Introduction
Breast cancer is the most frequent type of cancer
among women worldwide, with increasing incidence rates in the
majority of countries (1). In
Thailand, breast cancer is also the most common type of cancer
among women (2). Genetic alteration
is one of the key factors involved in breast cancer initiation and
progression. Human epidermal growth factor receptor 2
(HER2), an oncogene that is amplified and overexpressed in
breast cancer, has been correlated with more aggressive
characteristics, including negative estrogen receptor (ER) and
progesterone receptor (PR) status, higher histological grading,
lymph node involvement and resistance to chemotherapy.
In addition to oncogene alterations, angiogenesis,
the formation of new blood vessels, is of particular significance
in the process of cancer growth, invasion and metastasis (3,4). The most
important key modulator in this complex process is vascular
endothelial growth factor (VEGF). The expression of VEGF has been
correlated with the presence of higher microvessel density (MVD),
lymphovascular invasion (LVI) and shorter disease-free survival
(DFS) and overall survival (OS).
The analysis of plasma VEGF levels in metastatic
breast cancer patients receiving bevacizumab demonstrated that VEGF
levels >32.6 pg/ml were associated with shorter
time-to-progression (5). The
evaluation of VEGF in a randomized control trial on HER2-negative
metastatic breast cancer revealed that the pretreatment plasma
concentration of VEGF was correlated with a greater treatment
effect. In addition, patients with higher VEGF concentrations
exhibited lower hazard ratio (bevacizumab + docetaxel vs. placebo +
docetaxel) (6). A study of
VEGF polymorphisms in advanced breast cancer patients who
were treated with paclitaxel alone or paclitaxel+bevacizumab
(Eastern Cooperative Oncology Group 2100) revealed that
VEGF-2578 AA and -1154 AA were associated with better OS in
the combination arm (7).
HER2 activation is one of several mechanisms that
upregulate VEGF expression. The evaluation of VEGF along
with HER2, ER and PR status may provide useful information
regarding the aggressiveness of breast cancer and may help identify
patients who are suitable for anti-VEGF treatment.
Patients and methods
Study population
The patients were recruited from the Division of
Head-Neck and Breast Surgery, Department of Surgery, Faculty of
Medicine, Siriraj Hospital (Bangkok, Thailand), between 2002 and
2004. All the patients with histopathologically confirmed breast
carcinoma fulfilling the selection criteria were asked to be
participated in this study. Patients who were diagnosed with breast
cancer, aged ≥18 years and able to provide written informed
consent, were included in the study. Patients with history of other
cancers were excluded. At recruitment, informed consent was
obtained from all the subjects and each participant was interviewed
to collect detailed information on demographic characteristics and
family history of cancer.
This study's protocol was approved by the
Institutional Review Board of the Siriraj Hospital.
Immunohistochemistry
The expression levels of HER2 and MVD in breast
cancer tissue were assessed by immunohistochemical staining with
specific antibodies. Paraffin-embedded sections from each specimen
were stained with monoclonal rabbit antihuman HER2 antibody, clone
4B5 (ready to use, incubation time 8 h; catalog no. 790-289921;
Roche Diagnostics GmbH, Mannheim, Germany) and monoclonal mouse
anti-human antibody to transmembrane glycoprotein CD31, clone JC70A
(dilution 1:300, incubation time 16 h; catalog no. M082301; Dako
Denmark A/S, Glostrup, Denmark). The 3-µm sections were incubated
at 56°C overnight, deparaffinized and rehydrated. To block
endogenous peroxidase activity, the sections were incubated in 3%
H2O2 in deionized water for 10 min and then
washed with running distilled water for 5 min. Antigen retrieval
was performed by boiling the sections in 10 mmol/l citrate buffer
(pH 6.0). The sections were placed in phosphate-buffered saline
(PBS) for 10 min and then in 2% bovine serum albumin (BSA) for 30
min. The excess BSA was removed. The sections were stained with the
primary antibody at room temperature, washed twice with PBS for 5
min, incubated with secondary rabbit anti-mouse antibody (catalog
no. K500711; EnVision; Dako Denmark A/S) for 30 min. Following
incubation, the sections were washed twice with PBS for 5 min,
incubated in 3,3′-diaminobenzidine for 5 min and washed in tap
water for 5 min. The sections were counterstained with
haematoxylin, dehydrated, fixed and mounted. All the
immunohistochemical data were evaluated by two pathologists who
were blinded to the patients' characteristics and clinical
outcome.
Assessment of VEGF mRNA expression
levels
The levels of VEGF mRNA expression were
assayed by semiquantitative reverse transcription-polymerase chain
reaction, as previously described (8). Each RNA sample was assayed in duplicate
and in two separate settings.
Statistical analysis
Patient data on cancer recurrence and death were
retrieved through medical record review. The dates of recurrence
and death were recorded. The date of last contact was defined as
the date of the patient's last visit to the department where they
had received breast cancer therapy (Division of Head-Neck and
Breast Surgery, Department of Surgery; Division of Oncology,
Department of Medicine; and Division of Therapeutic Radiology,
Department of Radiology, Siriraj Hospital). The DFS analysis
endpoint was cancer recurrence/metastasis or breast cancer-related
death. DFS was defined as the time from diagnosis to the endpoint
(recurrence, metastasis, or breast cancer-related death), censoring
at the date of last contact or non-cancer death. The OS analysis
endpoint was breast cancer-related death. OS was defined as the
time from diagnosis to the endpoint of the study, censoring at the
date of last contact or non-cancer death. Survival curves were
constructed using the Kaplan-Meier product-limit method and
statistical significance was assessed using the log-rank test. A
multivariate analysis was performed to evaluate the effect of
prognostic factors on OS, using the Cox proportional hazards model.
The statistical analyses were conducted using SPSS software version
15.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate statistically significant differences.
Results
VEGF mRNA expression in breast cancer
tissue
A total of 99 breast cancer patients were recruited.
The patient characteristics are summarized in Table I. The mean age at diagnosis was 51.42
years (range, 39.49–63.35 years), with a median age of 50 years. A
total of 91 patients had invasive ductal carcinoma; 62 patients had
tumor size >20–50 mm; 55 patients had axillary nodal metastasis
and 6 patients had distant metastasis at the time of diagnosis. The
assessment of VEGF mRNA expression revealed that the mRNA
ratio ranged from 0 to 3.27, with a median mRNA ratio of 1.16. At
this cut-off value, 49 patients exhibited low and 50 patients high
VEGF mRNA expression.
 | Table I.Clinicopathological and demographic
characteristics of breast cancer patients. |
Table I.
Clinicopathological and demographic
characteristics of breast cancer patients.
| Characteristics | Patients, no. (%)
(n=99) |
|---|
| Age at diagnosis,
years |
|
|
<50 | 49 (49.50) |
| ≥50 | 50 (50.50) |
| Tumor type |
|
| Invasive
ductal carcinoma | 91 (91.92) |
| Invasive
lobular carcinoma | 3 (3.03) |
|
Others | 5 (5.05) |
| Tumor size, mm |
|
| ≤20 | 20 (20.20) |
|
20–50 | 62 (62.63) |
|
>50 | 17 (17.17) |
| Axillary nodal
metastasis |
|
| No | 44 (44.44) |
| Yes | 55 (55.56) |
| Distant
metastasis |
|
| No | 93 (93.94) |
| Yes | 6 (6.06) |
| Stage at
diagnosis |
|
| I | 12 (12.12) |
| II | 52 (52.53) |
| III | 29 (29.29) |
| IV | 6 (6.06) |
| Histological
differentiation |
|
| High | 3 (3.03) |
|
Moderate | 58 (58.59) |
| Poor | 35 (35.35) |
|
Unknown | 3 (3.03) |
| Lymphovascular
invasion |
|
|
Absent | 49 (49.49) |
|
Present | 46 (46.46) |
| Perineural
invasion |
|
|
Absent | 73 (73.74) |
|
Present | 15 (15.15) |
| Estrogen
receptor |
|
|
Negative | 42 (42.42) |
|
Positive | 57 (57.58) |
| Progesterone
receptor |
|
|
Negative | 57 (57.58) |
|
Positive | 42 (42.42) |
| HER2 |
|
|
Negative | 72 (72.73) |
|
Positive | 27 (27.27) |
Correlation between VEGF expression
and clinicopathological characteristics
On univariate analysis, high VEGF expression
was correlated with the presence of LVI [odds ratio (OR)=2.96, 95%
confidence interval (CI): 1.28–6.83; P=0.011]. High VEGF
expression tended to be correlated with locally advanced breast
cancer [stage III (except T3N1M0) and IV] (OR=2.30, 95% CI:
0.96–5.54; P=0.062). However, the multivariate analysis failed to
demonstrate the statistical significance of this correlation. The
distribution of VEGF expression status by different
clinicopathological characteristics is summarized in Table II. Breast cancer patients were
classified into 4 groups according to the ER, PR and HER2 status.
The numbers of patients with hormone
receptor-positive/HER2-negative, hormone
receptor-positive/HER2-positive, HER2-positive and triple-negative
breast cancer were 47 (47.47%), 10 (10.10%), 17 (17.17%) and 25
(25.25%), respectively.
 | Table II.Proportion of VEGF expression
among different clinicopathological characteristics. |
Table II.
Proportion of VEGF expression
among different clinicopathological characteristics.
|
| mRNA expression |
|
|---|
|
|
|
|
|---|
| Characteristics | Low (n=49) | High (n=50) | P-value |
|---|
| Age, years |
|
| 0.482 |
|
<50 | 26 | 23 |
|
| ≥50 | 23 | 27 |
|
| Tumor size, mm |
|
| 0.121 |
| ≤20 | 13 | 7 |
|
|
>20 | 36 | 43 |
|
| Axillary nodal
metastasis |
|
| 0.088 |
| No | 26 | 18 |
|
| Yes | 23 | 32 |
|
| Distant
metastasis |
|
| 0.097 |
| No | 48 | 45 |
|
| Yes | 1 | 5 |
|
| Early-stage
cancer |
|
| 0.060 |
| Yes | 38 | 30 |
|
| No | 11 | 20 |
|
| Histological
differentiation |
|
| 0.806 |
| High | 2 | 1 |
|
|
Moderate | 28 | 30 |
|
| Poor | 18 | 17 |
|
| Lymphovascular
invasion |
|
| 0.010 |
|
Absent | 30 | 19 |
|
|
Present | 16 | 30 |
|
| Perineural
invasion |
|
| 0.451 |
|
Absent | 37 | 36 |
|
|
Present | 6 | 9 |
|
| Estrogen
receptor |
|
| 0.257 |
|
Positive | 31 | 26 |
|
|
Negative | 18 | 24 |
|
| Progesterone
receptor |
|
| 0.191 |
|
Positive | 24 | 18 |
|
|
Negative | 25 | 32 |
|
| Hormone
receptor |
|
| 0.257 |
|
Positive | 31 | 26 |
|
|
Negative | 18 | 24 |
|
| HER2 |
|
| 0.101 |
|
Negative | 32 | 40 |
|
|
Positive | 17 | 10 |
|
In patients with hormone
receptor-positive/HER2-positive, HER2-positive and triple-negative
breast cancer, high VEGF expression was correlated with
axillary nodal metastasis (OR=3.56, 95% CI: 1.13–11.15; P=0.030).
High VEGF expression was also correlated with the presence
of LVI in patients with hormone receptor-positive/HER2-negative
(OR=3.75, 95% CI: 1.08–13.07; P=0.038).
Survival analysis
The median follow-up was 58.73 months (range,
1.23–93.03 months). The univariate analysis of survival by the
Kaplan-Meier method revealed that the presence of perineural
invasion (PNI), PR negativity and the presence of axillary nodal
metastasis were correlated with lower DFS rates (P<0.001,
P=0.017 and 0.043, respectively). The presence of PNI, PR
negativity, the presence of distant metastasis at the time of
diagnosis and advanced-stage breast cancer were correlated with
lower OS (P=0.011, 0.035, 0.003 and 0.009, respectively). The DFS
and OS rates by clinicopathological characteristics and levels of
VEGF expression are summarized in Table III. In the hormone
receptor-positive/HER2-positive, HER2-positive and triple-negative
groups, the presence of PNI was associated with lower DFS rates
(P<0.001). High VEGF expression, the presence of distant
metastasis at the time of diagnosis and advanced-stage breast
cancer were found to be correlated with lower OS rates (P=0.041,
<0.001 and 0.008, respectively; data not shown). In the hormone
receptor-positive/HER2-negative group, the presence of PNI and
distant metastasis at the time of diagnosis were correlated with
lower OS rates (P=0.019 and 0.013, respectively; data not shown).
However, the Cox regression analysis did not identify a significant
correlation of clinicopathological characteristics with DFS and OS.
The DFS and OS rates by VEGF expression in the hormone
receptor-positive/HER2-positive, HER2-positive and triple-negative
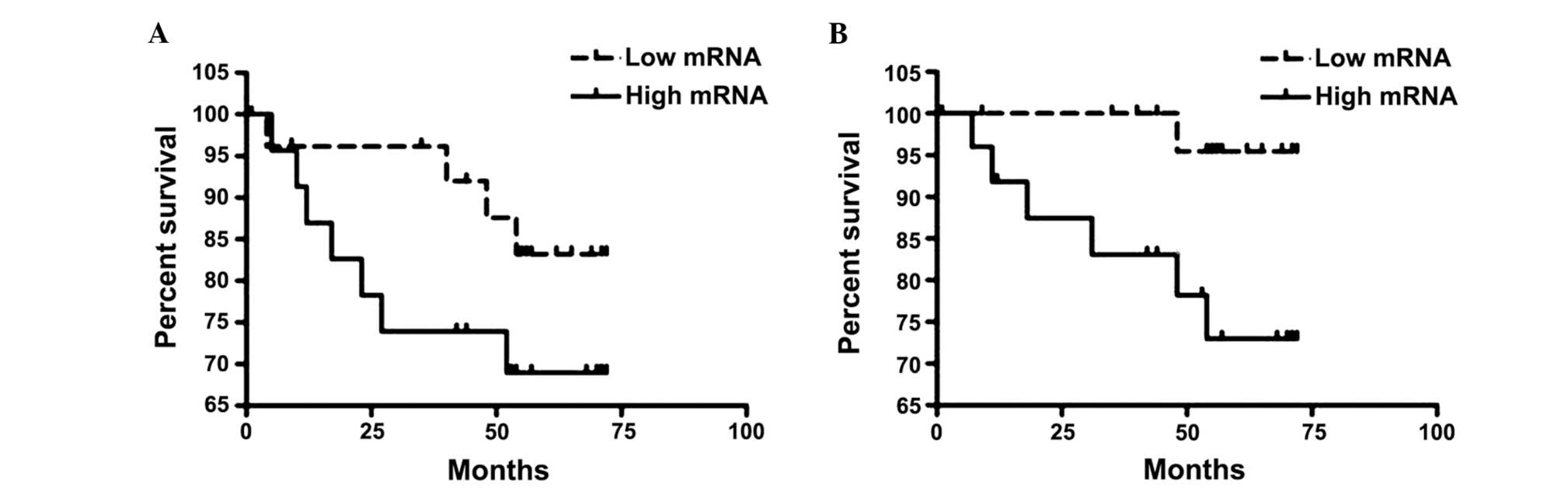
groups are shown in Fig. 1.
 | Table III.Disease-free survival (DFS) and
overall survival (OS) by clinicopathological characteristics and
VEGF expression level. |
Table III.
Disease-free survival (DFS) and
overall survival (OS) by clinicopathological characteristics and
VEGF expression level.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
Characteristics | Cases (n=90) | Events (n=20) | 5-year survival
(%) | P-value | Cases (n=96) | Events (n=9) | 5-year survival
(%) | P-value |
|---|
| Age, years |
|
|
| 0.679 |
|
|
| 0.719 |
|
<50 | 45 | 9 | 80.0 |
| 47 | 4 | 91.5 |
|
|
≥50 | 45 | 11 | 75.6 |
| 49 | 5 | 89.8 |
|
| Tumor size, mm |
|
|
| 0.059 |
|
|
| 0.117 |
|
≤20 | 28 | 1 | 94.4 |
| 19 | 0 | 100.0 |
|
|
>20 | 72 | 19 | 73.6 |
| 77 | 9 | 88.3 |
|
| Histological
differentiation |
|
|
| 0.449 |
|
|
| 0.862 |
|
High/moderate | 55 | 13 | 76.4 |
| 60 | 5 | 91.7 |
|
|
Poor | 33 | 6 | 81.8 |
| 33 | 3 | 90.9 |
|
| LVI |
|
|
| 0.354 |
|
|
| 0.314 |
|
Absent | 46 | 8 | 82.6 |
| 49 | 3 | 93.9 |
|
|
Present | 40 | 10 | 75.0 |
| 43 | 5 | 88.4 |
|
| PNI |
|
|
| <0.001 |
|
|
| 0.011 |
|
Absent | 67 | 9 | 86.6 |
| 72 | 3 | 95.8 |
|
|
Present | 13 | 7 | 46.2 |
| 14 | 3 | 78.6 |
|
| ER |
|
|
| 0.822 |
|
|
| 0.141 |
|
Positive | 52 | 11 | 78.8 |
| 56 | 3 | 94.6 |
|
|
Negative | 38 | 9 | 76.3 |
| 40 | 6 | 85.0 |
|
| PR |
|
|
| 0.017 |
|
|
| 0.035 |
|
Positive | 38 | 4 | 89.5 |
| 41 | 1 | 97.6 |
|
|
Negative | 52 | 16 | 69.2 |
| 55 | 8 | 85.5 |
|
| Hormone
receptor |
|
|
| 0.822 |
|
|
| 0.141 |
|
Positive | 52 | 11 | 78.8 |
| 56 | 3 | 94.6 |
|
|
Negative | 38 | 9 | 76.3 |
| 40 | 6 | 85.0 |
|
| HER2 |
|
|
| 0.457 |
|
|
| 0.245 |
|
Negative | 64 | 13 | 79.7 |
| 70 | 5 | 92.9 |
|
|
Positive | 26 | 7 | 73.1 |
| 26 | 4 | 84.6 |
|
| Subtype |
|
|
| 0.813 |
|
|
| 0.110 |
|
HR+HER2- | 42 | 9 | 78.6 |
| 46 | 2 | 95.7 |
|
|
Others | 48 | 11 | 77.1 |
| 50 | 7 | 86.0 |
|
| VEGF |
|
|
| 0.745 |
|
|
| 0.076 |
|
Low | 47 | 10 | 78.7 |
| 48 | 2 | 95.8 |
|
|
High | 43 | 10 | 76.7 |
| 48 | 7 | 85.4 |
|
| Axillary nodal
metastasis |
|
|
| 0.043 |
|
|
| 0.089 |
| No | 43 | 6 | 86.0 |
| 44 | 2 | 95.5 |
|
|
Yes | 47 | 14 | 70.2 |
| 52 | 7 | 86.5 |
|
| Distant
metastasis |
|
|
|
|
|
|
| 0.003 |
| No |
|
|
|
| 90 | 7 | 92.2 |
|
|
Yes |
|
|
|
| 6 | 2 | 66.7 |
| Early-stage
cancer |
|
|
| 0.090 |
|
|
| 0.009 |
|
Yes | 67 | 12 | 82.1 |
| 67 | 3 | 95.5 |
|
| No | 23 | 8 | 65.2 |
| 29 | 6 | 79.3 |
|
Discussion
The results of this study demonstrated an
association between high VEGF expression and the presence of
LVI. This finding was in concordance with those of several previous
studies, as reviewed elsewhere (9).
We also demonstrated a significant association of VEGF
expression with axillary nodal metastasis and lower OS in hormone
receptor-positi ve/HER2-positive, HER2-positive and triple-negative
breast cancer. However, due to the limited number of patients, the
multivariate analysis failed to demonstrate a statistically
significant difference.
Luminal B, HER2 and triple-negative subtypes were
found to be more aggressive compared with luminal A subtype by
tumor stage, lymph node status, or pathological type and also
exhibited worse DFS and OS (10,11). The
identification of high-risk patients and selection of an intensive
regimen may improve treatment outcome. The expression of VEGF was
found to be associated with reduced response to adjuvant endocrine
treatment. In a retrospective study of 699 breast cancer patients
conducted by Linderholm et al (12), the patients who received adjuvant
endocrine therapy and exhibited higher VEGF expression had
significantly shorter relapse-free survival and OS. In a study of
160 ER-positive advanced breast cancer patients who received
tamoxifen, an above median VEGF level was correlated with shorter
progression-free survival and post-relapse OS (13). In a randomized control trial of 224
breast cancer patients comparing 2 years of tamoxifen treatment
with no tamoxifen treatment, regardless of hormone receptor and
HER2 status, the patients with ER-positive and VEGF-negative tumors
significant benefited from tamoxifen after a 10-year follow-up,
whereas the patients with ER- and VEGF-positive tumors did not
benefit from tamoxifen treatment (14).
In a large study on 1,788 breast cancer patients,
higher frequency of VEGF expression was correlated with luminal B,
HER2 and basal-like subtypes. VEGF expression was associated with
increased risks of breast cancer-specific mortality and distant
recurrence among luminal A patients (15). In the present study, however, we did
not identify a significant difference in VEGF expression
frequency among breast cancer subtypes. In that study, conducted by
Liu et al (15), VEGF
immunohistochemistry was performed using VG1 antibody. VEGF
positivity was defined as any positive staining in the cytoplasm of
the tumor cells. By this definition, 72.5% of the patients were
positive for VEGF. In our study, the median of the VEGF ratio was
used as cut-off point. Using this definition, the patients were
evenly distributed into low and high VEGF expression groups.
The characteristics of the patients were also different, with
higher stage and lower age at diagnosis compared with those
reported earlier.
In conclusion, we demonstrated the role of
VEGF in non-luminal A (hormone
receptor-positive/HER2-positive, HER2-positive and triple-negative)
breast cancer. The assessment of the VEGF status in this
group of patients may help identify high-risk patients and may be
used to guide appropriate treatment selection.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute: Information
Technology Division, . http://www.nci.go.th/th/cancer_record/cancer_rec1.htmlAccessed.
November 1–2014
|
|
3
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burstein HJ, Chen YH, Parker LM, et al:
VEGF as a marker for outcome among advanced breast cancer patients
receiving anti-VEGF therapy with bevacizumab and vinorelbine
chemotherapy. Clin Cancer Res. 14:7871–7877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miles DW, de Haas SL, Dirix LY, et al:
Biomarker results from the AVADO phase 3 trial of first-line
bevacizumab plus docetaxel for HER2-negative metastatic breast
cancer. Br J Cancer. 108:1052–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schneider BP, Wang M, Radovich M, et al:
ECOG 2100: Association of vascular endothelial growth factor and
vascular endothelial growth factor receptor-2 genetic polymorphisms
with outcome in a trial of paclitaxel compared with paclitaxel plus
bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol.
26:4672–4678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O-Charoenrat P, Rhys-Evans P, Modjtahedi H
and Eccles SA: Vascular endothelial growth factor family members
are differentially regulated by c-erbB signaling in head and neck
squamous carcinoma cells. Clin Exp Metastasis. 18:155–161. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sa-Nguanraksa D and O-Charoenrat P: The
role of vascular endothelial growth factor a polymorphisms in
breast cancer. Int J Mol Sci. 13:14845–14864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng S, Song QK, Ren Y, et al: The
characteristics of breast cancer subtypes: Implications for
treatment guidelines and individualized treatment strategies in
China. Appl Immunohistochem Mol Morphol. 22:383–389. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dawood S, Hu R, Homes MD, Collins LC,
Schnitt SJ, Connolly J, Colditz GA and Tamimi RM: Defining breast
cancer prognosis based on molecular phenotypes: Results from a
large cohort study. Breast Cancer Res Treat. 126:185–192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linderholm B, Bergqvist J, Hellborg H,
Johansson U, Linderholm M, von Schoultz E, Elmberger G, Skoog L and
Bergh J: Shorter survival-times following adjuvant endocrine
therapy in oestrogen- and progesterone-receptor positive breast
cancer overexpressing HER2 and/or with an increased expression of
vascular endothelial growth factor. Med Oncol. 26:480–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berns EM, Klijn JG, Look MP, et al:
Combined vascular endothelial growth factor and TP53 status
predicts poor response to tamoxifen therapy in estrogen
receptor-positive advanced breast cancer. Clin Cancer Res.
9:1253–1258. 2003.PubMed/NCBI
|
|
14
|
Rydén L, Stendahl M, Jonsson H, Emdin S,
Bengtsson NO and Landberg G: Tumor-specific VEGF-A and VEGFR2 in
postmenopausal breast cancer patients with long-term follow-up.
Implication of a link between VEGF pathway and tamoxifen response.
Breast Cancer Res Treat. 89:135–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Tamimi RM, Collins LC, Schnitt SJ,
Gilmore HL, Connolly JL and Colditz GA: The association between
vascular endothelial growth factor expression in invasive breast
cancer and survival varies with intrinsic subtypes and use of
adjuvant systemic therapy: Results from the Nurses' Health Study.
Breast Cancer Res Treat. 129:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|