Introduction
Upper tract urothelial carcinoma (UTUC) is a rare
and highly aggressive urinary tumor, with a high recurrence rate
(mainly intravesical) and a poor prognosis. A bladder recurrence
rate of 44% was previously reported (1). Radical nephroureterectomy with bladder
cuff excision is recommend as the standard treatment by the
National Comprehensive Cancer Network and European Association of
Urology guidelines (2,3). However, the high rate of recurrence and
metastasis of UTUC pose a significant challenge for clinical
physicians. Neoadjuvant and adjuvant chemotherapy have been proven
to effectively decrease recurrence and improve survival (4,5).
Therefore, prognosis assessment is critical for designing a therapy
plan and increasing survival rate in patients with UTUC. However,
the number of studies investigating predictors of UTUC is limited,
due to its low incidence rate.
As a systematic inflammation-based biomarker, an
elevated C-reactive protein (CRP) level has been proven to be a
risk factor in several types of cancer (6). A previous meta-analysis demonstrated a
significant correlation between CRP level and renal cell carcinoma
(7). Therefore, the aim of this study
was to perform a systematic review and determine the prognostic
value of CRP in UTUC.
Materials and methods
Study selection process
A systematic literature search through PubMed,
Embase, Web of Science, the Cochrane Library and CBM databases was
conducted to identify the relevant clinical studies up to November,
2014. The search terms were as follows: (CRP OR C-reactive protein)
AND (ureteral neoplasms OR ureter cancer OR ureter carcinoma OR
renal pelvis cancer OR renal pelvic carcinoma OR upper tract
urothelial carcinoma OR UTUC). The reference lists of the retrieved
articles were searched for any additional relevant studies. The
initial selection was performed based on the title and abstract by
two independent investigators (Y. Luo and D.L. She). Thereafter,
the full text was reviewed according to the eligibility criteria.
To be eligible for this analysis, studies were required to evaluate
the association between preoperative CRP and UTUC prognosis,
including recurrence-free survival (RFS), cancer-specific survival
(CSS) or overall survival (OS). Studies with duplicated or
overlapping patient data and studies without sufficient available
data were excluded.
Quality assessment and data
extraction
The quality of the studies was independently
assessed by the Newcastle-Ottawa scale (NOS) (8). Data were extracted by two independent
investigators (Y. Luo and D.L. She) and cross-checked.
Disagreements were resolved through discussion. The extracted data
included the name of the first author, year of publication, source
of patients, cut-off value of CRP, prognostic outcomes delineated,
sample size, geographical region and follow-up time. The data were
extracted from the original articles. When required data were
missing, the original research data were requested directly from
the corresponding authors via e-mail. When the original data were
obtained, the cut-off value was set at 0.5 mg/dl; alternatively,
the provided survival or mortality curves were used to calculate
the hazard ratio (HR) and 95% confidence interval (CI), as
described by Tierney et al (9).
Statistical analysis
Review Manager 5.3 software (The Cochrane
Collaboration, Copenhagen, Denmark) was used for the analysis. As
the number of included studies was <10, quantitative publication
bias detection with Egger's regression intercept test or
meta-regression analysis was not performed (10–12). HR
was the time-to-event effect estimate for survival analysis and the
generic inverse variance method was used. First, Cochran's Q test
and Higgins I2 statistic were used to estimate
heterogeneity (11). when P≥0.1 and
I2≤50%, there was no significant heterogeneity and the
fixed-effects model was used. In any other case, the random-effects
model was used to calculate pooled HR. Univariate and multivariate
analyses were performed and two-tailed P-values of <0.05 were
considered to indicate statistically significant differences. All
the results are presented in the forest plots.
Results
Eligible studies and quality
assessment
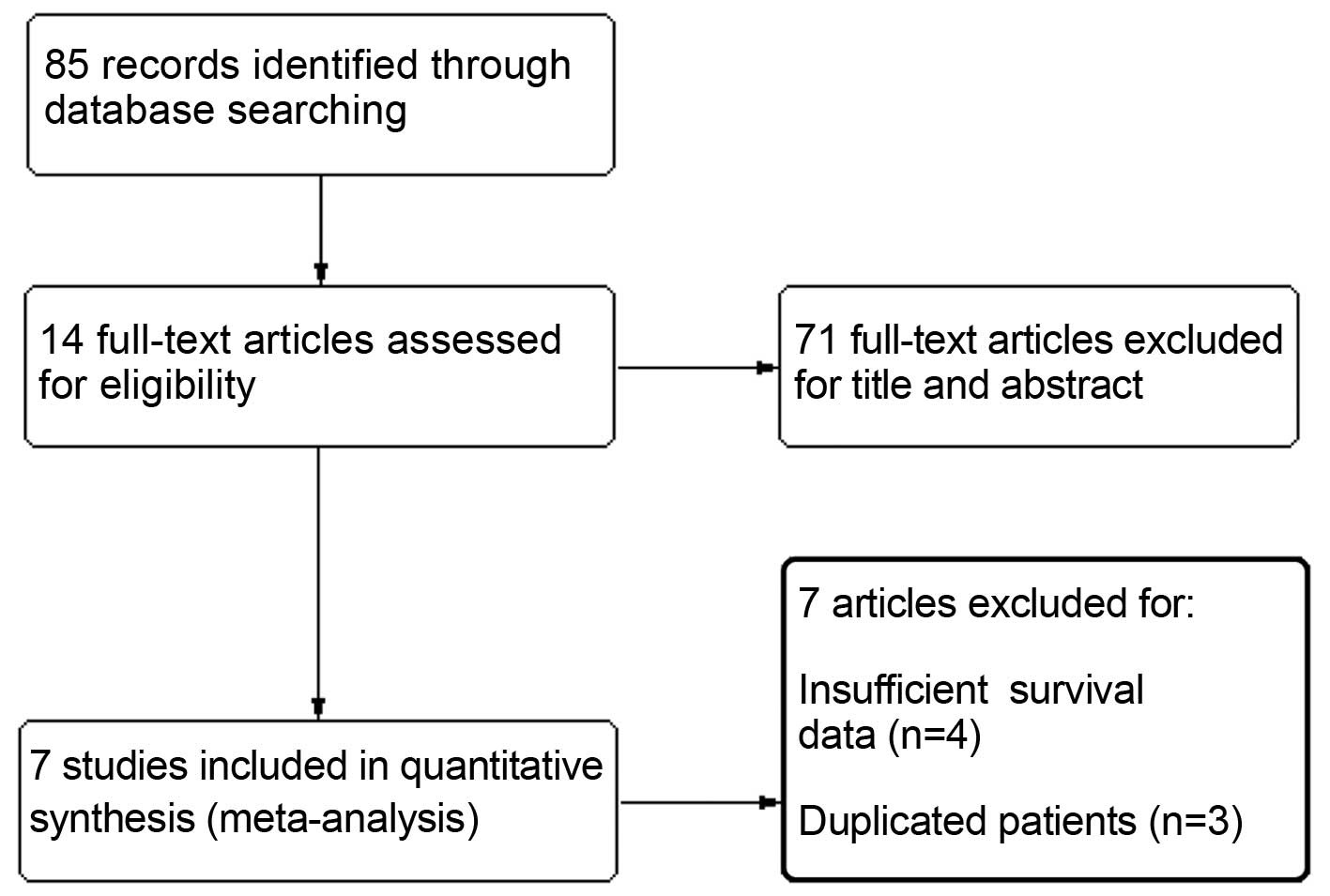
A total of 85 citations were selected by the initial
search strategy. After reading the title and abstract, 71 articles
were excluded for unrelated information. The remaining 14 articles
were read and relative information was extracted. A further 3
studies were excluded due to duplicated references or overlapping
patients and 4 studies due to insufficient survival data. Finally,
7 retrospective cohort studies including a total of 1,919 patients
were entered in the analysis (13–19). The
flow chart of the study selection process is presented in Fig. 1 and the basic characteristics of the
included studies are summarized in Table
I. The NOS scores were used to identify included studies of
high methodological quality. A total of 5 articles reported RFS, 6
reported CSS and only 2 reported OS.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| Author (year) | Country | Duration | Disease | No. of pts. | CRP cut.off
(mg/dl) | Outcomes | Median follow.up,
months (IQR/range) | NOS
scorea | (Refs.) |
|---|
| Saito et al
(2007) | Japan | 1990–2005 | Non-metastatic | 130 | 0.5 | RFS-CSS | 47 | 6 | (16) |
|
|
|
| UTUC |
|
|
| (3–190) |
|
|
| Azuma et al
(2013) | Japan | 1994–2008 | UTUC | 137 | 0.5 | RFS-CSS | 60.9 | 5 | (14) |
|
|
|
|
|
|
|
| (1.9–187.3) |
|
|
| Sakano et al
(2013) | Japan | 1995–2009 | Non-metastatic | 536 | 0.13 | CSS | 40.9 | 6 | (17) |
|
|
|
| UTUC |
|
|
| (3–200) |
|
|
| Stein et al
(2013) | Germany | 1981–2011 | UTUC | 115 | 0.5 | CSS | 15.1 | 7 | (18) |
|
|
|
|
|
|
|
| (7.2–37.7) |
|
|
| Aziz et al
(2014) | Germany | 1990–2012 | Non-metastatic | 265 | 0.9 | RFS-CSS-OS | 23 | 7 | (13) |
|
|
|
| UTUC |
|
|
| (10–48) |
|
|
| Cho et al
(2014) | Korea | 2004–2012 | Non-metastatic | 172 | 0.5 | RFS-OS | 33 | 5 | (15) |
|
|
|
| UTUC |
|
|
| (1–191) |
|
|
| Tanaka et al
(2014) | Japan | 1993–2010 | Non.metastatic,
locally confined or advanced | 564 | 0.5 | RFS-CSS | 32 | 7 | (19) |
|
|
|
| UTUC |
|
|
| (15–62) |
|
|
Meta-analysis
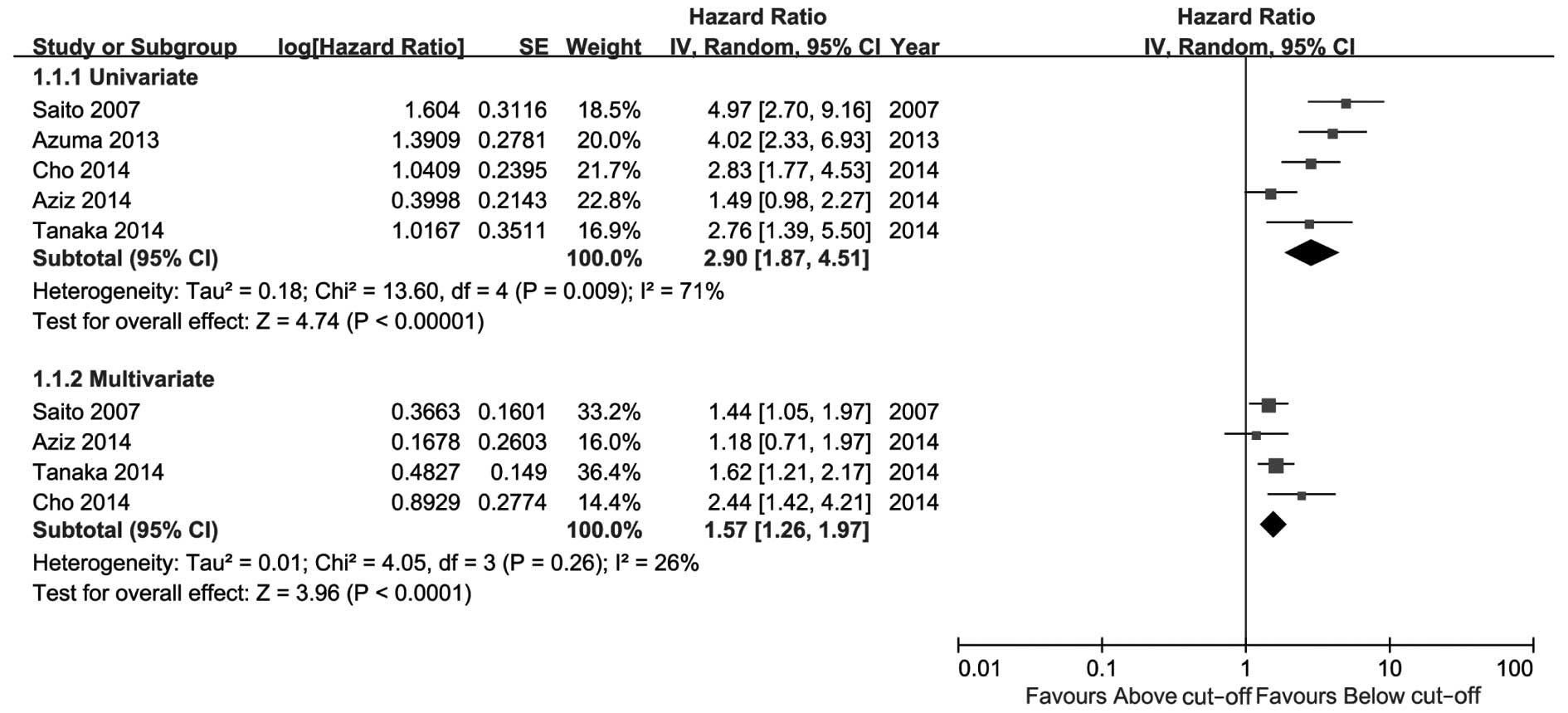
The meta-analysis results are presented in Figs. 2–4. As
shown in Fig. 2, high CRP level was a
significant risk factor for UTUC recurrence in the univariate as
well as the multivariate analysis. The comprehensive HR for RFS was
2.90 (95% CI: 1.87–4.51, P<0.00001) in the univariate and 1.57
(95% CI: 1.26–1.97, P<0.0001) in the multivariate analysis. As
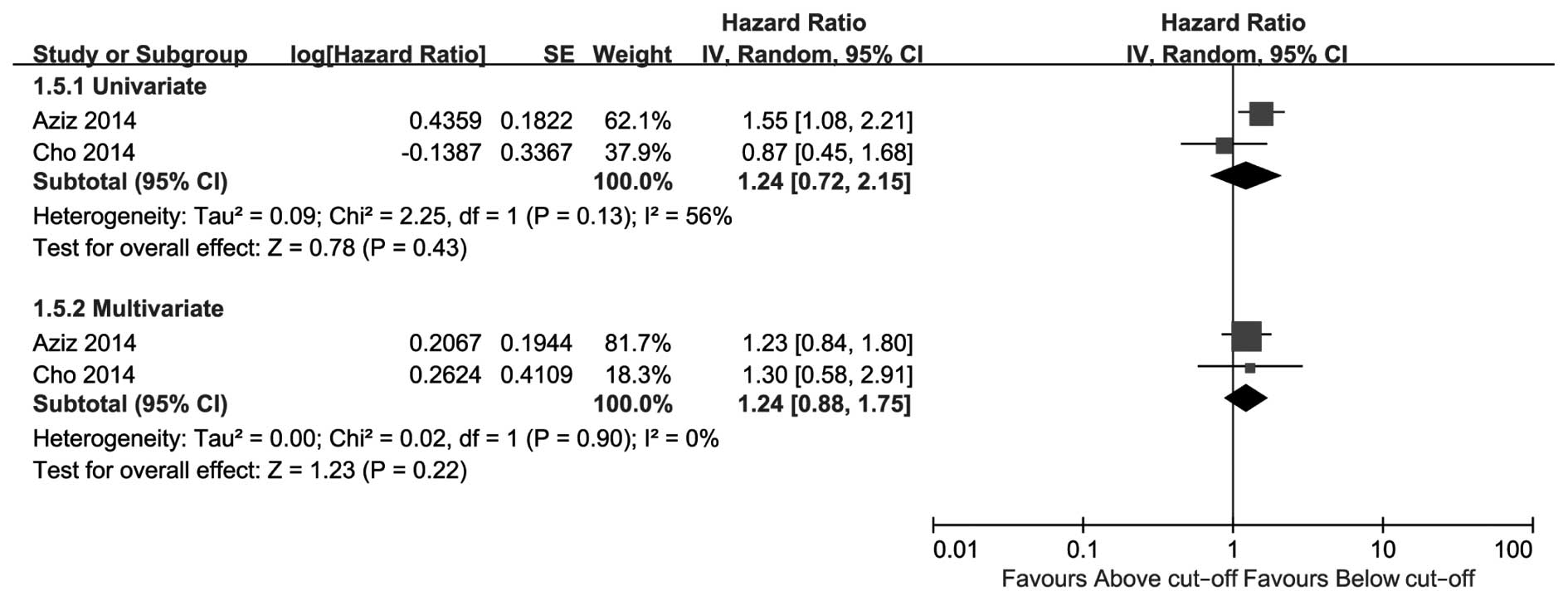
shown in Fig. 3, high CRP level was a
predictive risk factor for CSS of UTUC in the univariate as well as
the multivariate analysis, with pooled HRs of 2.78 (95% CI:
1.75–4.43, P<0.0001) and 1.64 (95% CI: 1.32–2.03, P<0.00001),
respectively. Patients with a high preoperative CRP level exhibited
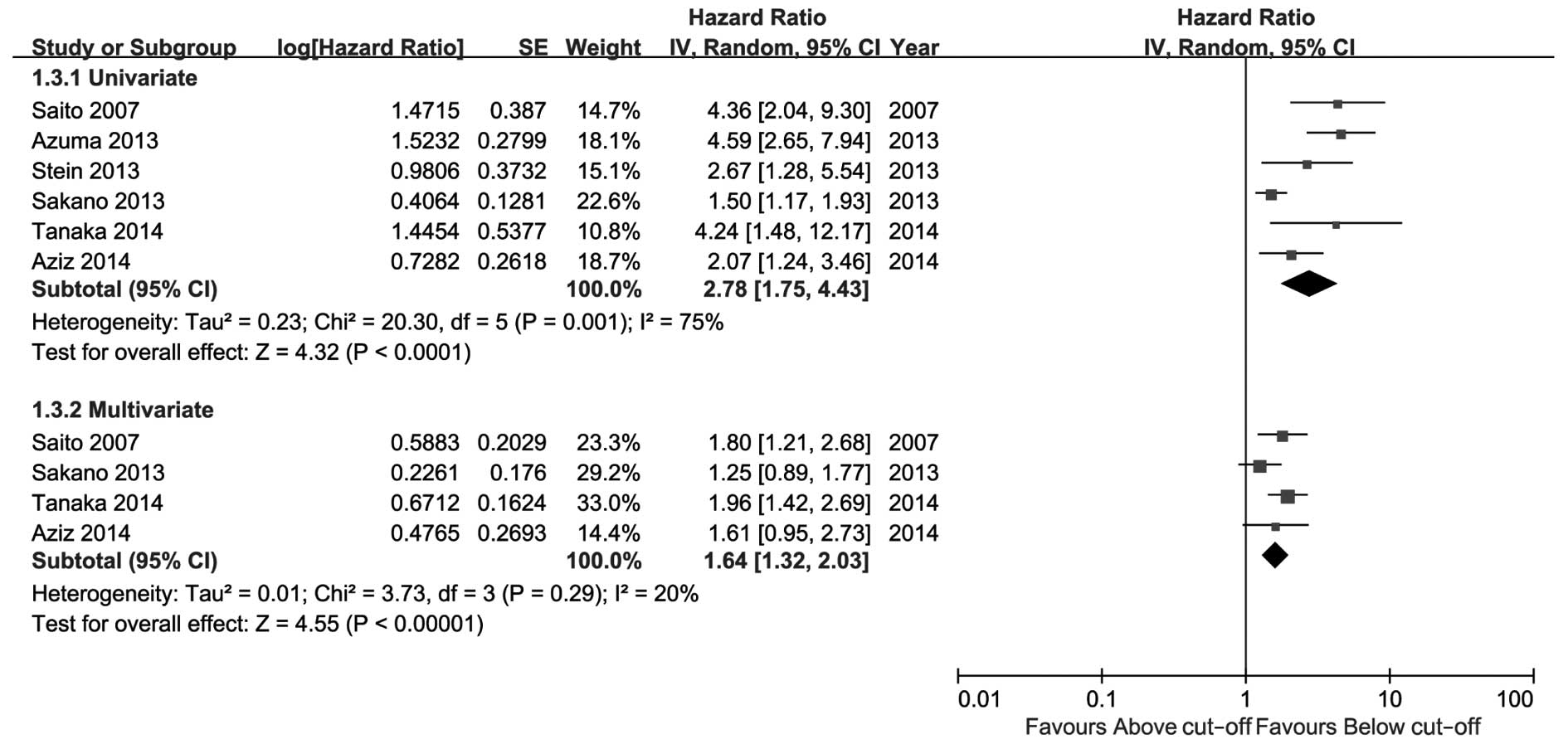
poorer CSS compared with patients with a low CRP level. Only 2
studies reported OS (Fig. 4); the
pooled HRs of univariate and multivariate analysis were not
significant [1.24 (95% CI: 0.72–2.15, P=0.43) and 1.24 (95% CI:
0.88–1.75, P=0.22), respectively].
Of note, 2 of the included studies utilized
inconsistent cut-off values for CRP level, which caused significant
heterogeneity as verified by a sensitivity analysis (data not
shown). However, the statistical significance remained the same
even following sensitivity analysis. A more significant effect may
have been observed if the cut-off value in these two studies was
set at 0.5 mg/dl (compared with 0.13 or 0.9 mg/dl; Table I).
Discussion
CRP is a non-specific biomarker of systemic
inflammation. Several studies have demonstrated an association
between CRP levels and tumor progression (6,7,20–26),
suggesting that a high CRP level predicts poor prognosis. This
association may be interpreted by the theory that the tumor itself
produces inflammatory cytokines, which promote tumor growth and
metastasis (19,27). If this theory is tenable, radical or
cytoreductive tumor resection may significantly decrease the levels
CRP and those of other inflammatory markers following surgery.
Should this hypothesis be validated, CRP may prove to be a valuable
response monitoring marker of UTUC or other tumors. However, no
relative studies were retrieved.
Several systematic reviews have verified CRP as a
tumor risk factor. A previous meta-analysis including 24 studies
demonstrated a significant association of elevated CRP levels with
tumor stage and grade in renal cell carcinoma, as well as with OS,
CSS and progression-free survival. The CRP level was directly
correlated with stage and grade and inversely correlated with
outcomes (7). Similar associations
were reported in lung (20),
colorectal (22), gastroesophageal
(23,25) and prostate cancer (21), hepatocellular carcinoma (26) and osteosarcoma (24). Our results are consistent with those
findings and amplify the evidence. In our meta-analysis, an
elevated CRP level indicated poor prognostic outcomes, but exerted
no significant effect on OS in terms of the current evidence.
CRP was not the unique predictor of prognosis; other
prognostic risk factors were found to be significantly associated
with UTUC prognosis. Lughezzani et al (28) summarized the prognostic factors of
UTUC, including clinical performance factors, pathological and
biomarkers. Clinicopathological parameters were the primary
prognostic risk factors of UTUC (19). In fact, certain pre- or intraoperative
manipulations may lead to metastasis. It was previously
demonstrated that preoperative diagnostic ureteroscopy may increase
intravesical seeding by irrigation and instrument adhesion and,
thus, lead to bladder cancer recurrence (29,30).
Although our results revealed a significant
association between CRP and UTUC patient survival, there were
several limitations to our study. In the forest plots, 2 studies
did not report the multivariate HRs; the stepwise regression model
was used in these studies, the results of which are not displayed
if they are not significant risk factors. Although the respective
corresponding authors were contacted for the original data, there
have been no replies. Thus, this may result in inevitable reporting
bias. Additionally, there was a small-study effect due to our
limited study sample.
In conclusion, our meta-analysis demonstrated that
CRP is a predictive serum biomarker for UTUC survival and a high
preoperative CRP level is significantly associated with poor
prognosis. However, considering the potential reporting bias risk
and small sample, further studies with more detailed information
are required to reach a definitive conclusion.
Acknowledgements
The authors are grateful to Dr Eu Chang Hwang,
Department of Urology, Chonnam National University Hwasun Hospital
(Chonnam National University Medical School, Republic of Korea),
for the original clinical data cited in this study (15).
References
|
1
|
Raman JD, Sosa RE, Vaughan ED Jr and
Scherr DS: Pathologic features of bladder tumors after
nephroureterectomy or segmental ureterectomy for upper urinary
tract transitional cell carcinoma. Urology. 69:251–254. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark PE, Agarwal N, Biagioli MC, et al:
National Comprehensive Cancer Network (NCCN): Bladder cancer. J
Natl Compr Canc Netw. 11:446–475. 2013.PubMed/NCBI
|
|
3
|
Rouprêt M, Babjuk M, Compérat E, et al:
European Association of Urology: European guidelines on upper tract
urothelial carcinomas: 2013 update. Eur Urol. 63:1059–1071. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leow JJ, Martin-Doyle W, Fay AP, Choueiri
TK, Chang SL and Bellmunt J: A systematic review and meta-analysis
of adjuvant and neoadjuvant chemotherapy for upper tract urothelial
carcinoma. Eur Urol. 66:529–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porten S, Siefker-Radtke AO, Xiao L,
Margulis V, Kamat AM, Wood CG, Jonasch E, Dinney CP and Matin SF:
Neoadjuvant chemotherapy improves survival of patients with upper
tract urothelial carcinoma. Cancer. 120:1794–1799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo YZ, Pan L, Du CJ, Ren DQ and Xie XM:
Association between C-reactive protein and risk of cancer: A
meta-analysis of prospective cohort studies. Asian Pac J Cancer
Prev. 14:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Q, Gou Y, Sun C, et al: The prognostic
value of C reactive protein in renal cell carcinoma: A systematic
review and meta-analysis. Urol Oncol. 32:50.e1–e8. 2014. View Article : Google Scholar
|
|
8
|
Wells GA, Shea B, O'Connell D, et al: The
Newcastle Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed.
December 20–2014
|
|
9
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. Version 5.1.0. updated.
March;2011.http://handbook.cochrane.org/Accessed. December
20–2014
|
|
12
|
Ioannidis JP and Trikalinos TA: The
appropriateness of asymmetry tests for publication bias in
meta-analyses: A large survey. CMAJ. 176:1091–1096. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aziz A, Rink M, Gakis G, et al:
Preoperative C-reactive protein in the serum: A prognostic
biomarker for upper urinary tract urothelial carcinoma treated with
radical nephroureterectomy. Urol Int. 93:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azuma T, Matayoshi Y, Odani K, Sato Y,
Sato Y, Nagase Y and Oshi M: Preoperative neutrophil-lymphocyte
ratio as an independent prognostic marker for patients with upper
urinary tract urothelial carcinoma. Clin Genitourin Cancer.
11:337–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho YH, Seo YH, Chung SJ, et al:
Predictors of intravesical recurrence after radical
nephroureterectomy for upper urinary tract urothelial carcinoma: An
inflammation-based prognostic score. Korean J Urol. 55:453–459.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saito K, Kawakami S, Ohtsuka Y, Fujii Y,
Masuda H, Kumagai J, Kobayashi T, Kageyama Y and Kihara K: The
impact of preoperative serum C-reactive protein on the prognosis of
patients with upper urinary tract urothelial carcinoma treated
surgically. BJU Int. 100:269–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakano S, Matsuyama H, Kamiryo Y, et al:
Yamaguchi Uro-Oncology Group: Risk group stratification based on
preoperative factors to predict survival after nephroureterectomy
in patients with upper urinary tract urothelial carcinoma. Ann Surg
Oncol. 20:4389–4396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein B, Schrader AJ, Wegener G, Seidel C,
Kuczyk MA and Steffens S: Preoperative serum C-reactive protein: A
prognostic marker in patients with upper urinary tract urothelial
carcinoma. BMC Cancer. 13:1012013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka N, Kikuchi E, Shirotake S, et al:
The predictive value of C-reactive protein for prognosis in
patients with upper tract urothelial carcinoma treated with radical
nephroureterectomy: A multi-institutional study. Eur Urol.
65:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin Y, Sun Y, Shi X, Zhao J, Shi L and Yu
X: Prognostic value of circulating C-reactive protein levels in
patients with non-small cell lung cancer: A systematic review with
meta-analysis. J Cancer Res Ther. 10:(Suppl). C160–C166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX,
Chen YJ and Xu Q: Prognostic role of C-reactive protein in prostate
cancer: A systematic review and meta-analysis. Asian J Androl.
16:467–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh PP, Zeng ISL, Srinivasa S, Lemanu
DP, Connolly AB and Hill AG: Systematic review and meta-analysis of
use of serum C-reactive protein levels to predict anastomotic leak
after colorectal surgery. Br J Surg. 101:339–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warschkow R, Tarantino I, Ukegjini K,
Beutner U, Müller SA, Schmied BM and Steffen T: Diagnostic study
and meta-analysis of C-reactive protein as a predictor of
postoperative inflammatory complications after gastroesophageal
cancer surgery. Langenbecks Arch Surg. 397:727–736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi JH, Wang D, Li ZY, Hu J, Niu XF and Liu
XL: C-reactive protein as a prognostic factor for human
osteosarcoma: A meta-analysis and literature review. PLoS One.
9:e946322014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Q, Yu XF, Zhang SD, Wang HH, Wang HY
and Teng LS: Prognostic role of C-reactive protein in gastric
cancer: A meta-analysis. Asian Pac J Cancer Prev. 14:5735–5740.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Z, Zhou L, Gao S, Yang Z, Yao J and
Zheng S: Prognostic role of C-reactive protein in hepatocellular
carcinoma: A systematic review and meta-analysis. Int J Med Sci.
10:653–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lughezzani G, Burger M, Margulis V, Matin
SF, Novara G, Roupret M, Shariat SF, Wood CG and Zigeuner R:
Prognostic factors in upper urinary tract urothelial carcinomas: A
comprehensive review of the current literature. Eur Urol.
62:100–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishikawa S, Abe T, Shinohara N, et al:
Impact of diagnostic ureteroscopy on intravesical recurrence and
survival in patients with urothelial carcinoma of the upper urinary
tract. J Urol. 184:883–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo HL, Kang CH, Chen YT, Chuang YC, Lee
WC, Cheng YT and Chiang PH: Diagnostic ureteroscopy independently
correlates with intravesical recurrence after nephroureterectomy
for upper urinary tract urothelial carcinoma. Ann Surg Oncol.
20:3121–3126. 2013. View Article : Google Scholar : PubMed/NCBI
|