10%. The number of clinically significant discrepancies
was extremely low (22.8 and 1%, respectively for the two considered
variables) and they were identified only during the initial period
of time.
 | Table II.Clinically significant discrepancies
between the results of the echocardiograms performed by
cardiologists and oncologists. |
Table II.
Clinically significant discrepancies
between the results of the echocardiograms performed by
cardiologists and oncologists.
| Characteristics | Global, n (%) | First third, n
(%) | Second third, n
(%) | Final third, n
(%) |
|---|
| LVEF difference
>10% | 23 (22.8) | 10 (30.3) | 11 (33.3) | 2 (5.7) |
| LVEF < or
>50% | 1 (1.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) |
Discussion
The present study shows for the first time that
there is a good concordance between the use of a hand-held
echocardiographic device by a breast cancer medical oncologist and
a ‘premium’ system by a cardiologist for the simple, but highly
important, evaluations in patients under chemotherapy, mainly the
evaluation of LVEF. Therefore, these results show the possibility
of limited and focused echocardiographic studies, known as
echoscopic studies, to be performed by physicians different to the
cardiologist. Thus, these results show that following a short
training, an oncologist may be able to obtain echocardiographic
images and evaluate and measure them in order to take clinical
decisions on the patients regarding the use or maintenance of
chemotherapy drugs.
Currently, the best tool for the follow-up appears
to be echocardiography. It has the advantage of being accessible
and innocuous, but a recognizable decrease in LVEF is when damage
has already occurred, and with a normal LVEF it is not possible to
reject the possibility of cardiotoxicity absolutely. Training
should be a ‘must’ for any physician who is required to perform
echocardiographic studies. The training period for the oncologist,
according to the present results, may consist of a first one-week
theoretical-practical period followed by a 4-month practical
period, tailored by an expert cardiologist. During this practical
period, the cardiologist should be accessible to the oncologist to
consult any doubt. This recommendation is based on the fact that,
in the present study, the agreement between cardiologists and
oncologists considerably increases after 4 months of practice,
reaching a good level of agreement and a considerably low level of
clinically significant discrepancies, as is shown in Figs. 2–4.
Previous experiences with hand-held
echocardiographic systems have shown that these devices may improve
the cost-effectiveness of the service provided by the echo
laboratory and avoid patient discomfort derived from prolonged
waiting times prior and subsequent to the examination (9). Other studies show that the use of
hand-held echo systems improves workflow in the echo laboratory,
avoiding the requirement for porters, patient transfer and waiting
times (10–12). Furthermore, the cardiovascular imaging
units should perform a large number of advanced examinations, such
as stress echocardiograms and transesophageal echocardiograms. A
reduction in the workload could increase the time slots dedicated
to this type of examination.
The present results demonstrate that echoscopy in an
oncology department may be as accurate as a conventional
echocardiographic examination for a targeted evaluation.
Furthermore, it may reduce the requirement for porters and the
required patient time. This fact has important economic
implications. Badano et al (14) published the improvement in the
cost-effectiveness of the service provided by the echo laboratory
for inpatients. Performing the studies in the hospital ward instead
of in the echo laboratory avoids a long waiting time for patients
in the echo laboratory prior and subsequent to the examination,
decreases the number of days waiting for the examination and
increases sonographer and echo laboratory productivity. All these
improvements translate into a reduced cost of echocardiograms by
29% (14).
Thus, these hand-held devices in the hands of
well-trained oncologists, may became a new standard in the
evaluation of oncology patients, avoiding unnecessary waiting times
and reducing the overload in the echo laboratorys.
The present study had certain limitations. The
capabilities of the echo-portable devices are inferior to that of
standard complete systems. Therefore, echoscopic evaluations should
not replace conventional studies performed in the echo laboratory,
although it is sufficient in focused evaluations (1). Of note, new prognostic markers have
appeared for this type of patients and they are not evaluated by
these basic studies (15).
Furthermore, a learning period to acquire skills in obtaining and
measuring the echocardiographic images is strongly recommended and
it is necessary to provide high-quality instructions. Finally, an
expert cardiologist should always be available to aid in case of
doubts or technical limitations.
In conclusion, heart echoscopy performed by an
oncologist with a portable device may lead to a similar clinical
management as a study performed by an expert cardiologist with a
‘premium’ system in patients undergoing chemotherapy after a
relatively short training period. These results may lead to a
decrease in the echo laboratorys waiting lists and reduce patient
discomfort and echo-derived costs.
References
|
1
|
Quiles J, Garcia-Fernandez MA, Almeida PB,
Perez-David E, Bermejo J, Moreno M and Avanzas P: Portable spectral
Doppler echocardiographic device: Overcoming limitations. Heart.
89:1014–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fiuza M: Cardiotoxicity associated with
trastuzumab treatment of HER2+ breast cancer. Adv Ther.
26:(Suppl 1). S9–S17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen MH, Kerkela R and Force T: Mechanisms
of cardiac dysfunction associated with tyrosine kinase inhibitor
cancer therapeutics. Circulation. 118:84–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bria E, Cuppone F, Milella M, Verma S,
Carlini P, Nistico C, Vaccaro V, Rossi A, Tonini G, Cognetti F, et
al: Trastuzumab cardiotoxicity: Biological hypotheses and clinical
open issues. Expert Opin Biol Ther. 8:1963–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costa RB, Kurra G, Greenberg L and Geyer
CE: Efficacy and cardiac safety of adjuvant trastuzumab-based
chemotherapy regimens for HER2-positive early breast cancer. Ann
Oncol. 21:2153–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong RA, Iimura T, Sumida KN and Eager RM:
Cardio-oncology/onco-cardiology. Clin Cardiol. 33:733–737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin M, Esteva FJ, Alba E, Khandheria B,
Perez-Isla L, Garcia-Saenz JA, Marquez A, Sengupta P and Zamorano
J: Minimizing cardiotoxicity while optimizing treatment efficacy
with trastuzumab: Review and expert recommendations. Oncologist.
14:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray LJ, Ramakrishnan S, O'Toole L,
Manifold IH, Purohit OP and Coleman RE: Adjuvant trastuzumab in
routine clinical practice and the impact of cardiac monitoring
guidelines on treatment delivery. Breast. 19:339–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spencer KT, Anderson AS, Bhargava A, Bales
AC, Sorrentino M, Furlong K and Lang RM: Physician-performed
point-of-care echocardiography using a laptop platform compared
with physical examination in the cardiovascular patient. J Am Coll
Cardiol. 37:2013–2018. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawai J, Tanabe K, Yagi T, Fujii Y, Konda
T, Sumida T, Okada M, Yamaguchi K, Tani T, Yamabe K, et al:
Assessment of the clinical feasibility of OptiGo for hand-held
echocardiography. J Cardiol. 41:81892003.(In Japanese). PubMed/NCBI
|
|
11
|
Xie T, Chamoun AJ, McCulloch M, Tsiouris
N, Birnbaum Y and Ahmad M: Rapid screening of cardiac patients with
a miniaturized hand-held ultrasound imager-comparisons with
physical examination and conventional two-dimensional
echocardiography. Clin Cardiol. 27:241–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia Fernandez MA: Is it possible to
train non-cardiologists to perform echocardiography? Rev Esp
Cardiol (Engl Ed). 67:168–170. 2014.PubMed/NCBI
|
|
13
|
Lang RM, Bierig M, Devereux RB,
Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward
J, Shanewise JS, et al: Chamber Quantification Writing Group;
American Society of Echocardiography's Guidelines and Standards
Committee; European Association of Echocardiography:
Recommendations for chamber quantification: A report from the
American Society of Echocardiography's Guidelines and Standards
Committee and the Chamber Quantification Writing Group, developed
in conjunction with the European Association of Echocardiography, a
branch of the European Society of Cardiology. J Am Soc
Echocardiogr. 18:1440–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Badano LP, Nucifora G, Stacul S, Gianfagna
P, Pericoli M, Del Mestre L, Buiese S, Compassi R, Tonutti G, Di
Benedetto L, et al: Improved workflow, sonographer productivity and
cost-effectiveness of echocardiographic service for inpatients by
using miniaturized systems. Eur J Echocardiogr. 10:537–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plana JC, Galderisi M, Barac A, Ewer MS,
Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP,
et al: Expert consensus for multimodality imaging evaluation of
adult patients during and after cancer therapy: A report from the
American Society of Echocardiography and the European Association
of Cardiovascular Imaging. J Am Soc Echocardiogr. 27:911–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|